Abstract
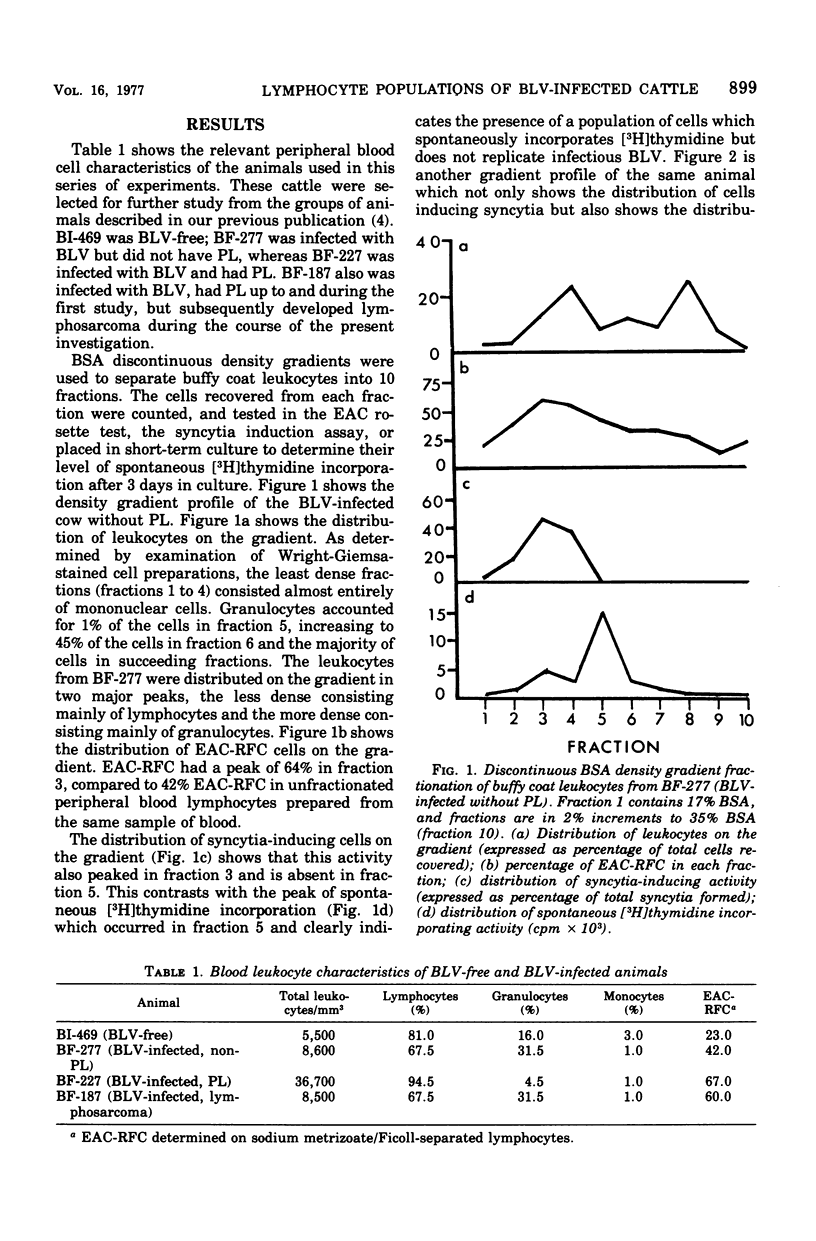
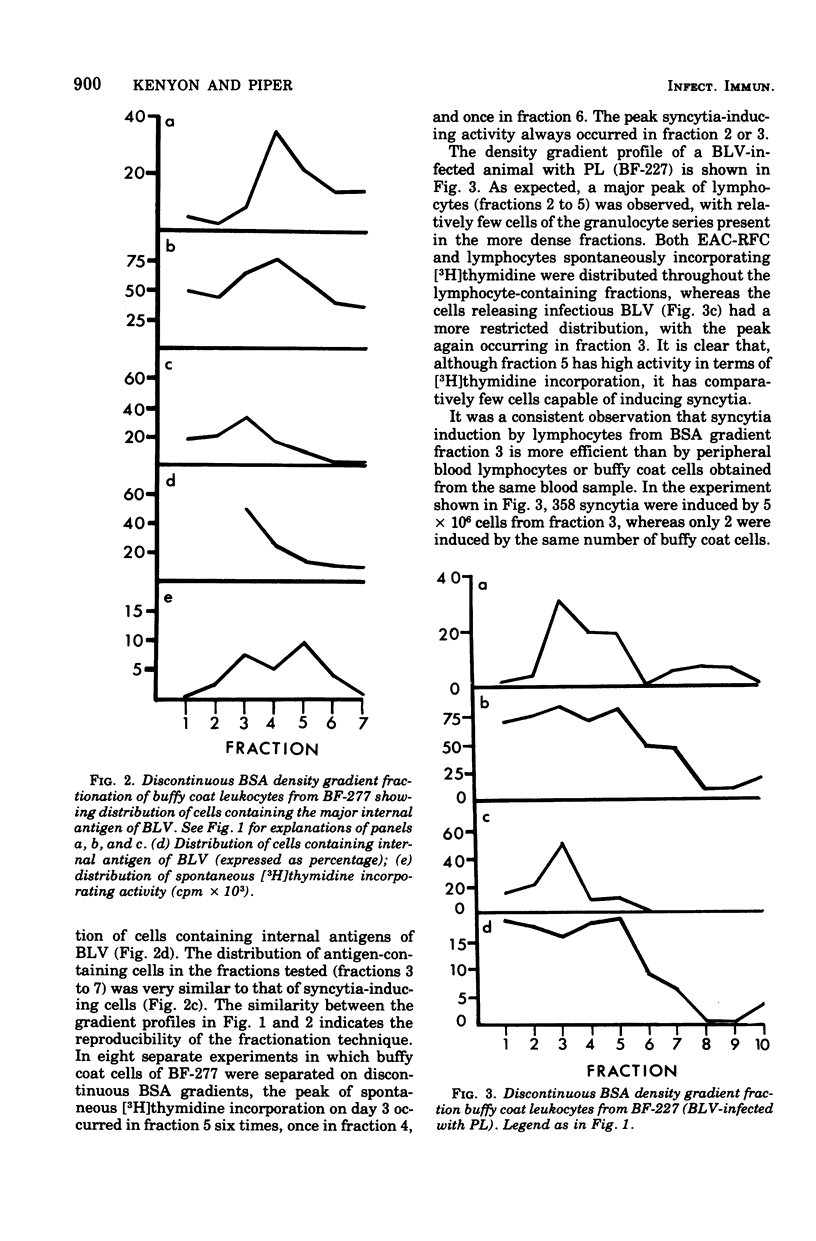
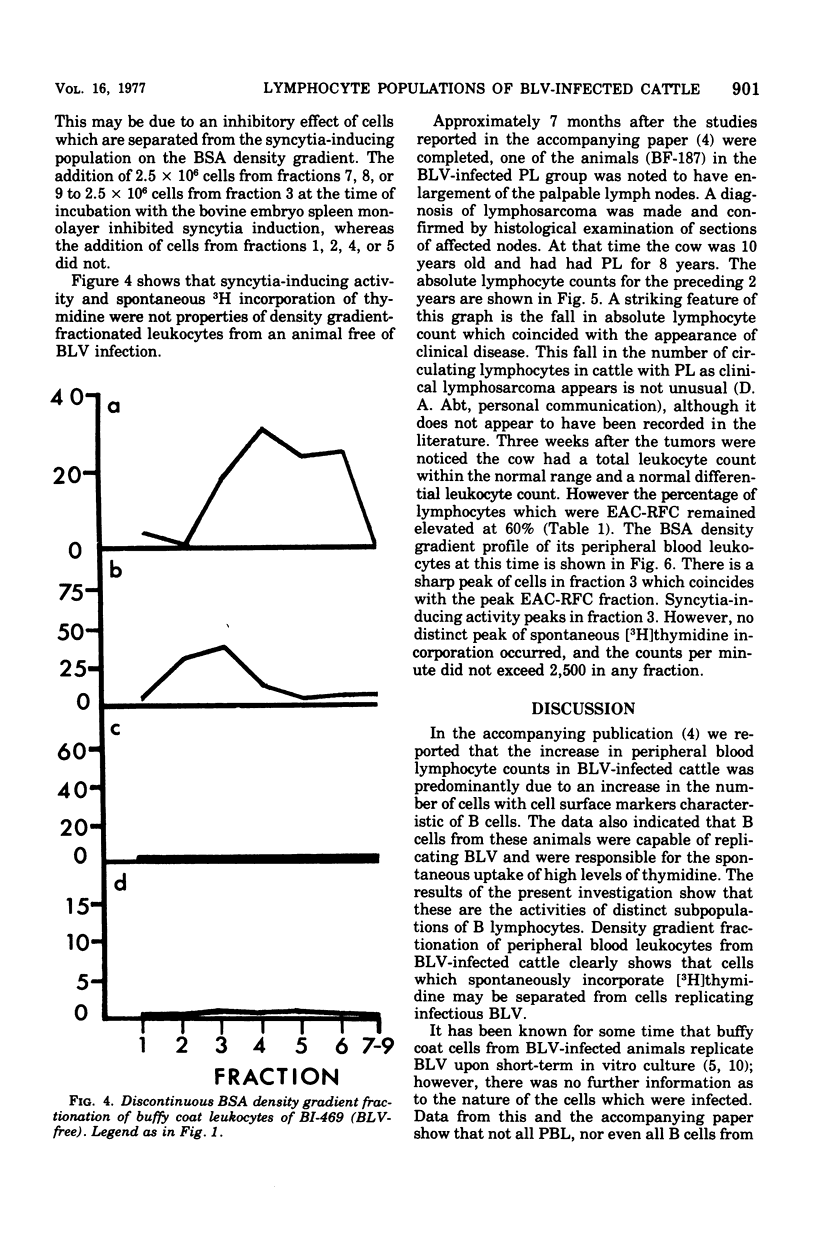
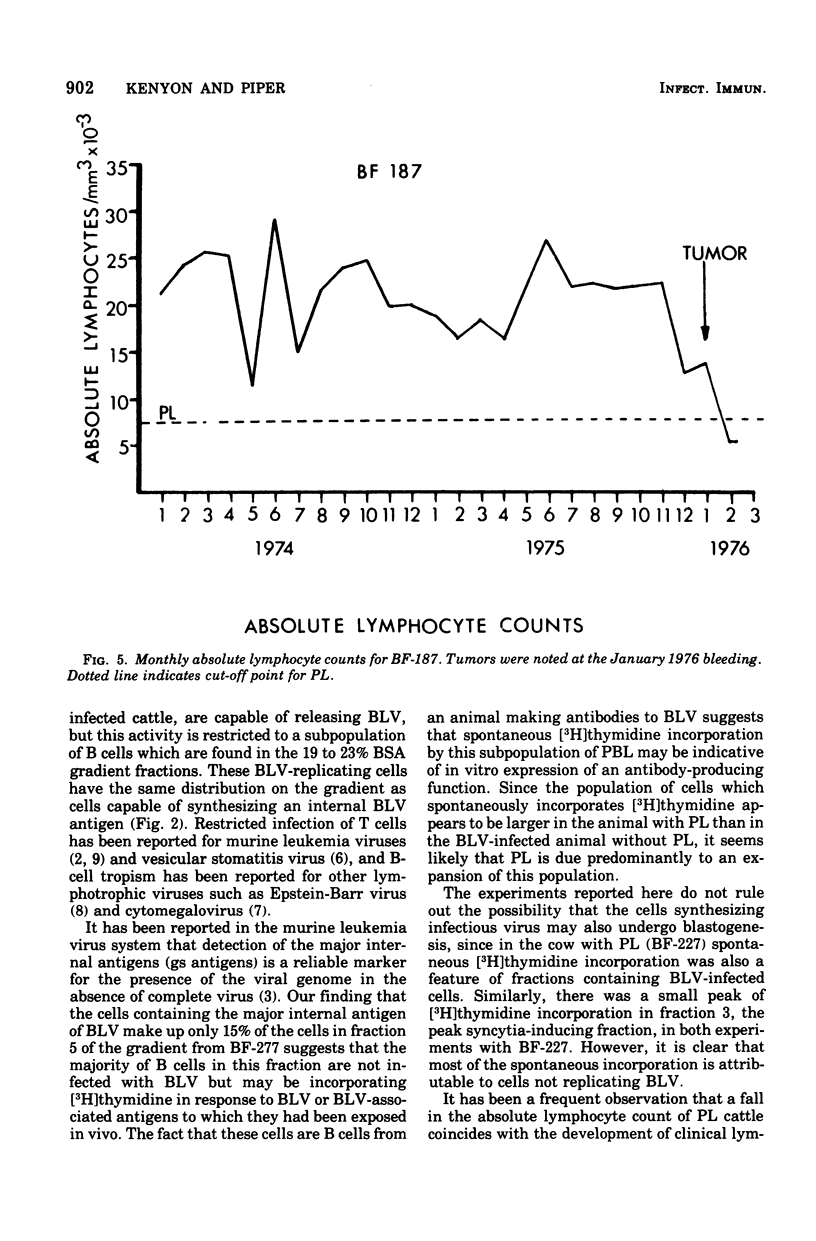
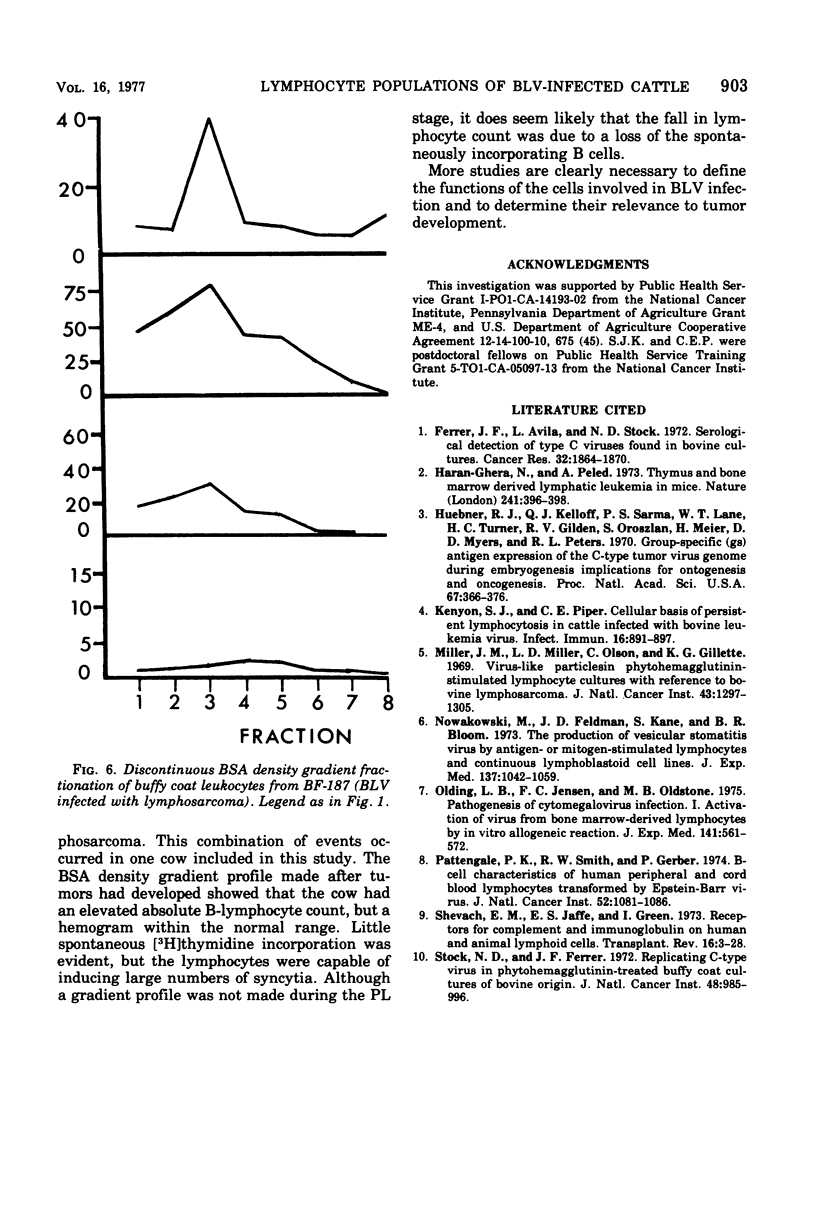
Discontinuous bovine serum albumin gradients were used to fractionate peripheral blood leukocytes from bovine leukemia virus (BLV)-free and BLV-infected cows. The release of infectious BLV and spontaneous incorporation of [3H]thymidine were not properties of density gradient-fractionated leukocytes from a BLV-free cow. When leukocytes from BLV-infected cattle were fractionated, B lymphocytes which spontaneously incorporated [3H]thymidine could be separated as a distinct subpopulation from B lymphocytes which replicated infectious BLV. Density gradient fractionation of leukocytes from a cow with lymphosarcoma is also reported. A fall in lymphocyte count at the time of tumor development is attributed to the loss of B lymphocytes which spontaneously incorporate [3H]thymidine.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ferrer J. F., Avila L., Stock N. D. Serological detection of type C viruses found in bovine cultures. Cancer Res. 1972 Sep;32(9):1864–1870. [PubMed] [Google Scholar]
- Haran-Chera N., Peled A. Thymus and bone marrow derived lymphatic leukaemia in mice. Nature. 1973 Feb 9;241(5389):396–398. doi: 10.1038/241396a0. [DOI] [PubMed] [Google Scholar]
- Huebner R. J., Kelloff G. J., Sarma P. S., Lane W. T., Turner H. C., Gilden R. V., Oroszlan S., Meier H., Myers D. D., Peters R. L. Group-specific antigen expression during embryogenesis of the genome of the C-type RNA tumor virus: implications for ontogenesis and oncogenesis. Proc Natl Acad Sci U S A. 1970 Sep;67(1):366–376. doi: 10.1073/pnas.67.1.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon S. J., Piper C. E. Cellular basis of persistent lymphocytosis in cattle infected with bovine leukemia virus. Infect Immun. 1977 Jun;16(3):891–897. doi: 10.1128/iai.16.3.891-897.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. M., Miller L. D., Olson C., Gillette K. G. Virus-like particles in phytohemagglutinin-stimulated lymphocyte cultures with reference to bovine lymphosarcoma. J Natl Cancer Inst. 1969 Dec;43(6):1297–1305. [PubMed] [Google Scholar]
- Nowakowski M., Feldman J. D., Kano S., Bloom B. R. The production of vesicular stomatitis virus by antigen- or mitogen-stimulated lymphocytes and continuous lymphoblastoid lines. J Exp Med. 1973 Apr 1;137(4):1042–1059. doi: 10.1084/jem.137.4.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olding L. B., Jensen F. C., Oldstone M. B. Pathogenesis of of cytomegalovirus infection. I. Activation of virus from bone marrow-derived lymphocytes by in vitro allogenic reaction. J Exp Med. 1975 Mar 1;141(3):561–572. doi: 10.1084/jem.141.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattengale P. K., Smith R. W., Gerber P. B-cell characteristics of human peripheral and cord blood lymphocytes transformed by Epstein-Barr virus. J Natl Cancer Inst. 1974 Apr;52(4):1081–1086. doi: 10.1093/jnci/52.4.1081. [DOI] [PubMed] [Google Scholar]
- Shevach E. M., Jaffe E. S., Green I. Receptors for complement and immunoglobulin on human and animal lymphoid cells. Transplant Rev. 1973;16:3–28. doi: 10.1111/j.1600-065x.1973.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Stock N. D., Ferrer J. F. Replicating C-type virus in phytohemagglutinin-treated buffy-coat cultures of bovine origin. J Natl Cancer Inst. 1972 Apr;48(4):985–996. [PubMed] [Google Scholar]


