Abstract
Gradients of soluble attractants as well as extracellular matrix (ECM) proteins serve as cues for directional cell movement. Such “chemotaxis” and “haptotaxis” steers migration of cells during embryonic development, wound healing, and immune responses. In this issue, Chan et al. (2014. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201404067) show that the tumor suppressor LKB1 controls haptotaxis through the microtubule affinity-regulating kinase (MARK) family, one of the many substrates of the LKB1 master kinase. In the absence of this pathway, melanoma cells migrate irrespective of ECM gradients, which may explain the increased metastatic spread observed in LKB1-deficient tumors.
LKB1 is a serine/threonine kinase that is highly conserved throughout evolution. Its activity is allosterically controlled by interactions with the scaffold protein MO25 and the pseudokinase STRAD (Rajakulendran and Sicheri, 2010). LKB1 acts as a master kinase that phosphorylates 14 kinases within a shared consensus motif (Hardie and Alessi, 2013). Germline mutations in the LKB1 gene cause Peutz-Jeghers syndrome, which is associated with increased cancer risk (Hemminki et al., 1998; Jenne et al., 1998), and inactivating somatic LKB1 mutations are found in several sporadic cancers, including melanoma (Guldberg et al., 1999; Rowan et al., 1999). Moreover, evidence from mouse models points to a prominent role for LKB1 as a suppressor of metastasis in lung cancer and melanoma (Ji et al., 2007; Liu et al., 2012).
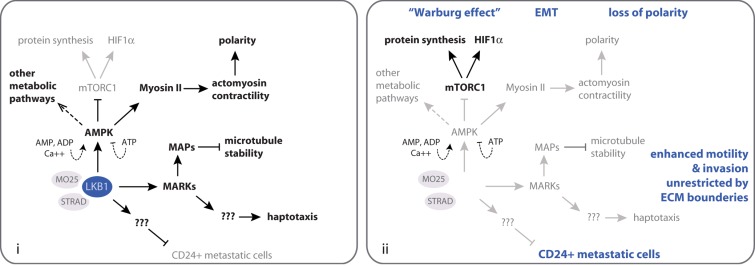
The contribution of the different LKB1 substrates to tumor suppression is poorly understood, with the exception of AMP-activated protein kinase (AMPK). AMPK is activated in an LKB1-dependent manner in metabolically stressed cells, and, in turn, phosphorylates key metabolic enzymes and transcription factors, causing a shift from anabolic (ATP-consuming) to catabolic (ATP-producing) metabolism (Hardie and Alessi, 2013; Fig. 1). This represents a crucial cellular response to metabolic stress that arrests cell proliferation and biosynthesis of macromolecules to restore energy homeostasis. As part of this response, AMPK inhibits the nutrient/energy/redox sensor mTORC1, which controls protein synthesis. Unrestrained mTORC1 activity in the absence of LKB1-AMPK activity leads to continued translation of HIF1α, a transcription factor that helps tumor cells to switch from mitochondrial oxidative metabolism to aerobic glycolysis, a process referred to as “the Warburg effect” (Vander Heiden et al., 2009). Thus, loss of LKB1-AMPK signaling allows cancer cells to disregard metabolic stress and continue proliferating.
Figure 1.
Summary of known and predicted tumor- and metastasis-suppressing functions of LKB1. (i) Pathways restricted by LKB1 activity are indicated in gray, and pathways promoted by LKB1 are indicated in black. (ii) Using the same color scheme as in i, the effects of LKB1 loss in cancer cells are shown. The resulting known and predicted consequences of LKB1 loss for aspects of tumorigenesis and metastasis are indicated in blue. Note that only a limited subset of LKB1 and AMPK substrates is shown. See the text for further details.
In addition to its role in metabolism, LKB1 controls cell division orientation and polarity (Baas et al., 2004; Mirouse and Billaud, 2011). Several LKB1 substrates have been implicated in this process, but activation of AMPK may be the predominant pathway (Zhang et al., 2006; Lee et al., 2007; Zheng and Cantley, 2007). AMPK directly or indirectly phosphorylates the myosin II regulatory light chain, thereby activating the myosin II motor protein that drives actomyosin contractility, which is essential for cell polarity (Lee et al., 2007). Consequently, loss of LKB1-AMPK activity not only allows cancer cells to rewire metabolic signaling networks but also causes loss of epithelial polarity, which contributes to tumorigenesis (Martin-Belmonte and Perez-Moreno, 2012).
Attenuated AMPK-mediated signaling could also be involved in the increased metastatic potential of LKB1-deficient cancers. Loss of polarity not only contributes to tumorigenesis but also equips cancer cells with a morphology fit for invasion of surrounding tissues and dissemination to distant organs. The increased abundance of HIF1α, in addition to metabolic rewiring, transcriptionally activates programs involved in epithelial-to-mesenchymal transition (EMT), matrix remodeling, and growth factor signaling that promote cell migration, local invasion, and dissemination (Lu and Kang, 2010). However, AMPK phosphorylates the microtubule plus-end-tracking protein CLIP-170 to support microtubule dynamics and cell migration (Nakano et al., 2010). It is not known to what extent changes in these LKB1-AMPK–regulated processes contribute to metastasis of LKB1-deficient tumors. In melanoma and lung cancer where genetic mouse studies implicate LKB1 in metastasis suppression, the relevant LKB1 substrate is unidentified, but in both cases expansion of a CD24+ highly metastatic cell population is observed (Ji et al., 2007; Liu et al., 2012).
The work by Chan et al. adds a new function to LKB1 that may underlie its role as a metastasis suppressor. In several melanoma cell models the authors test how depletion or reconstitution of LKB1 affects cell migration. The presence of LKB1 limits cell migration on 2D and in 3D ECM substrates. Cells lacking LKB1 do not pause at ECM boundaries and migrate randomly with respect to 2D or 3D ECM gradients, whereas chemotaxis is fully intact. Notably, LKB1 does not regulate signaling by the integrin family of ECM receptors, secretion of ECM-degrading proteases, or formation of pro-invasive cell substratum contacts called invadopodia. The authors show that haptotaxis requires membrane-targeted, kinase-active LKB1. They use RNA interference and pharmacological inhibitors to exclude involvement of AMPK and several other LKB1 substrates expressed in the melanoma cells. Instead, they establish that the microtubule affinity-regulating kinases (MARKs) are essential mediators of LKB1-dependent haptotaxis. MARKs are known to phosphorylate microtubule-associated proteins (MAPs), thereby destabilizing microtubules (Illenberger et al., 1996). However, strikingly, the LKB1-MARK–mediated haptotaxis identified by Chan et al. (2014) does not depend on MAP phosphorylation, nor does it require intact microtubules.
This work adds a new branch to the LKB1 signaling network that may be specifically important for its role as a metastasis suppressor. Future studies will have to unravel how LKB1-MARK signaling controls haptotaxis, if not through MAP-mediated control of microtubule dynamics. What is the relevant MARK substrate for LKB1-controlled haptotaxis? Moreover, as is the case for most LKB1-controlled processes, it is unknown to what extent these findings are context dependent. Is the role for LKB1 in haptotaxis specific for melanoma cells? How is it affected by the repertoire of mutated oncogenes and tumor suppressors in a given cancer? And, perhaps most urgent, is this pathway really responsible for the strong metastatic potential of LKB1-deficient melanomas? The authors provide evidence that MARKs limit melanoma cell invasion in 3D ECM substrates, but the question of whether LKB1-MARK–mediated haptotaxis represents a metastasis-suppressing process remains to be answered.
Acknowledgments
I acknowledge support from the Dutch Cancer Society (UL 2010-4670).
The author declares no competing financial interests.
References
- Baas, A.F., Kuipers J., van der Wel N.N., Batlle E., Koerten H.K., Peters P.J., and Clevers H.C.. 2004. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 116:457–466. 10.1016/S0092-8674(04)00114-X [DOI] [PubMed] [Google Scholar]
- Chan, K.T., Asokan S.B., King S.J., Bo T., Dubose E.S., Liu W., Berginski M.E., Simon J.M., Davis I.J., Gomez S.M., et al. 2014. LKB1 loss in melanoma disrupts directional migration toward extracellular matrix cues. J. Cell Biol. 207:299–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldberg, P., thor Straten P., Ahrenkiel V., Seremet T., Kirkin A.F., and Zeuthen J.. 1999. Somatic mutation of the Peutz-Jeghers syndrome gene, LKB1/STK11, in malignant melanoma. Oncogene. 18:1777–1780. 10.1038/sj.onc.1202486 [DOI] [PubMed] [Google Scholar]
- Hardie, D.G., and Alessi D.R.. 2013. LKB1 and AMPK and the cancer-metabolism link - ten years after. BMC Biol. 11:36. 10.1186/1741-7007-11-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki, A., Markie D., Tomlinson I., Avizienyte E., Roth S., Loukola A., Bignell G., Warren W., Aminoff M., Höglund P., et al. 1998. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 391:184–187. 10.1038/34432 [DOI] [PubMed] [Google Scholar]
- Illenberger, S., Drewes G., Trinczek B., Biernat J., Meyer H.E., Olmsted J.B., Mandelkow E.M., and Mandelkow E.. 1996. Phosphorylation of microtubule-associated proteins MAP2 and MAP4 by the protein kinase p110mark. Phosphorylation sites and regulation of microtubule dynamics. J. Biol. Chem. 271:10834–10843. 10.1074/jbc.271.18.10834 [DOI] [PubMed] [Google Scholar]
- Jenne, D.E., Reimann H., Nezu J., Friedel W., Loff S., Jeschke R., Müller O., Back W., and Zimmer M.. 1998. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet. 18:38–43. 10.1038/ng0198-38 [DOI] [PubMed] [Google Scholar]
- Ji, H., Ramsey M.R., Hayes D.N., Fan C., McNamara K., Kozlowski P., Torrice C., Wu M.C., Shimamura T., Perera S.A., et al. 2007. LKB1 modulates lung cancer differentiation and metastasis. Nature. 448:807–810. 10.1038/nature06030 [DOI] [PubMed] [Google Scholar]
- Lee, J.H., Koh H., Kim M., Kim Y., Lee S.Y., Karess R.E., Lee S.H., Shong M., Kim J.M., Kim J., and Chung J.. 2007. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 447:1017–1020. 10.1038/nature05828 [DOI] [PubMed] [Google Scholar]
- Liu, W., Monahan K.B., Pfefferle A.D., Shimamura T., Sorrentino J., Chan K.T., Roadcap D.W., Ollila D.W., Thomas N.E., Castrillon D.H., et al. 2012. LKB1/STK11 inactivation leads to expansion of a prometastatic tumor subpopulation in melanoma. Cancer Cell. 21:751–764. 10.1016/j.ccr.2012.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, X., and Kang Y.. 2010. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin. Cancer Res. 16:5928–5935. 10.1158/1078-0432.CCR-10-1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte, F., and Perez-Moreno M.. 2012. Epithelial cell polarity, stem cells and cancer. Nat. Rev. Cancer. 12:23–38 [DOI] [PubMed] [Google Scholar]
- Mirouse, V., and Billaud M.. 2011. The LKB1/AMPK polarity pathway. FEBS Lett. 585:981–985. 10.1016/j.febslet.2010.12.025 [DOI] [PubMed] [Google Scholar]
- Nakano, A., Kato H., Watanabe T., Min K.D., Yamazaki S., Asano Y., Seguchi O., Higo S., Shintani Y., Asanuma H., et al. 2010. AMPK controls the speed of microtubule polymerization and directional cell migration through CLIP-170 phosphorylation. Nat. Cell Biol. 12:583–590. 10.1038/ncb2060 [DOI] [PubMed] [Google Scholar]
- Rajakulendran, T., and Sicheri F.. 2010. Allosteric protein kinase regulation by pseudokinases: insights from STRAD. Sci. Signal. 3:pe8. 10.1126/scisignal.3111pe8 [DOI] [PubMed] [Google Scholar]
- Rowan, A., Bataille V., MacKie R., Healy E., Bicknell D., Bodmer W., and Tomlinson I.. 1999. Somatic mutations in the Peutz-Jeghers (LKB1/STKII) gene in sporadic malignant melanomas. J. Invest. Dermatol. 112:509–511. 10.1046/j.1523-1747.1999.00551.x [DOI] [PubMed] [Google Scholar]
- Vander Heiden, M.G., Cantley L.C., and Thompson C.B.. 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 324:1029–1033. 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L., Li J., Young L.H., and Caplan M.J.. 2006. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc. Natl. Acad. Sci. USA. 103:17272–17277. 10.1073/pnas.0608531103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, B., and Cantley L.C.. 2007. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc. Natl. Acad. Sci. USA. 104:819–822. 10.1073/pnas.0610157104 [DOI] [PMC free article] [PubMed] [Google Scholar]