Abstract
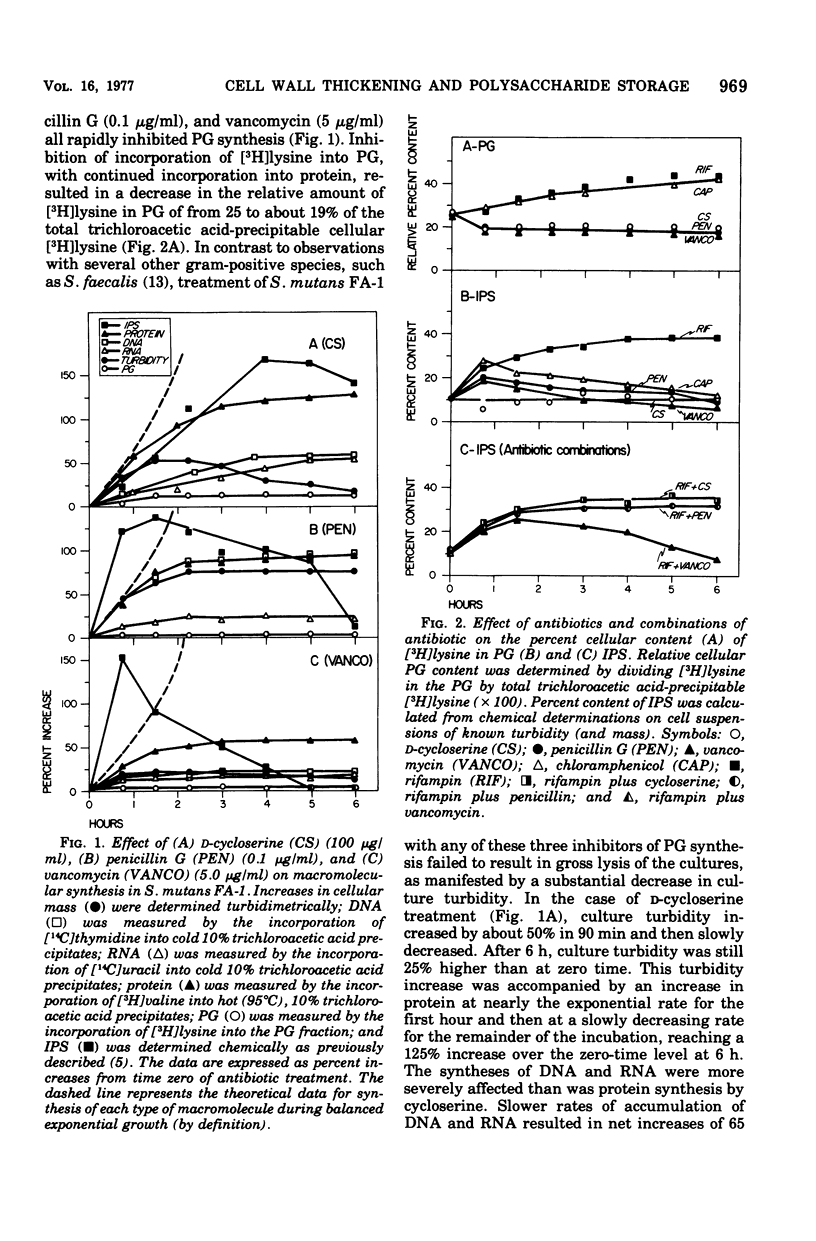
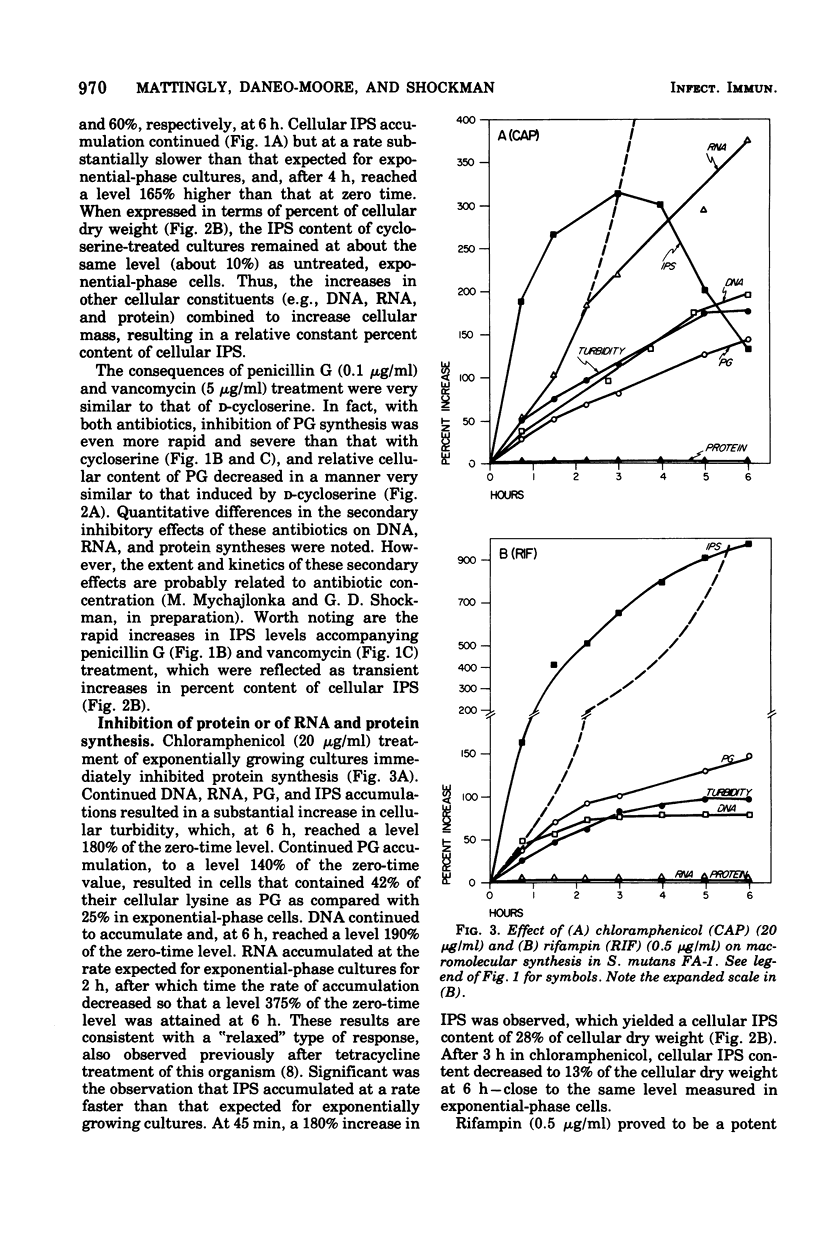
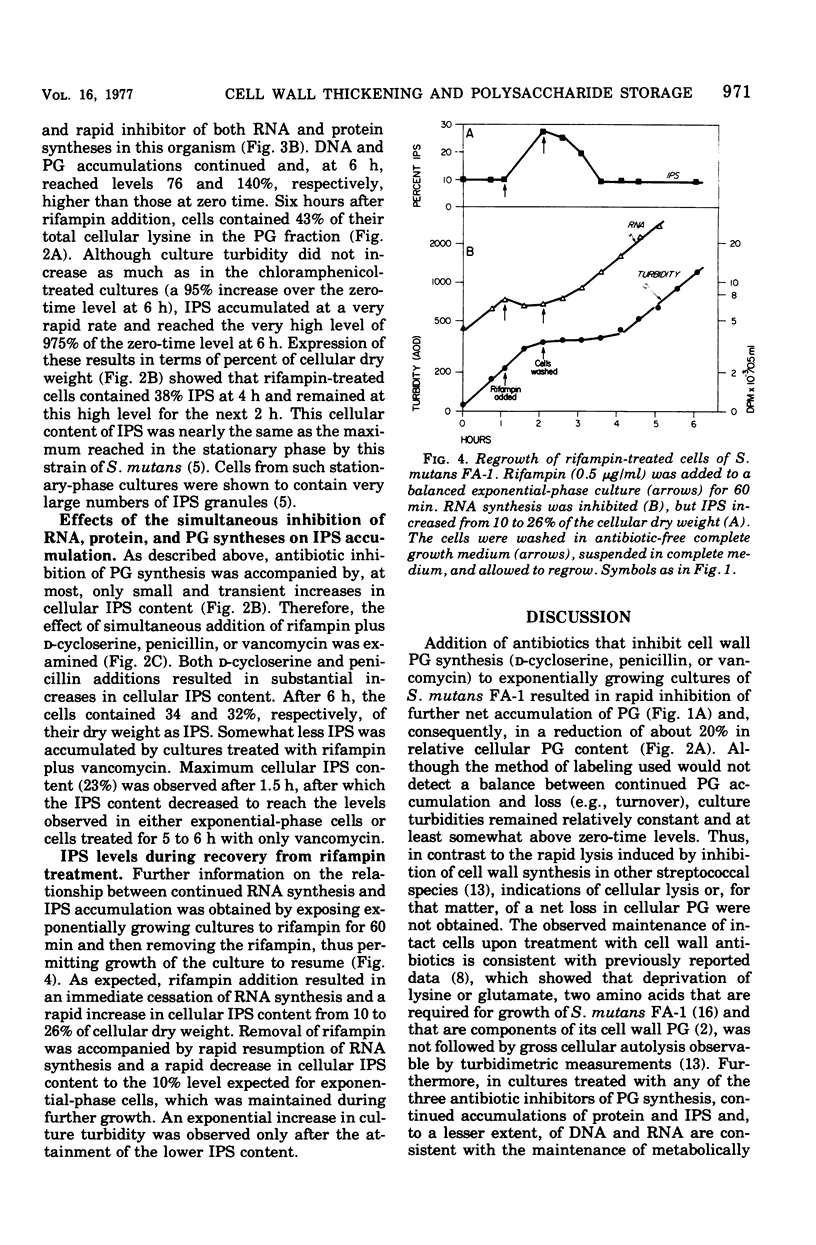
The effects of a series of different antibiotics on the synthesis and accumulation of deoxyribonucleic acid (DNA), ribonucleic acid (RNA), protein, cell wall peptidoglycan (PG), and intracellular iodophilic polysaccharide (IPS) in Streptococcus mutans FA-1 were examined. d-Cycloserine, penicillin G, or vancomycin treatment resulted in rapid inhibitions of PG synthesis and a consequent decrease in the relative amount of lysine found in PG fractions. Decreases in culture turbidity, an indicator of gross cellular lysis, were not observed. Secondary inhibitions of the rates and extent of syntheses of DNA, RNA, and protein were observed. With all three inhibitors of PG synthesis, IPS synthesis continued for varying time intervals but, at most, resulted in only relatively small and transient increases in cellular IPS content. Chloramphenicol inhibited protein synthesis but permitted continued synthesis of RNA and PG. After 6 h, the cells contained 42% of their [3H] lysine in the PG fraction compared with 25% in exponential-phase cells, a good indication of thickened cell walls. In the presence of chloramphenicol, cellular IPS content increased about 2.5-fold during the first 45 min and then decreased to a level (13%) at 6 h very similar to that of exponential-phase cells (about 10%). Rifampin inhibition of RNA (and, consequently, also protein) synthesis resulted in accumulation of cellular PG and IPS. After 6 h, IPS accounted for 38% of the cellular dry weight, and the cells contained 43% of their lysine in PG. Thus, rifampin-inhibited cells appear to have both thickened walls and a high IPS content. The correlation between inhibition of RNA synthesis and IPS accumulation was confirmed by exposing cultures to rifampin for 60 min and then removing the drug, thus permitting the cells to regrow. Upon removal of rifampin and resumption of RNA synthesis, cellular IPS content rapidly decreased to the level expected for exponentialphase cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Bleiweis A. S., Craig R. A., Zinner D. D., Jablon J. M. Chemical composition of purified cell walls of cariogenic streptococci. Infect Immun. 1971 Jan;3(1):189–191. doi: 10.1128/iai.3.1.189-191.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Higgins M. L., Coyette J., Shockman G. D. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973 Mar 25;248(6):2161–2169. [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Shockman G. D. A rapid, guantitative, and selective estimation of radioactively labeled peptidoglycan in gram-positive bacteria. Anal Biochem. 1971 Dec;44(2):645–653. doi: 10.1016/0003-2697(71)90255-7. [DOI] [PubMed] [Google Scholar]
- DiPersio J. R., Mattingly S. J., Higgins M. L., Shockman G. D. Measurement of intracellular iodophilic polysaccharide in two cariogenic strains of Streptococcus mutans by cytochemical and chemical methods. Infect Immun. 1974 Sep;10(3):597–604. doi: 10.1128/iai.10.3.597-604.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govons S., Gentner N., Greenberg E., Preiss J. Biosynthesis of bacterial glycogen. XI. Kinetic characterization of an altered adenosine diphosphate-glucose synthase from a "glycogen-excess" mutant of Escherichia coli B. J Biol Chem. 1973 Mar 10;248(5):1731–1740. [PubMed] [Google Scholar]
- Mattingly S. J., Dipersio J. R., Higgins M. L., Shockman G. D. Unbalanced growth and macromolecular synthesis in Streptococcus mutans FA-1. Infect Immun. 1976 Mar;13(3):941–948. doi: 10.1128/iai.13.3.941-948.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G. S., Shockman G. D., Daneo-Moore L. Balanced macromolecular biosynthesis in "protoplasts" of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):710–717. doi: 10.1128/jb.105.3.710-717.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOCKMAN G. D. Reversal of cycloserine inhibition by D-alanine. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:693–695. doi: 10.3181/00379727-101-25064. [DOI] [PubMed] [Google Scholar]
- Sayare M., Daneo-Moore L., Shockman G. D. Influence of macromolecular biosynthesis on cellular autolysis in Streptococcus faecalis. J Bacteriol. 1972 Oct;112(1):337–344. doi: 10.1128/jb.112.1.337-344.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L. C., Atkinson D. E. Regulation of adenosine diphosphate glucose synthase from Escherichia coli. Interactions of adenylate energy charge and modifier concentrations. J Biol Chem. 1970 Aug 10;245(15):3996–4000. [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Phillips P. M., Riley L. S., Toennies G. LYSIS OF STREPTOCOCCUS FAECALIS. J Bacteriol. 1961 Jan;81(1):36–43. doi: 10.1128/jb.81.1.36-43.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Shockman G. D. Amino acid requirements of Streptococcus mutans and other oral streptococci. Infect Immun. 1975 Apr;11(4):656–664. doi: 10.1128/iai.11.4.656-664.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houte J., Saxton C. A. Cell wall thickening and intracellular polysaccharide in microorganisms of the dental plaque. Caries Res. 1971;5(1):30–43. doi: 10.1159/000259730. [DOI] [PubMed] [Google Scholar]


