Abstract
Epithelia are polarized layers of adherent cells that are the building blocks for organ and appendage structures throughout animals. To preserve tissue architecture and barrier function during both homeostasis and rapid growth, individual epithelial cells divide in a highly constrained manner. Building on decades of research focused on single cells, recent work is probing the mechanisms by which the dynamic process of mitosis is reconciled with the global maintenance of epithelial order during development. These studies reveal how symmetrically dividing cells both exploit and conform to tissue organization to orient their mitotic spindles during division and establish new adhesive junctions during cytokinesis.
The association of large numbers of cells in tightly organized epithelial layers is a unique and defining feature of Metazoa. Although classical studies of development once labeled distinct embryonic regions as territories, fields, layers, placodes, and primordia, we now know many of these structures to be primarily constructed from epithelial sheets. Epithelial structure and function are critically dependent on cell polarization, which is coupled to the targeted assembly of adhesive junctions along the apicolateral membranes of adjacent cells (Tepass et al., 2001; Cavey and Lecuit, 2009). In brief, the plasma membrane of epithelial cells is polarized into apical and basolateral domains, each enriched with distinct lipid and protein components (Fig. 1; Rodriguez-Boulan et al., 2005; St Johnston and Ahringer, 2010). At the molecular level, E-cadherins are the major class of adhesion proteins that establish cell–cell connections through homophilic interaction across cell membranes (Takeichi, 1991, 2011; Halbleib and Nelson, 2006; Harris and Tepass, 2010). Whereas E-cadherin is apically enriched in invertebrate epithelia, it is localized along the lateral domain of vertebrate epithelial cells. In both cases, E-cadherin interacts with cytoplasmic actin filaments via the catenin class of adaptor proteins, thus coupling intercellular adhesive contacts to the cytoskeleton (Cavey and Lecuit, 2009; Harris and Tepass, 2010; Gomez et al., 2011). Within this framework, the maintenance of both polarity and cell–cell adhesion are essential for epithelial barrier function and tissue architecture during growth and morphogenesis (Papusheva and Heisenberg, 2010; Guillot and Lecuit, 2013b).
Figure 1.
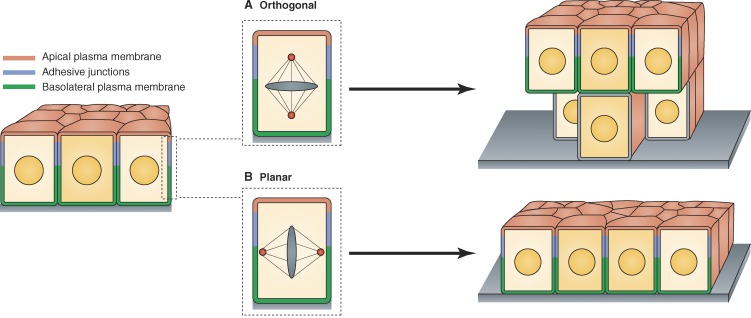
Architectural implications of orthogonal and planar spindle orientations during epithelial cell division. (A) Programmed orthogonal orientation of the mitotic spindle can promote epithelial stratification, although the remodeling of adhesion and polarity complexes during this process remains an important area for further study. (B) Planar spindle orientation is coordinated with the overall cell polarity machinery and thus facilitates conservation of monolayer organization during rapid cell proliferation.
During development, epithelia expand by the combined effects of cell growth (increase in cell size) and cell division (increase in cell numbers). Division events are typically oriented either parallel or orthogonal to the plane of the layer and less frequently at oblique angles (Gillies and Cabernard, 2011). When cells divide orthogonally (perpendicular to the plane of the epithelium), the two daughters will be at least initially nonequivalent with respect to position within the cell layer (Fig. 1 A). Under normal conditions, such programmed orthogonal divisions can be used to effect asymmetric segregation of cell fates or to establish distinct cell types, such as in the developing cortex (Fietz et al., 2010; Hansen et al., 2010) or during morphogenesis of stratified epithelia (Lechler and Fuchs, 2005; Williams et al., 2011). Conversely, when cells divide parallel to the plane of the epithelium (planar orientation; Fig. 1 B), both daughter cells are equivalent with respect to mother cell polarity and tightly integrated in the growing monolayer (Morin and Bellaïche, 2011).
During planar division, epithelial cells typically round up, constrict in the middle to form the cytokinetic furrow, and divide symmetrically with respect to the apicobasal axis to produce two equal daughter cells. These daughters construct new cell–cell junctions at their nascent interface, thus integrating into the monolayer (Fig. 2, A–G). Although the intricate relationship between cell polarity and cell division has been explored for many years in the context of asymmetric cell division (Rhyu and Knoblich, 1995; Siller and Doe, 2009; Williams and Fuchs, 2013), recent studies have also begun to explore how epithelia maintain their morphology, integrity, and barrier function during continuous rounds of planar cell division and junction assembly. In this review, we highlight recent findings that provide new insights into the problem of symmetric planar cell division in diverse polarized epithelia, with a focus on two crucial mitotic events: (1) the orientation of cell division and (2) the formation of new cell junctions.
Figure 2.
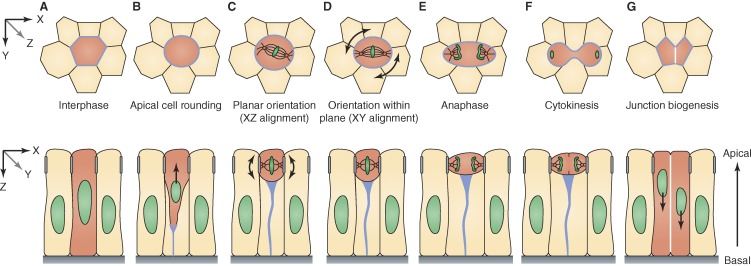
Progression of planar cell division in an epithelial monolayer. Apical cross section (xy, top row) and longitudinal (xz, bottom row) view of a dividing cell (red). (A) At the level of apical junctions, cells are packed in a polygonal cell arrangement during interphase. (B) In prophase, the dividing nucleus begins to translocate apically as the cell rounds up and maintains a thin basal projection enriched with nonmuscle myosin II and actin (light blue). Notably, this type of nuclear migration is typically observed in pseudostratified columnar epithelia and does not occur in cuboidal and squamous epithelial tissues. (C) Localized molecular landmarks (apical complexes marked as gray bars on cell sides) direct orientation of the mitotic spindle to the plane of the epithelium (arrows). (D) Within the plane of the cell layer, the spindle can be further oriented (arrows) in response to molecular cues, global tissue tension, and local cell geometry. (E and F) After chromosome segregation during anaphase, the cell constricts in the middle and cleaves orthogonal to the plane of the monolayer. (G) After cytokinesis, daughter nuclei move basally and daughter cells form new junctions at their nascent interface (white) while elongating along the apicobasal axis.
Mitotic spindle position and orientation in epithelial cells
Planar orientation of epithelial cell division requires coordinated interaction between the cell polarization machinery and the mitotic spindle itself (Morin and Bellaïche, 2011). In animal cells, the spindle is organized by two symmetrically positioned poles or centrosomes, which nucleate three forms of microtubules (Tanaka, 2010): kinetochore microtubules that attach to the chromosomes, polar microtubules that overlap in an antiparallel fashion over the midplane, and astral microtubules that extend to the cell cortex, which is the actin-rich layer beneath the cell membrane (Lancaster and Baum, 2014). Work in Drosophila melanogaster and vertebrates reveals that at least three factors influence the orientation of this spindle machinery with respect to polarized epithelial architecture: cytoskeletal forces, localized cortical cues, and tissue tension.
Cytoskeletal forces position mitotic nuclei near the apical cell membrane.
In columnar and pseudostratified epithelia where cells elongate along their apicobasal axes, mitotic events are typically restricted to the apical domain of the epithelium (corresponding to the apical membrane of each cell; Fig. 2, C–F). How does the mitotic nucleus achieve the correct apical position? In Drosophila wing discs and zebrafish neuroepithelia, mitotic nuclei and the bulk of the cell cytoplasm are driven apically by actomyosin-dependent cortical contractility at prophase entry (Norden et al., 2009; Leung et al., 2011; Meyer et al., 2011). These events are fundamentally similar to mitotic cell rounding in tissue culture cells (Kunda and Baum, 2009; Lancaster et al., 2013; Lancaster and Baum, 2014). In many epithelia, as the cell rounds up and the nucleus translocates apically, a thin actin-rich projection maintains contact with the basal lamina (Fig. 2, B and C). It remains poorly understood how this structure behaves during cleavage and whether this basal process plays any role in the correct reintegration of the postmitotic daughter cells into the monolayer. Although actomyosin may be the primary driver of apical rounding in many cases, evidence also supports a role for microtubule-based mechanisms in the positioning of premitotic nuclei. In chicken neural tube and mouse cerebral cortex, nuclei migrate apically on microtubules before actomyosin-dependent rounding (Spear and Erickson, 2012a). Centrosomes provide directionality to the microtubules on which the nucleus migrates and organize the spindle once the mitotic chromatin reaches the apical domain (Peyre et al., 2011; Spear and Erickson, 2012a; Nakajima et al., 2013). Collectively, current evidence suggests that both actomyosin- and microtubule-dependent forces conspire to effect mitotic nuclear translocation in a highly context- and species-specific manner. One possibility is that the varying physical dimensions of epithelial cells require varying mechanisms for apical nuclear translocation. For example, highly elongated radial glial cells require active transport of the nucleus on microtubules before mitotic rounding, whereas cortical actomyosin contractility may be sufficient in less elongated cells (Spear and Erickson, 2012b). A major outstanding problem is how cortical contractility triggers cell rounding that is polarized along the apicobasal axis of the cell. Whereas centrosomes function as an apical landmark for nuclei moving on microtubules, it remains unclear what provides the directionality for the basal-to-apical actomyosin contraction. One hypothesis is that certain proteins can restrict the localization of nonmuscle myosin II at the basal domain of epithelial cells. The microcephaly protein Asp interacts with myosin II and regulates its polarized localization along the apicobasal axis in the fly optic lobe neuroepithelium. In asp mutant flies, myosin II is enriched apically instead of basally. Many dividing nuclei fail to reach the apical domain and are thus broadly distributed along the apicobasal axis of the epithelium, leading to a disorganized tissue (Rujano et al., 2013). Interestingly, Asp also interacts with microtubules, associates with spindle poles, and is essential for positioning the spindle in fly and vertebrate epithelia (Saunders et al., 1997; do Carmo Avides and Glover, 1999; Wakefield et al., 2001; Fish et al., 2006). Elucidating the function of proteins such as Asp at the interface of microtubules and actomyosin will be essential to our understanding of how the cytoskeleton drives apical mitotic rounding.
Localized molecular landmarks direct planar spindle orientation.
In most animal cells, the mitotic spindle is anchored to the cell cortex by astral microtubules (Fig. 2, C–E; Théry and Bornens, 2006). Translocation of the dynein–dynactin motor toward the astral microtubule minus ends provides a pulling force on centrosomes and is essential for spindle orientation and pole separation during cell division (Dujardin and Vallee, 2002; Kotak et al., 2012). Molecular cues embedded in the cortex can thus determine spindle orientation by anchoring the dynein–dynactin complex in restricted domains. In cultured MDCK and chick neuroepithelia cells, the Gαi–LGN–nuclear mitotic apparatus (NuMA) complex serves this function (Busson et al., 1998; Hao et al., 2010; Zheng et al., 2010; Peyre et al., 2011). Knockdown or mislocalization of these factors leads to spindle orientation defects that ultimately lead to removal of cell progenitors from the monolayer (Peyre et al., 2011). LGN (Pins in Drosophila) localizes to the lateral cell cortex by binding to the membrane-bound Gαi and enforces spindle orientation by recruiting NuMA (Mud in Drosophila), which binds directly to the dynein–dynactin motor. In certain epithelia, including MDCK cells and Drosophila wing discs, LGN is excluded from the apical domain by atypical PKC (aPKC) phosphorylation, thus restricting it at the lateral cell cortex (Konno et al., 2008; Hao et al., 2010; Zheng et al., 2010; Guilgur et al., 2012). In chick neuroepithelia, however, LGN is restricted at the lateral cortex independently of aPKC, suggesting that other cues control its localization (Morin et al., 2007; Peyre et al., 2011). In the mouse embryonic neocortex, the actin–membrane linkers ERM (ezrin/radixin/moesin) promote the association of LGN with NuMA (Machicoane et al., 2014), indicating that organized cortical actin is critical for correct LGN localization.
Cell–cell junctions have been implicated in planar cell division in mammalian epithelia, suggesting a possible direct link between the polarity apparatus and the spindle machinery (Reinsch and Karsenti, 1994; den Elzen et al., 2009). Interfering with E-cadherin function or reducing E-cadherin levels abolishes junctional localization of APC (adenomatous polyposis coli), a microtubule-interacting protein that is required for planar spindle orientation and chromosome alignment (Green et al., 2005; den Elzen et al., 2009). However, spindle orientation may not directly depend on E-cadherin or adherens junctions (AJs) in all cases. In Drosophila follicle cells and imaginal discs as well as Xenopus laevis embryonic epithelia, mitotic spindles exhibit planar orientation but do not align with the AJs (Woolner and Papalopulu, 2012; Bergstralh et al., 2013; Nakajima et al., 2013). Moreover, disruption of AJs in Drosophila follicle cells does not affect spindle position (Bergstralh et al., 2013).
In Drosophila wing discs, the spindle poles localize in close proximity to septate junctions, which are positioned immediately basal to AJs (Nakajima et al., 2013). Septate junctions are enriched with many proteins, including the neoplastic tumor suppressors SCRIB (Scribbled) and DLG1 (Discs large 1; Bilder and Perrimon, 2000; Bilder et al., 2000). In asymmetrically dividing cells, such as Drosophila sensory organ precursors and neuroblasts, DLG1 interacts with LGN at the cortex and is required for proper spindle orientation (Bellaïche et al., 2001; Siegrist and Doe, 2005; Johnston et al., 2009). Recent findings indicate that DLG1 is also essential for planar spindle orientation in the symmetric division of epithelial cells. In wing discs, knockdown of scrib or dlg1 leads to randomized spindle orientations. scrib knockdown wing discs exhibit diffuse DLG1 localization but no obvious apicobasal polarity defect, suggesting that epithelial disorganization could be a consequence of aberrant spindle orientation (Nakajima et al., 2013). However, it is not clear whether the septate junctions themselves are important. In Drosophila follicle epithelial cells where septate junctions do not form until relatively late in development (Oshima and Fehon, 2011), DLG1 is localized at the lateral cell cortex and is essential for planar spindle orientation (Bergstralh et al., 2013). Interestingly, dlg1 mutant follicle cells display misoriented divisions yet normal epithelial polarity and tissue organization. In this case, planar spindle orientation appears to be independent of junctions per se but still depends on a DLG1–LGN–NuMA complex, similar to asymmetrically dividing cells (Bergstralh et al., 2013).
Global stress and local cell geometry influence mitotic spindle orientation within the plane of the epithelium.
During planar divisions, the mitotic spindle aligns to the plane of the epithelium (xz; Fig. 2 C) and also within the plane of the cell layer (xy; Fig. 2 D). Studies in gastrulating zebrafish embryos revealed a role for the Wnt–Frizzled–planar cell polarity signaling pathway in orienting cell divisions (Concha and Adams, 1998; Gong et al., 2004). Similarly, the atypical cadherins Fat and Dachsous are involved in orienting cell divisions in the Drosophila wing and in developing mouse kidneys (Baena-López et al., 2005; Saburi et al., 2008). Although both of these pathways have been reviewed elsewhere (Morin and Bellaïche, 2011), recent studies also point to at least two other mechanisms that may independently influence spindle orientation within the plane of the monolayer: (1) global tissue stress and (2) local epithelial cell geometry.
Epithelial cell shape and spindle orientation are modulated by global stress that accumulates during tissue growth. In Drosophila wing discs, cells in the center of the wing blade primordium proliferate at a faster rate than in the periphery. Consequently, cells in the periphery are mechanically stretched, and cells in the center are compressed. As a result of stretching, peripheral cells localize myosin II at their cortex and align their mitotic spindle with the stretch axis (LeGoff et al., 2013; Mao et al., 2013). Similarly, epithelial cells of the enveloping cell layer in gastrulating zebrafish embryos elongate and orient their spindle along the direction of tension generated by spreading during epiboly (Campinho et al., 2013). It is unclear whether myosin II directly conveys cell tension to the mitotic apparatus, and it will be necessary to dissect whether cell elongation alone or additional mechanosensing pathways signal cell tension to the mitotic spindle. Keratinocytes from the mammalian epidermis reorient their mitotic spindle in response to mechanical stretch in a NuMA-dependent manner. The mitotic spindle aligns with the cortical NuMA-localized crescent upon stretch and fails to orient when NuMA levels are reduced (Seldin et al., 2013). In summary, global tension generated by growth and cell spreading impact division orientation, suggesting that shape changes in proximity to dividing cells may also lead to a similar effect.
Although variations certainly exist, the apical surfaces of proliferating epithelia tend to feature a consistent percentage of hexagonal, pentagonal, heptagonal, and octagonal cell shapes (Gibson et al., 2006; Farhadifar et al., 2007; Aegerter-Wilmsen et al., 2010). In Drosophila imaginal discs, these local patterns of cell packing may systematically influence spindle orientation, as mitotic cells are biased toward cleaving their common interfaces with subhexagonal neighbors (less than six sides) and avoid cleaving their interfaces with superhexagonal neighbors (more than six sides; Gibson et al., 2011). Although the mechanisms underlying the effect of local cell geometry remain elusive, cell packing influences mitotic cell shape and the distribution of adhesive cues, both of which could, in turn, bias spindle orientation. Indeed, dividing cells maintain contacts with their neighbors, which can influence the cell cortex and direct spindle orientation (Goldstein, 1995; Wang et al., 1997). The distribution of adhesions between epithelial cells may also alter the position or action of cortical force generators that interact with spindle microtubules in the mitotic cell. In support of this idea, when single cells are placed on micropatterned substrates, they orient their spindle relative to the geometry of their adhesion pattern and not their cell shape (Théry et al., 2005, 2007). Alternatively, neighbors of different polygonal shapes could stretch the mitotic cell, thus imposing a bias on its long axis. Indeed, sea urchin embryos orient their spindles to divide their longest axis (Hertwig, 1884) and can even sense complex cell geometries to orient their spindles accordingly (Minc et al., 2011). Still, precisely how the interphase morphology of epithelial cells might impinge on mitotic spindle orientation remains an open question.
Genesis of nascent junctions during epithelial cell division
After spindle orientation, the essential processes of cytokinesis and abscission are driven by the assembly and contraction of an actomyosin ring positioned in the cleavage plane (Fededa and Gerlich, 2012). In epithelia, ring contraction accompanied by membrane invagination ultimately gives rise to a new junctional interface between nascent daughter cells. Precisely how this new interface forms remains poorly understood. Recent studies in Drosophila epithelia reveal that, during cytokinesis, (a) E-cadherin levels are reduced at the interface between the cleavage furrow of dividing cells and their neighbors (Fig. 3), and (b) neighbor tension and midbody position guide establishment of new AJs in context with local epithelial geometry (Fig. 4).
Figure 3.
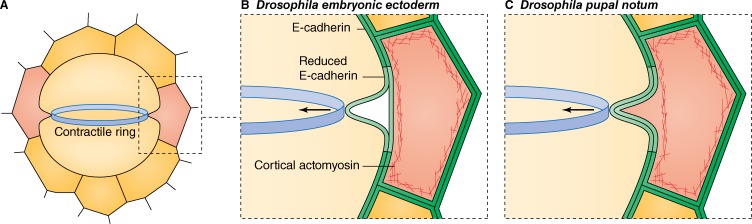
Cytokinetic membrane dynamics in epithelial cells. (A) Cytokinesis of a dividing epithelial cell (yellow) presents several unique structural considerations not addressed by the analysis of single cells. Recent studies (Founounou et al., 2013; Guillot and Lecuit, 2013a; Herszterg et al., 2013, 2014) report a local reduction of E-cadherin levels in proximity to the contractile ring in the dividing cell and its neighbor (red). How cytokinesis is resolved from there may vary in a context-dependent manner. (B) In Drosophila embryos, ring contraction leads to E-cadherin disengagement, and a gap forms between the mitotic cell and its neighbor (Guillot and Lecuit, 2013a). (C) In the Drosophila pupal notum, the contractile ring pulls the neighbor cell plasma membrane into the cleavage furrow, perhaps enabled by uncoupling of the membrane and the cortex in the neighbor (Herszterg et al., 2013, 2014).
Figure 4.
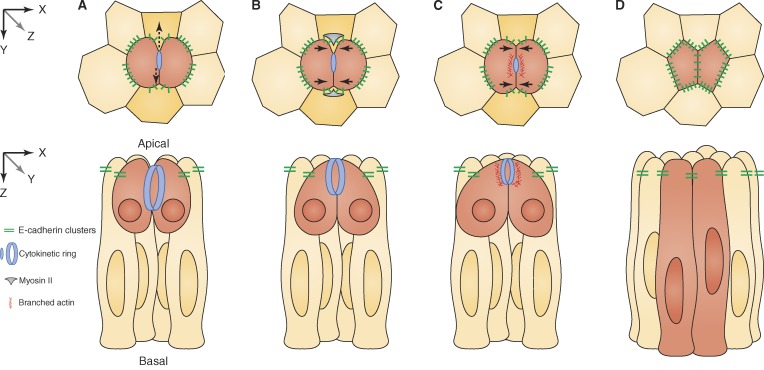
New AJ formation in dividing epithelial cells. Apical cross section (xy, top row) and longitudinal (xz, bottom row) view of a dividing epithelial cell (red). (A) Opposing forces (black vertical arrows) develop between the contractile ring in the dividing cell and the two neighboring cells (orange) in proximity to the cleavage furrow. E-cadherin clusters are reduced at the furrow/neighbor interface. (B) Myosin II and tension build up in the neighboring cell, causing the nascent daughter cells to juxtapose their plasma membranes at the presumptive site of junction assembly (black horizontal arrows). (C) Arp2/3 and Rac1 drive actin polymerization at the daughter cell interface around the midbody, stabilizing the nascent junction as the neighboring cell membrane withdraws. (D) The new junction is complete and of suitable length in context with the local epithelial geometry.
Mitotic cells remodel their adhesion junctions during cytokinesis.
Two kinds of forces are at work during cytokinesis: an active force in the dividing cell caused by ring contraction and a reactive force in contacting neighbors caused by their resistance to pulling to maintain their shape (Fig. 3 and Fig. 4 A; Founounou et al., 2013; Guillot and Lecuit, 2013a; Herszterg et al., 2013). Recent results indicate that these opposing forces can lead to a transient and partial reduction of cell adhesion after mitotic exit. In Drosophila epithelia, E-cadherin levels are reduced at the interface between the cleavage furrow of the dividing cell and its neighbors (Fig. 3, B and C; Founounou et al., 2013; Guillot and Lecuit, 2013a; Herszterg et al., 2013; Morais-de-Sá and Sunkel, 2013). Specifically in embryonic epithelia, the local reduction of E-cadherin facilitates membrane separation, and a gap appears between the dividing cell and its neighbors (Fig. 3 B; Guillot and Lecuit, 2013a). In the dorsal thorax, in contrast, the neighbor cell plasma membrane detaches from the cortex and is drawn into the cleavage furrow (Fig. 3 C; Herszterg et al., 2013, 2014). What triggers E-cadherin modulation in cells after mitotic exit? The loss of overall cell polarity is one possible mechanism. During mitosis in Drosophila, follicular epithelial cells lose cortical enrichment of some apical polarity proteins (aPKC, Crumbs, and Bazooka/Par3; Bergstralh et al., 2013; Morais-de-Sá and Sunkel, 2013), and embryonic cells lose localization of lethal giant larvae, a basolateral cortical protein (Huang et al., 2009). Contrasting with these observations, however, MDCK cells and Drosophila embryonic and dorsal thorax epithelial cells appear to maintain apicobasal polarity as they divide (Reinsch and Karsenti, 1994; Founounou et al., 2013; Guillot and Lecuit, 2013a; Herszterg et al., 2013). Furthermore, E-cadherin reduction is limited to the furrow/neighbor interface and is not observed in other areas of cell contact. Therefore, an alternative mechanism that explains local E-cadherin modulation is mechanical tension that arises precisely at the area between the contractile ring and the neighboring cell membrane (Founounou et al., 2013; Guillot and Lecuit, 2013a).
Does E-cadherin modulation serve a functional role in mitotic cells? In Drosophila embryonic and dorsal thorax epithelia, E-cadherin decrease leads to a local adhesion disengagement proposed to facilitate the formation of new AJs between daughter cells (Founounou et al., 2013; Guillot and Lecuit, 2013a). It has been previously reported that cells maintain their AJs throughout division. For example, intercellular junctions are maintained in dividing cells of human colonic mucosal crypt cells and basal keratinocytes (Baker and Garrod, 1993). Similarly, mitotic MDCK cultured cells maintain tight junctions apically and E-cadherin basolaterally (Reinsch and Karsenti, 1994). The E-cadherin loss in certain Drosophila epithelia may be either a tissue-specific phenomenon or a highly dynamic process only observable with the temporal resolution of live-cell imaging. Moreover, dividing cells in the Drosophila dorsal thorax show decreased levels of E-cadherin yet maintain their cohesiveness (Herszterg et al., 2013). Interestingly, E-cadherin is internalized in mitotic MDCK cells (Bauer et al., 1998). It will therefore be important to investigate whether loss of E-cadherin leads to adhesion disengagement in other epithelial tissues and whether tension alone or in combination with biochemical pathways is responsible for E-cadherin modulation.
Epithelial neighbors exert tension on daughter cell membranes to facilitate new AJ formation.
How new junctional contacts form during mitosis is a poorly understood problem at the heart of epithelial cell biology. In Drosophila, new membrane interfaces between nascent daughter cells initially show only a weak level of E-cadherin clusters (Guillot and Lecuit, 2013a; Herszterg et al., 2013). Subsequently, the daughter cells assemble their AJs de novo. How is the length of these new junctions determined with respect to cell geometry? Recent evidence indicates that AJ length is a function of local cell packing within the epithelium. In dividing cells of the Drosophila dorsal thorax, the contractile ring triggers tension and accumulation of myosin II in neighbors at the furrow/neighbor interface (Fig. 4 B; Founounou et al., 2013; Herszterg et al., 2013). Myosin II in the neighboring cells in turn contracts and creates tension at the furrowing membrane of the nascent daughter cells, keeping them tightly pressed against each other (Fig. 4, B and C). This local membrane juxtaposition facilitates AJ formation. To allow expansion of the daughter cell interface and maintain AJ length, branched actin polymerization via Rac1 and Arp2/3 is oriented to the midbody, which serves as a positional landmark for new AJs (Fig. 4 C; Herszterg et al., 2013). The midbody is a narrow intercellular bridge that remains after the contracted cytokinetic ring has driven membrane invagination, and it recruits the abscission factors that will eventually separate the daughter cells (Fededa and Gerlich, 2012). Interestingly, the midbody is positioned apically as a result of the presence of AJs. In Drosophila follicular epithelia, the midbody also provides cues for the formation of the apical daughter cell interface, suggesting that it plays a role in both AJ and epithelial cell polarity establishment and maintenance in dividing epithelial cells (Morais-de-Sá and Sunkel, 2013). Thus, examples from Drosophila epithelia show that cohesion between dividing cells and their neighbors together with the apically positioned midbody provides a spatial template and polarized positional cue for de novo AJ assembly (Herszterg et al., 2013; Morais-de-Sá and Sunkel, 2013). Further work on other epithelial tissues may provide alternative mechanisms of junction biogenesis.
Growth and order in the epithelium: Thinking outside the cell
During development, epithelial monolayers have the remarkable capacity to maintain specialized morphologies and barrier functions during rapid cell proliferation. Mitotic cells remain adherent to their neighbors throughout cell division. Cell cohesion enables local geometry and global tissue tension to instruct mitotic cells where to position their cleavage plane and how to assemble their junctions. However, local tension may also lead to a transient disengagement of dividing cells from their neighbors after mitotic exit. How is global and local tension conveyed to protein complexes in mitotic cells so that different outcomes take place? Moreover, it is unclear whether and how tissue tension instructs synchronously dividing epithelial cells how to divide and reestablish their junctions after division. Clearly, this is a fundamental problem for the maintenance of epithelial order and may be linked to the origin of epithelial cancers, in which cells undergo rapid proliferation but fail to remain integrated into the monolayer.
The selected studies discussed here hint at the remarkable level of coordination that occurs during epithelial cell division, recasting mitosis as a truly multicellular process. Looking ahead, understanding the interface between cells, proteins, and mechanical forces that each operate on different scales will require creative multidisciplinary approaches in diverse organismal systems. Indeed, epithelial organization is widespread in nature and is encountered among even the most basal animals, including sponges and cnidarians as well as the fruiting body of the nonmetazoan social amoeba Dictyostelium discoideum (Wood, 1959; Ereskovsky et al., 2009; Houliston et al., 2010; Dickinson et al., 2011; Meyer et al., 2011). Combined, future interdisciplinary studies and a fresh look at diverse animal models should yield new insight into epithelial cell division for many years to come.
Acknowledgments
We are grateful to Antony Jose, Yuichiro Nakajima, Boris Rubinstein, and Kendra Marr for helpful discussions and their comments on the manuscript. We also thank the anonymous reviewers for their constructive and critical suggestions.
This work was supported by the Stowers Institute for Medical Research. Illustrations were provided by Neil Smith, www.neilsmithillustration.co.uk.
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- AJ
- adherens junction
- aPKC
- atypical PKC
- NuMA
- nuclear mitotic apparatus
References
- Aegerter-Wilmsen, T., Smith A.C., Christen A.J., Aegerter C.M., Hafen E., and Basler K.. 2010. Exploring the effects of mechanical feedback on epithelial topology. Development. 137:499–506. 10.1242/dev.041731 [DOI] [PubMed] [Google Scholar]
- Baena-López, L.A., Baonza A., and García-Bellido A.. 2005. The orientation of cell divisions determines the shape of Drosophila organs. Curr. Biol. 15:1640–1644. 10.1016/j.cub.2005.07.062 [DOI] [PubMed] [Google Scholar]
- Baker, J., and Garrod D.. 1993. Epithelial cells retain junctions during mitosis. J. Cell Sci. 104:415–425 [DOI] [PubMed] [Google Scholar]
- Bauer, A., Lickert H., Kemler R., and Stappert J.. 1998. Modification of the E-cadherin-catenin complex in mitotic Madin-Darby canine kidney epithelial cells. J. Biol. Chem. 273:28314–28321. 10.1074/jbc.273.43.28314 [DOI] [PubMed] [Google Scholar]
- Bellaïche, Y., Radovic A., Woods D.F., Hough C.D., Parmentier M.L., O’Kane C.J., Bryant P.J., and Schweisguth F.. 2001. The Partner of Inscuteable/Discs-large complex is required to establish planar polarity during asymmetric cell division in Drosophila. Cell. 106:355–366. 10.1016/S0092-8674(01)00444-5 [DOI] [PubMed] [Google Scholar]
- Bergstralh, D.T., Lovegrove H.E., and St Johnston D.. 2013. Discs large links spindle orientation to apical-basal polarity in Drosophila epithelia. Curr. Biol. 23:1707–1712. 10.1016/j.cub.2013.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder, D., and Perrimon N.. 2000. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 403:676–680. 10.1038/35001108 [DOI] [PubMed] [Google Scholar]
- Bilder, D., Li M., and Perrimon N.. 2000. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 289:113–116. 10.1126/science.289.5476.113 [DOI] [PubMed] [Google Scholar]
- Busson, S., Dujardin D., Moreau A., Dompierre J., and De Mey J.R.. 1998. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr. Biol. 8:541–544. 10.1016/S0960-9822(98)70208-8 [DOI] [PubMed] [Google Scholar]
- Campinho, P., Behrndt M., Ranft J., Risler T., Minc N., and Heisenberg C.P.. 2013. Tension-oriented cell divisions limit anisotropic tissue tension in epithelial spreading during zebrafish epiboly. Nat. Cell Biol. 15:1405–1414. 10.1038/ncb2869 [DOI] [PubMed] [Google Scholar]
- Cavey, M., and Lecuit T.. 2009. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb. Perspect. Biol. 1:a002998. 10.1101/cshperspect.a002998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha, M.L., and Adams R.J.. 1998. Oriented cell divisions and cellular morphogenesis in the zebrafish gastrula and neurula: a time-lapse analysis. Development. 125:983–994 [DOI] [PubMed] [Google Scholar]
- den Elzen, N., Buttery C.V., Maddugoda M.P., Ren G., and Yap A.S.. 2009. Cadherin adhesion receptors orient the mitotic spindle during symmetric cell division in mammalian epithelia. Mol. Biol. Cell. 20:3740–3750. 10.1091/mbc.E09-01-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson, D.J., Nelson W.J., and Weis W.I.. 2011. A polarized epithelium organized by β- and α-catenin predates cadherin and metazoan origins. Science. 331:1336–1339. 10.1126/science.1199633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo Avides, M., and Glover D.M.. 1999. Abnormal spindle protein, Asp, and the integrity of mitotic centrosomal microtubule organizing centers. Science. 283:1733–1735. 10.1126/science.283.5408.1733 [DOI] [PubMed] [Google Scholar]
- Dujardin, D.L., and Vallee R.B.. 2002. Dynein at the cortex. Curr. Opin. Cell Biol. 14:44–49. 10.1016/S0955-0674(01)00292-7 [DOI] [PubMed] [Google Scholar]
- Ereskovsky, A.V., Borchiellini C., Gazave E., Ivanisevic J., Lapébie P., Perez T., Renard E., and Vacelet J.. 2009. The Homoscleromorph sponge Oscarella lobularis, a promising sponge model in evolutionary and developmental biology: model sponge Oscarella lobularis. BioEssays. 31:89–97. 10.1002/bies.080058 [DOI] [PubMed] [Google Scholar]
- Farhadifar, R., Röper J.C., Aigouy B., Eaton S., and Jülicher F.. 2007. The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr. Biol. 17:2095–2104. 10.1016/j.cub.2007.11.049 [DOI] [PubMed] [Google Scholar]
- Fededa, J.P., and Gerlich D.W.. 2012. Molecular control of animal cell cytokinesis. Nat. Cell Biol. 14:440–447. 10.1038/ncb2482 [DOI] [PubMed] [Google Scholar]
- Fietz, S.A., Kelava I., Vogt J., Wilsch-Bräuninger M., Stenzel D., Fish J.L., Corbeil D., Riehn A., Distler W., Nitsch R., and Huttner W.B.. 2010. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat. Neurosci. 13:690–699. 10.1038/nn.2553 [DOI] [PubMed] [Google Scholar]
- Fish, J.L., Kosodo Y., Enard W., Pääbo S., and Huttner W.B.. 2006. Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proc. Natl. Acad. Sci. USA. 103:10438–10443. 10.1073/pnas.0604066103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Founounou, N., Loyer N., and Le Borgne R.. 2013. Septins regulate the contractility of the actomyosin ring to enable adherens junction remodeling during cytokinesis of epithelial cells. Dev. Cell. 24:242–255. 10.1016/j.devcel.2013.01.008 [DOI] [PubMed] [Google Scholar]
- Gibson, M.C., Patel A.B., Nagpal R., and Perrimon N.. 2006. The emergence of geometric order in proliferating metazoan epithelia. Nature. 442:1038–1041. 10.1038/nature05014 [DOI] [PubMed] [Google Scholar]
- Gibson, W.T., Veldhuis J.H., Rubinstein B., Cartwright H.N., Perrimon N., Brodland G.W., Nagpal R., and Gibson M.C.. 2011. Control of the mitotic cleavage plane by local epithelial topology. Cell. 144:427–438. 10.1016/j.cell.2010.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies, T.E., and Cabernard C.. 2011. Cell division orientation in animals. Curr. Biol. 21:R599–R609. 10.1016/j.cub.2011.06.055 [DOI] [PubMed] [Google Scholar]
- Goldstein, B.1995. Cell contacts orient some cell division axes in the Caenorhabditis elegans embryo. J. Cell Biol. 129:1071–1080. 10.1083/jcb.129.4.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, G.A., McLachlan R.W., and Yap A.S.. 2011. Productive tension: force-sensing and homeostasis of cell-cell junctions. Trends Cell Biol. 21:499–505. 10.1016/j.tcb.2011.05.006 [DOI] [PubMed] [Google Scholar]
- Gong, Y., Mo C., and Fraser S.E.. 2004. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 430:689–693. 10.1038/nature02796 [DOI] [PubMed] [Google Scholar]
- Green, R.A., Wollman R., and Kaplan K.B.. 2005. APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment. Mol. Biol. Cell. 16:4609–4622. 10.1091/mbc.E05-03-0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilgur, L.G., Prudêncio P., Ferreira T., Pimenta-Marques A.R., and Martinho R.G.. 2012. Drosophila aPKC is required for mitotic spindle orientation during symmetric division of epithelial cells. Development. 139:503–513. 10.1242/dev.071027 [DOI] [PubMed] [Google Scholar]
- Guillot, C., and Lecuit T.. 2013a. Adhesion disengagement uncouples intrinsic and extrinsic forces to drive cytokinesis in epithelial tissues. Dev. Cell. 24:227–241. 10.1016/j.devcel.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Guillot, C., and Lecuit T.. 2013b. Mechanics of epithelial tissue homeostasis and morphogenesis. Science. 340:1185–1189. 10.1126/science.1235249 [DOI] [PubMed] [Google Scholar]
- Halbleib, J.M., and Nelson W.J.. 2006. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 20:3199–3214. 10.1101/gad.1486806 [DOI] [PubMed] [Google Scholar]
- Hansen, D.V., Lui J.H., Parker P.R., and Kriegstein A.R.. 2010. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 464:554–561. 10.1038/nature08845 [DOI] [PubMed] [Google Scholar]
- Hao, Y., Du Q., Chen X., Zheng Z., Balsbaugh J.L., Maitra S., Shabanowitz J., Hunt D.F., and Macara I.G.. 2010. Par3 controls epithelial spindle orientation by aPKC-mediated phosphorylation of apical Pins. Curr. Biol. 20:1809–1818. 10.1016/j.cub.2010.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, T.J., and Tepass U.. 2010. Adherens junctions: from molecules to morphogenesis. Nat. Rev. Mol. Cell Biol. 11:502–514. 10.1038/nrm2927 [DOI] [PubMed] [Google Scholar]
- Herszterg, S., Leibfried A., Bosveld F., Martin C., and Bellaiche Y.. 2013. Interplay between the dividing cell and its neighbors regulates adherens junction formation during cytokinesis in epithelial tissue. Dev. Cell. 24:256–270. 10.1016/j.devcel.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Herszterg, S., Pinheiro D., and Bellaïche Y.. 2014. A multicellular view of cytokinesis in epithelial tissue. Trends Cell Biol. 24:285–293. 10.1016/j.tcb.2013.11.009 [DOI] [PubMed] [Google Scholar]
- Hertwig, O.1884. Das Problem der Befruchtung und der Isotropie des Eies, eine Theory der Vererbung. Jenaische Zeitschrift fuer Naturwissenschaft. 18:21–23 [Google Scholar]
- Houliston, E., Momose T., and Manuel M.. 2010. Clytia hemisphaerica: a jellyfish cousin joins the laboratory. Trends Genet. 26:159–167. 10.1016/j.tig.2010.01.008 [DOI] [PubMed] [Google Scholar]
- Huang, J., Zhou W., Dong W., Watson A.M., and Hong Y.. 2009. From the Cover: Directed, efficient, and versatile modifications of the Drosophila genome by genomic engineering. Proc. Natl. Acad. Sci. USA. 106:8284–8289. 10.1073/pnas.0900641106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, C.A., Hirono K., Prehoda K.E., and Doe C.Q.. 2009. Identification of an Aurora-A/PinsLINKER/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell. 138:1150–1163. 10.1016/j.cell.2009.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno, D., Shioi G., Shitamukai A., Mori A., Kiyonari H., Miyata T., and Matsuzaki F.. 2008. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat. Cell Biol. 10:93–101. 10.1038/ncb1673 [DOI] [PubMed] [Google Scholar]
- Kotak, S., Busso C., and Gönczy P.. 2012. Cortical dynein is critical for proper spindle positioning in human cells. J. Cell Biol. 199:97–110. 10.1083/jcb.201203166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunda, P., and Baum B.. 2009. The actin cytoskeleton in spindle assembly and positioning. Trends Cell Biol. 19:174–179. 10.1016/j.tcb.2009.01.006 [DOI] [PubMed] [Google Scholar]
- Lancaster, O.M., and Baum B.. 2014. Shaping up to divide: Coordinating actin and microtubule cytoskeletal remodelling during mitosis. Semin. Cell Dev. Biol. 34:109–115. 10.1016/j.semcdb.2014.02.015 [DOI] [PubMed] [Google Scholar]
- Lancaster, O.M., Le Berre M., Dimitracopoulos A., Bonazzi D., Zlotek-Zlotkiewicz E., Picone R., Duke T., Piel M., and Baum B.. 2013. Mitotic rounding alters cell geometry to ensure efficient bipolar spindle formation. Dev. Cell. 25:270–283. 10.1016/j.devcel.2013.03.014 [DOI] [PubMed] [Google Scholar]
- Lechler, T., and Fuchs E.. 2005. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 437:275–280. 10.1038/nature03922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGoff, L., Rouault H., and Lecuit T.. 2013. A global pattern of mechanical stress polarizes cell divisions and cell shape in the growing Drosophila wing disc. Development. 140:4051–4059. 10.1242/dev.090878 [DOI] [PubMed] [Google Scholar]
- Leung, L., Klopper A.V., Grill S.W., Harris W.A., and Norden C.. 2011. Apical migration of nuclei during G2 is a prerequisite for all nuclear motion in zebrafish neuroepithelia. Development. 138:5003–5013. 10.1242/dev.071522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machicoane, M., de Frutos C.A., Fink J., Rocancourt M., Lombardi Y., Garel S., Piel M., and Echard A.. 2014. SLK-dependent activation of ERMs controls LGN–NuMA localization and spindle orientation. J. Cell Biol. 205:791–799. 10.1083/jcb.201401049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, Y., Tournier A.L., Hoppe A., Kester L., Thompson B.J., and Tapon N.. 2013. Differential proliferation rates generate patterns of mechanical tension that orient tissue growth. EMBO J. 32:2790–2803. 10.1038/emboj.2013.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, E.J., Ikmi A., and Gibson M.C.. 2011. Interkinetic nuclear migration is a broadly conserved feature of cell division in pseudostratified epithelia. Curr. Biol. 21:485–491. 10.1016/j.cub.2011.02.002 [DOI] [PubMed] [Google Scholar]
- Minc, N., Burgess D., and Chang F.. 2011. Influence of cell geometry on division-plane positioning. Cell. 144:414–426. 10.1016/j.cell.2011.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais-de-Sá, E., and Sunkel C.. 2013. Adherens junctions determine the apical position of the midbody during follicular epithelial cell division. EMBO Rep. 14:696–703. 10.1038/embor.2013.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin, X., and Bellaïche Y.. 2011. Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev. Cell. 21:102–119. 10.1016/j.devcel.2011.06.012 [DOI] [PubMed] [Google Scholar]
- Morin, X., Jaouen F., and Durbec P.. 2007. Control of planar divisions by the G-protein regulator LGN maintains progenitors in the chick neuroepithelium. Nat. Neurosci. 10:1440–1448. 10.1038/nn1984 [DOI] [PubMed] [Google Scholar]
- Nakajima, Y., Meyer E.J., Kroesen A., McKinney S.A., and Gibson M.C.. 2013. Epithelial junctions maintain tissue architecture by directing planar spindle orientation. Nature. 500:359–362. 10.1038/nature12335 [DOI] [PubMed] [Google Scholar]
- Norden, C., Young S., Link B.A., and Harris W.A.. 2009. Actomyosin is the main driver of interkinetic nuclear migration in the retina. Cell. 138:1195–1208. 10.1016/j.cell.2009.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima, K., and Fehon R.G.. 2011. Analysis of protein dynamics within the septate junction reveals a highly stable core protein complex that does not include the basolateral polarity protein Discs large. J. Cell Sci. 124:2861–2871. 10.1242/jcs.087700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papusheva, E., and Heisenberg C.P.. 2010. Spatial organization of adhesion: force-dependent regulation and function in tissue morphogenesis. EMBO J. 29:2753–2768. 10.1038/emboj.2010.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyre, E., Jaouen F., Saadaoui M., Haren L., Merdes A., Durbec P., and Morin X.. 2011. A lateral belt of cortical LGN and NuMA guides mitotic spindle movements and planar division in neuroepithelial cells. J. Cell Biol. 193:141–154. 10.1083/jcb.201101039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinsch, S., and Karsenti E.. 1994. Orientation of spindle axis and distribution of plasma membrane proteins during cell division in polarized MDCKII cells. J. Cell Biol. 126:1509–1526. 10.1083/jcb.126.6.1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyu, M.S., and Knoblich J.A.. 1995. Spindle orientation and asymmetric cell fate. Cell. 82:523–526. 10.1016/0092-8674(95)90022-5 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan, E., Kreitzer G., and Müsch A.. 2005. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 6:233–247. 10.1038/nrm1593 [DOI] [PubMed] [Google Scholar]
- Rujano, M.A., Sanchez-Pulido L., Pennetier C., le Dez G., and Basto R.. 2013. The microcephaly protein Asp regulates neuroepithelium morphogenesis by controlling the spatial distribution of myosin II. Nat. Cell Biol. 15:1294–1306. 10.1038/ncb2858 [DOI] [PubMed] [Google Scholar]
- Saburi, S., Hester I., Fischer E., Pontoglio M., Eremina V., Gessler M., Quaggin S.E., Harrison R., Mount R., and McNeill H.. 2008. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat. Genet. 40:1010–1015. 10.1038/ng.179 [DOI] [PubMed] [Google Scholar]
- Saunders, R.D., Avides M.C., Howard T., Gonzalez C., and Glover D.M.. 1997. The Drosophila gene abnormal spindle encodes a novel microtubule-associated protein that associates with the polar regions of the mitotic spindle. J. Cell Biol. 137:881–890. 10.1083/jcb.137.4.881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin, L., Poulson N.D., Foote H.P., and Lechler T.. 2013. NuMA localization, stability, and function in spindle orientation involve 4.1 and Cdk1 interactions. Mol. Biol. Cell. 24:3651–3662. 10.1091/mbc.E13-05-0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist, S.E., and Doe C.Q.. 2005. Microtubule-induced Pins/Gαi cortical polarity in Drosophila neuroblasts. Cell. 123:1323–1335. 10.1016/j.cell.2005.09.043 [DOI] [PubMed] [Google Scholar]
- Siller, K.H., and Doe C.Q.. 2009. Spindle orientation during asymmetric cell division. Nat. Cell Biol. 11:365–374. 10.1038/ncb0409-365 [DOI] [PubMed] [Google Scholar]
- Spear, P.C., and Erickson C.A.. 2012a. Apical movement during interkinetic nuclear migration is a two-step process. Dev. Biol. 370:33–41. 10.1016/j.ydbio.2012.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear, P.C., and Erickson C.A.. 2012b. Interkinetic nuclear migration: a mysterious process in search of a function. Dev. Growth Differ. 54:306–316. 10.1111/j.1440-169X.2012.01342.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston, D., and Ahringer J.. 2010. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 141:757–774. 10.1016/j.cell.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Takeichi, M.1991. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 251:1451–1455. 10.1126/science.2006419 [DOI] [PubMed] [Google Scholar]
- Takeichi, M.2011. Self-organization of animal tissues: cadherin-mediated processes. Dev. Cell. 21:24–26. 10.1016/j.devcel.2011.06.002 [DOI] [PubMed] [Google Scholar]
- Tanaka, T.U.2010. Kinetochore-microtubule interactions: steps towards bi-orientation. EMBO J. 29:4070–4082. 10.1038/emboj.2010.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass, U., Tanentzapf G., Ward R., and Fehon R.. 2001. Epithelial cell polarity and cell junctions in Drosophila. Annu. Rev. Genet. 35:747–784. 10.1146/annurev.genet.35.102401.091415 [DOI] [PubMed] [Google Scholar]
- Théry, M., and Bornens M.. 2006. Cell shape and cell division. Curr. Opin. Cell Biol. 18:648–657. 10.1016/j.ceb.2006.10.001 [DOI] [PubMed] [Google Scholar]
- Théry, M., Racine V., Pépin A., Piel M., Chen Y., Sibarita J.B., and Bornens M.. 2005. The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol. 7:947–953. 10.1038/ncb1307 [DOI] [PubMed] [Google Scholar]
- Théry, M., Jiménez-Dalmaroni A., Racine V., Bornens M., and Jülicher F.. 2007. Experimental and theoretical study of mitotic spindle orientation. Nature. 447:493–496. 10.1038/nature05786 [DOI] [PubMed] [Google Scholar]
- Wakefield, J.G., Bonaccorsi S., and Gatti M.. 2001. The Drosophila protein asp is involved in microtubule organization during spindle formation and cytokinesis. J. Cell Biol. 153:637–648. 10.1083/jcb.153.4.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S.W., Griffin F.J., and Clark W.H. Jr. 1997. Cell-cell association directed mitotic spindle orientation in the early development of the marine shrimp Sicyonia ingentis. Development. 124:773–780 [DOI] [PubMed] [Google Scholar]
- Williams, S.E., and Fuchs E.. 2013. Oriented divisions, fate decisions. Curr. Opin. Cell Biol. 25:749–758. 10.1016/j.ceb.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, S.E., Beronja S., Pasolli H.A., and Fuchs E.. 2011. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 470:353–358. 10.1038/nature09793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, R.L.1959. Intercellular attachment in the epithelium of Hydra as revealed by electron microscopy. J. Biophys. Biochem. Cytol. 6:343–352. 10.1083/jcb.6.3.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolner, S., and Papalopulu N.. 2012. Spindle position in symmetric cell divisions during epiboly is controlled by opposing and dynamic apicobasal forces. Dev. Cell. 22:775–787. 10.1016/j.devcel.2012.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Z., Zhu H., Wan Q., Liu J., Xiao Z., Siderovski D.P., and Du Q.. 2010. LGN regulates mitotic spindle orientation during epithelial morphogenesis. J. Cell Biol. 189:275–288. 10.1083/jcb.200910021 [DOI] [PMC free article] [PubMed] [Google Scholar]