Abstract
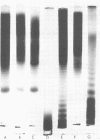
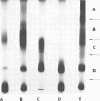
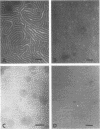
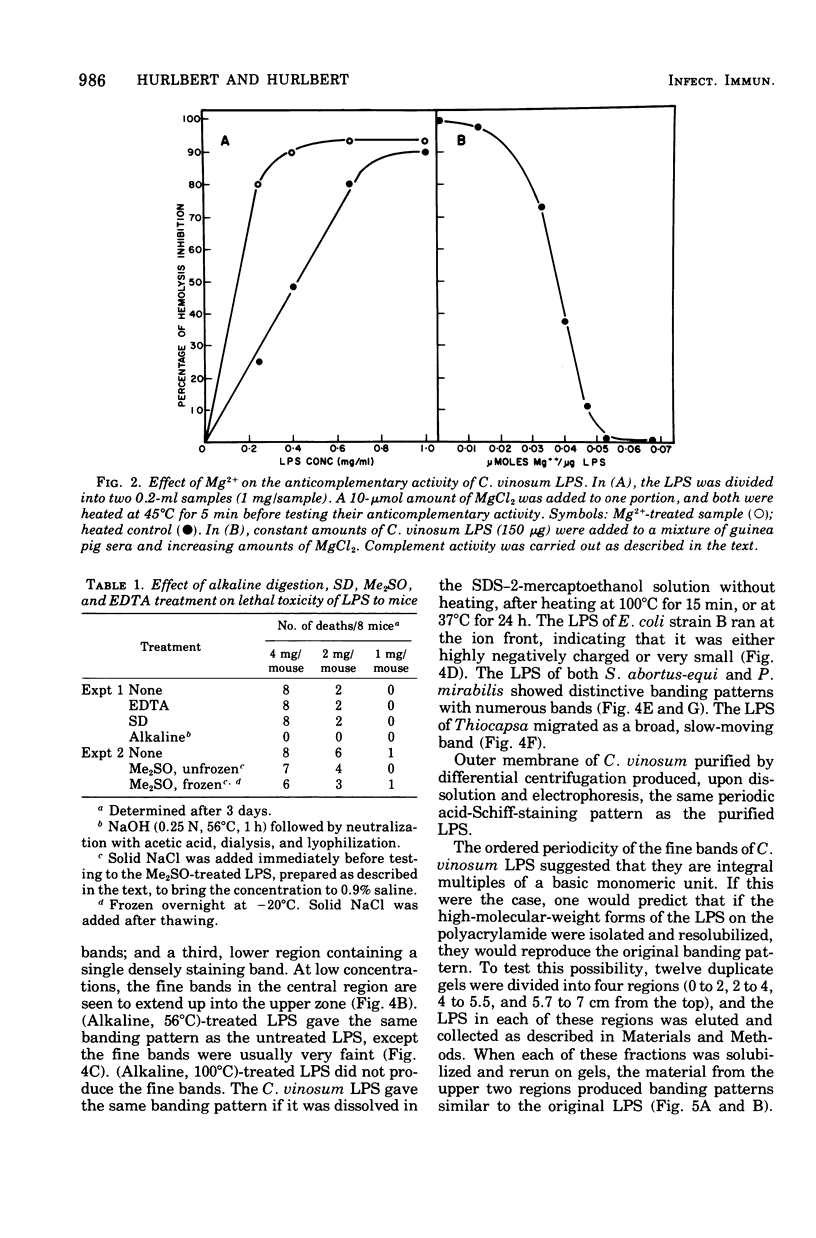
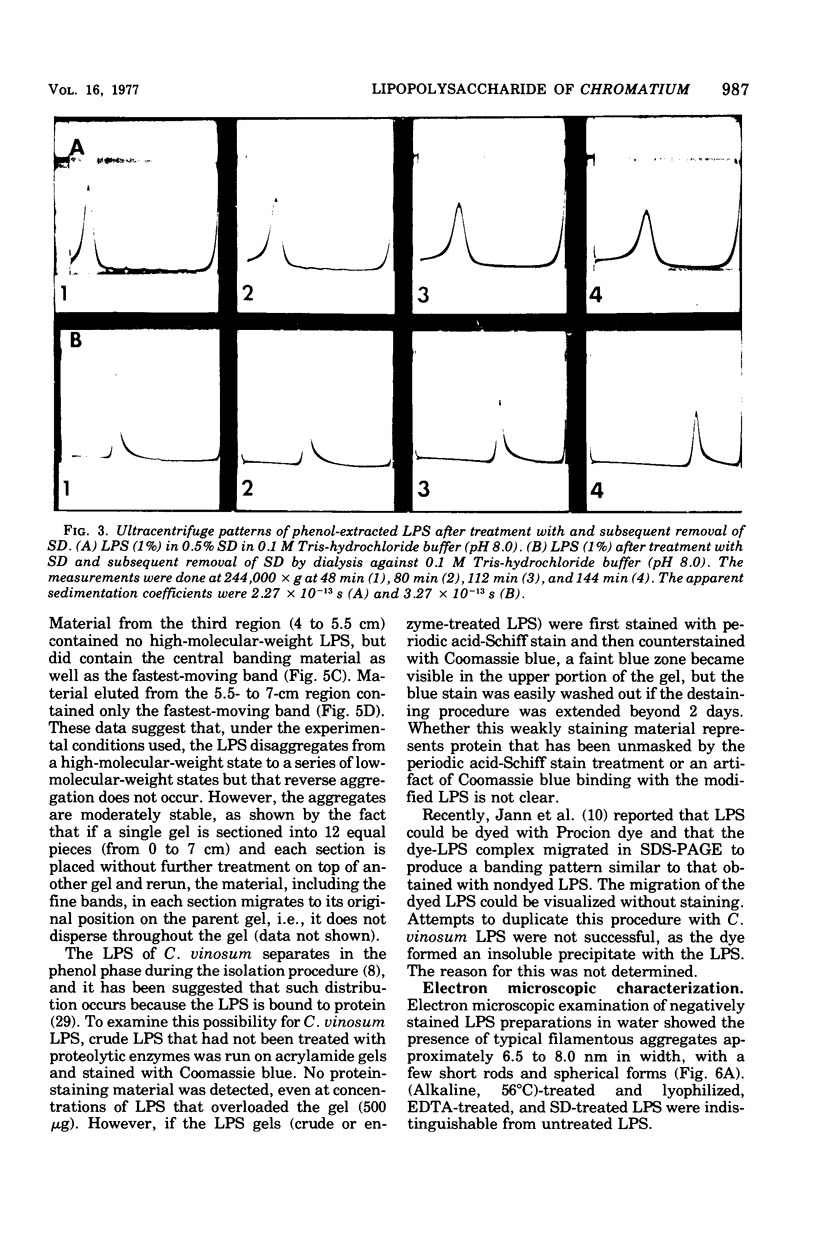
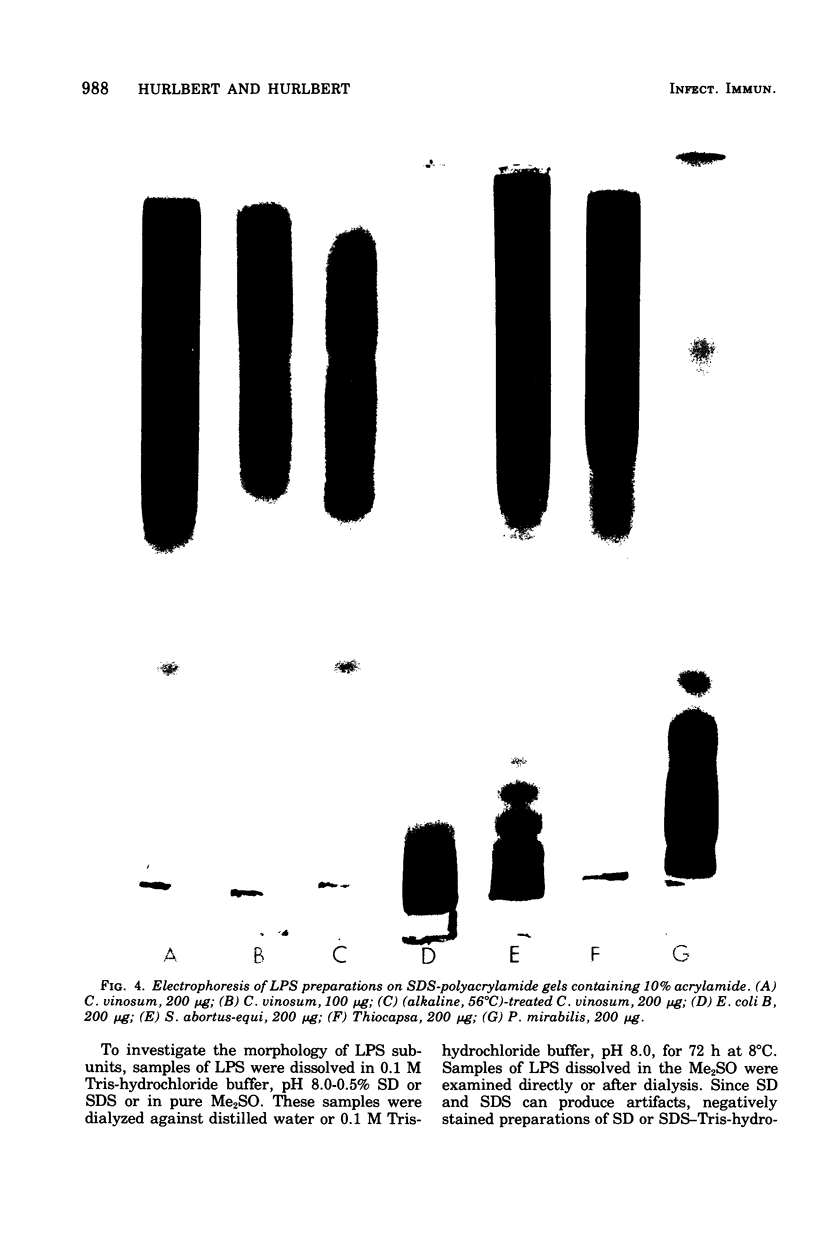
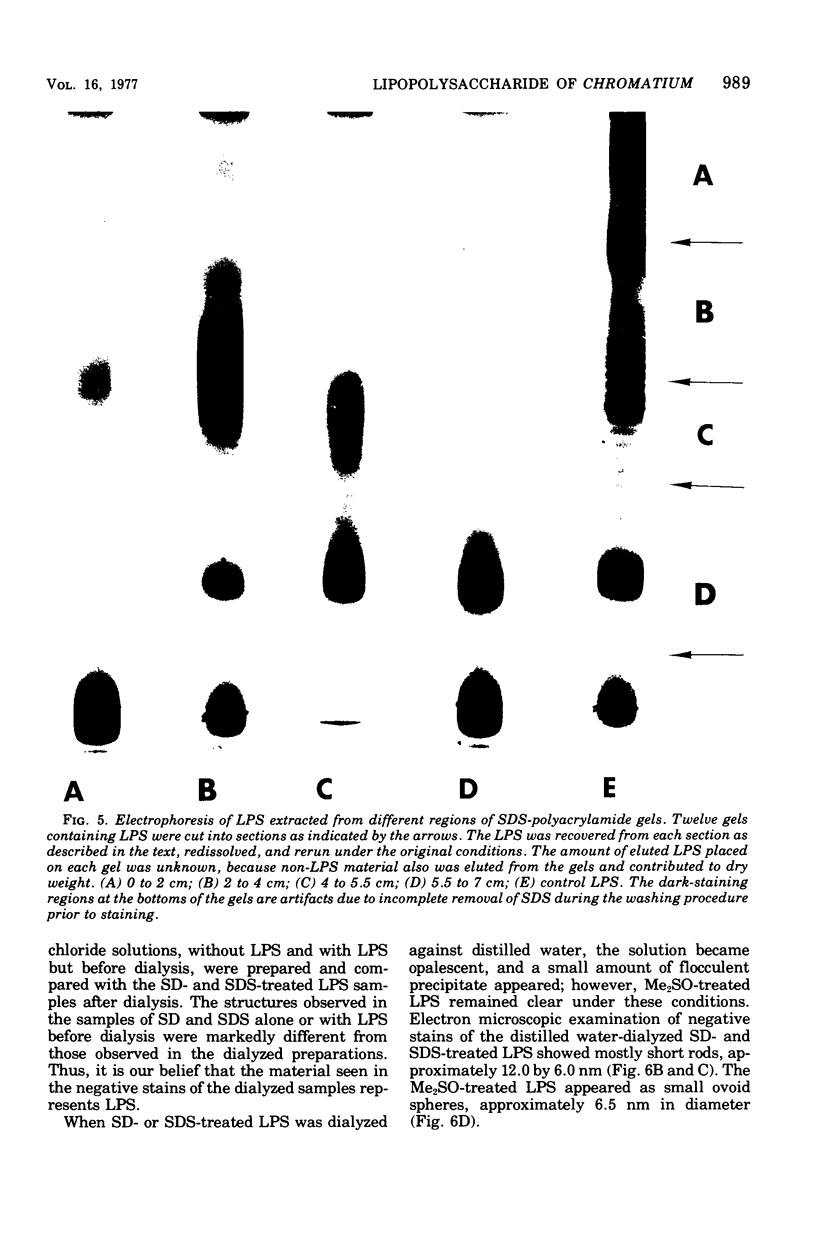
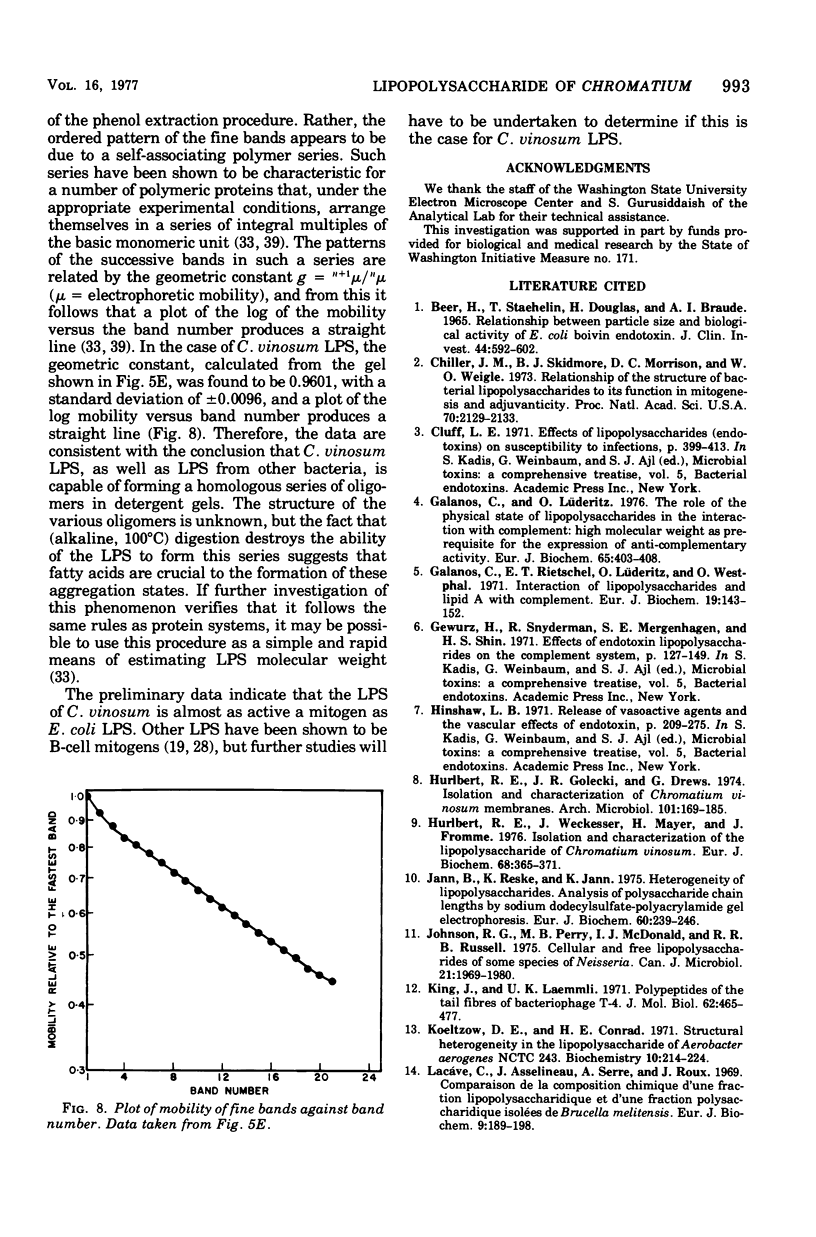
The lipopolysaccharide (LPS) of Chromatium vinosum has anticomplementary activity. This anticomplementary activity is destroyed by alkaline digestion of the LPS and is suppressed by both Mg2+ and Ca2+ ions. Treatment of the LPS with ethylenediaminetetraacetic acid, sodium deoxycholate, or dimethyl sulfoxide did not affect its toxicity toward mice; however, alkaline-treated LPS was not toxic. Treatment of the LPS with sodium deoxycholate, dimethyl sulfoxide, or sodium dodecyl sulfate resulted in reversible dissociation into subunits. Aggregation of the subunits into the original form was achieved by removing the dispersing agent by dialysis against distilled water followed by freezing and thawing. Electron micrographs of phenol-extracted LPS showed long filaments. Electron micrographs of sodium deoxycholate- and sodium dodecyl sulfate-treated and dialyzed LPS showed a mixture of small subunits and short filaments, whereas dimethyl sulfoxide-treated and dialyzed LPS contained only small ovoid spheres. The LPS produced an ordered series of multiple bands on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. A similar banding pattern was observed for Salmonella abortus-equi and Proteus mirabilis LPS. The C. vinosum LPS appears to be mitogenic for mouse spleen cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEER H., STAEHELIN T., DOUGLAS H., BRAUDE A. I. RELATIONSHIP BETWEEN PARTICLE SIZE AND BIOLOGICAL ACTIVITY OF E. COLI BOIVIN ENDOTOXIN. J Clin Invest. 1965 Apr;44:592–602. doi: 10.1172/JCI105172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiller J. M., Skidmore B. J., Morrison D. C., Weigle W. O. Relationship of the structure of bacterial lipopolysaccharides to its function in mitogenesis and adjuvanticity. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2129–2133. doi: 10.1073/pnas.70.7.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. The role of the physical state of lipopolysaccharides in the interaction with complement. High molecular weight as prerequisite for the expression of anti-complementary activity. Eur J Biochem. 1976 Jun 1;65(2):403–408. doi: 10.1111/j.1432-1033.1976.tb10354.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O. Interaction of lipopolysaccharides and lipid A with complement. Eur J Biochem. 1971 Mar 1;19(1):143–152. doi: 10.1111/j.1432-1033.1971.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Hurlbert R. E. Isolation and characterization of Chromatium vinosum membranes. Arch Microbiol. 1974;101(2):169–186. doi: 10.1007/BF00455937. [DOI] [PubMed] [Google Scholar]
- Hurlbert R. E., Weckesser J., Mayer H., Fromme I. Isolation and characterization of the lipopolysaccharide of Chromatium vinosum. Eur J Biochem. 1976 Sep 15;68(2):365–371. doi: 10.1111/j.1432-1033.1976.tb10823.x. [DOI] [PubMed] [Google Scholar]
- Jann B., Reske K., Jann K. Heterogeneity of lipopolysaccharides. Analysis of polysaccharide chain lengths by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Eur J Biochem. 1975 Dec 1;60(1):239–246. doi: 10.1111/j.1432-1033.1975.tb20996.x. [DOI] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B., McDonald I. J., Russel R. R. Cellular and free lipopolysaccharides of some species of Neisseria. Can J Microbiol. 1975 Dec;21(12):1969–1980. doi: 10.1139/m75-285. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Koeltzow D. E., Conrad H. E. Structural heterogeneity in the lipopolysaccharide of Aerobacter aerogenes NCTC 243. Biochemistry. 1971 Jan 19;10(2):214–224. doi: 10.1021/bi00778a004. [DOI] [PubMed] [Google Scholar]
- Lacave C., Asselineau J., Serre A., Roux J. Comparaison de la composition chimique d'une fraction lipopolysaccharidique et d'une fraction polysaccharidique isolées de Brucella melitensis. Eur J Biochem. 1969 Jun;9(2):189–198. doi: 10.1111/j.1432-1033.1969.tb00594.x. [DOI] [PubMed] [Google Scholar]
- MILNER K. C., ANACKER R. L., FUKUSHI K., HASKINS W. T., LANDY M., MALMGREN B., RIBI E. SYMPOSIUM ON RELATIONSHIP OF STRUCTURE OF MICROORGANISMS TO THEIR IMMUNOLOGICAL PROPERTIES. III. STRUCTURE AND BIOLOGICAL PROPERTIES OF SURFACE ANTIGENS FROM GRAM-NEGATIVE BACTERIA. Bacteriol Rev. 1963 Dec;27:352–368. doi: 10.1128/br.27.4.352-368.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- NETER E., WESTPHAL O., LUDERITZ O., GORZYNSKI E. A., EICHENBERGER E. Studies of enterobacterial lipopolysaccharides; effects of heat and chemicals on erythrocyte-modifying, antigenic, toxic and pyrogenic properties. J Immunol. 1956 May;76(5):377–385. [PubMed] [Google Scholar]
- Niwa M., Milner K. C., Ribi E., Rudbach J. A. Alteration of physical, chemical, and biological properties of endotoxin by treatment with mild alkali. J Bacteriol. 1969 Mar;97(3):1069–1077. doi: 10.1128/jb.97.3.1069-1077.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny A., Cundy K. R., Neale N. L., Nowotny A. M., Radvany R., Thomas S. P., Tripodi D. J. Relation of structure to function in bacterial O-antigens. IV. Fractionation of the components. Ann N Y Acad Sci. 1966 Jun 30;133(2):586–603. doi: 10.1111/j.1749-6632.1966.tb52391.x. [DOI] [PubMed] [Google Scholar]
- OROSZLAN S. I., MORA P. T. DISSOCIATION AND RECONSTITUTION OF AN ENDOTOXIN. Biochem Biophys Res Commun. 1963 Aug 14;12:345–349. doi: 10.1016/0006-291x(63)90102-5. [DOI] [PubMed] [Google Scholar]
- Olins A. L., Warner R. C. Physicochemical studies on a lipopolysaccharide from the cell wall of Azotobacter vinelandii. J Biol Chem. 1967 Nov 10;242(21):4994–5001. [PubMed] [Google Scholar]
- Ribi E., Anacker R. L., Brown R., Haskins W. T., Malmgren B., Milner K. C., Rudbach J. A. Reaction of endotoxin and surfactants. I. Physical and biological properties of endotoxin treated with sodium deoxycholate. J Bacteriol. 1966 Nov;92(5):1493–1509. doi: 10.1128/jb.92.5.1493-1509.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R., Johnson K. G., McDonald I. J. Envelope proteins in Neisseria. Can J Microbiol. 1975 Oct;21(10):1519–1534. doi: 10.1139/m75-224. [DOI] [PubMed] [Google Scholar]
- Russell R. R., Johnson K. G. SDS-polyacrylamide gel electrophoresis of lipopolysaccharides. Can J Microbiol. 1975 Dec;21(12):2013–2018. doi: 10.1139/m75-289. [DOI] [PubMed] [Google Scholar]
- Scurzi W., Fishbein W. N. The geometric mobility sequence in polyacrylamide gel electrophoresis of polymeric proteins: its significance for polymer geometry and electrophoretic theory. Trans N Y Acad Sci. 1973 May;35(5):396–416. doi: 10.1111/j.2164-0947.1973.tb01979.x. [DOI] [PubMed] [Google Scholar]
- Tripodi D., Nowotny A. Relation of structure to function in bacterial O-antigens. V. Nature of active sites in endotoxic lipopolysaccharides of Serratia marcescens. Ann N Y Acad Sci. 1966 Jun 30;133(2):604–621. doi: 10.1111/j.1749-6632.1966.tb52392.x. [DOI] [PubMed] [Google Scholar]
- Ugel A. R., Chrambach A., Rodbard D. Fractionation and characterization of an oligomeric series of bovine keratohyalin by polyacrylamide gel electrophoresis. Anal Biochem. 1971 Oct;43(2):410–426. doi: 10.1016/0003-2697(71)90271-5. [DOI] [PubMed] [Google Scholar]
- Weckesser J., Drews G., Ladwig R. Localization and biological and physicochemical properties of the cell wall lipopolysaccharide of Rhodopseudomonas capsulata. J Bacteriol. 1972 Apr;110(1):346–353. doi: 10.1128/jb.110.1.346-353.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]