Abstract
Summary
We evaluated the prevalence and geographic variation of short-interval (repeated in under 2 years) dual-energy X-ray absorptiometry tests (DXAs) among Medicare beneficiaries. Short-interval DXA use varied across regions (coefficient of variation=0.64), and unlike other DXAs, rates decreased with payment cuts.
Introduction
The American College of Rheumatology, through the Choosing Wisely initiative, identified measuring bone density more often than every 2 years as care “physicians and patients should question.” We measured the prevalence and described the geographic variation of short-interval (repeated in under 2 years) DXAs among Medicare beneficiaries and estimated the cost of this testing and its responsiveness to payment change.
Methods
Using 100 % Medicare claims data, 2006–2011, we identified DXAs and short-interval DXAs for female Medicare beneficiaries over age 66. We determined the population rate of DXAs and short-interval DXAs, as well as Medicare spending on short-interval DXAs, nationally and by hospital referral region (HRR).
Results
DXA use was stable 2008–2011 (12.4 to 11.5 DXAs per 100 women). DXA use varied across HRRs: in 2011, overall DXA use ranged from 6.3 to 23.0 per 100 women (coefficient of variation = 0.18), and short-interval DXAs ranged from 0.3 to 8.0 per 100 women (coefficient of variation=0.64). Short-interval DXA use fluctuated substantially with payment changes; other DXAs did not. Short-interval DXAs, which represented 10.1 % of all DXAs, cost Medicare approximately US$16 million in 2011.
Conclusions
One out of ten DXAs was administered in a time frame shorter than recommended and at a substantial cost to Medicare. DXA use varied across regions. Short-interval DXA use was responsive to reimbursement changes, suggesting carefully designed policy and payment reform may reduce this care identified by rheumatologists as low value.
Keywords: Bone densitometry, Health services research, Medicare
Introduction
In February 2013, the American College of Rheumatology identified measuring bone density more often than once every 2 years as low-value care [1]. This position was published as part of the American Board of Internal Medicine Foundation’s “Choosing Wisely” campaign, which encourages medical specialty societies to identify specific examples of commonly used care “whose necessity should be questioned and discussed” [2]. The ultimate aim of this campaign is not simply to list but to reduce the use of such care. While rheumatologists chose this example of low-value care, it is important to all physicians who order bone density tests. The North American Menopause Society and the US Preventive Services Task Force have made similar recommendations [3, 4]. We do not yet know, however, how short-interval bone density testing is distributed across the USA, what it costs, or how sensitive it is to changes in reimbursement.
Short-interval dual-energy X-ray absorptiometry scans (DXAs) are considered low-value because changes in bone density over a short time period are generally smaller than the measurement error inherent in the tests themselves [5]. As a result, in most patients, changes reported in a time interval of 2 years or less are likely to lack clinical significance and should not influence treatment decisions; they reflect wasted time and money [4]. Even at longer time intervals of 4 and 8 years, repeat tests may not improve prediction of fracture risk made from initial DXA tests [4, 6-9]. Until 2011, Medicare beneficiaries were responsible for a 20 % cost share for screening and monitoring bone density tests; since January 1, 2011, DXAs have been exempt from patient cost share [10]. The full cost is borne by taxpayers.
Recent changes in Medicare reimbursement complicate the study of DXA rates, costs, and value. Medicare reimbursement for DXAs administered outside the hospital decreased substantially (from US$139 in 2006 to US$82 in 2007 and 2008, then to US$72 in 2009) under the Deficit Reduction Act of 2005 [11, 12]. In 2011, and retroactively for 2010, reimbursement was increased to 70 % of 2006 payment rates, or approximately US$98 per test [13]. Reimbursements for hospital-based testing were stable over this time frame [14]. These payment changes have been associated with a shift in testing from nonhospital to hospital settings and an overall slowing in the growth of DXA testing [14-16, 12]. The impact of these changes on health outcomes of patients is unclear [12]. It is equally unclear if payment changes resulted in slowed growth for all DXA testing or if the impact differed for more valuable, initial or longer-interval testing compared to low-value, clinically meaningless, short-interval repeat tests.
We studied DXA use among Medicare beneficiaries to quantify the prevalence of low-value, short-interval testing. We examined variation in this care across the USA to understand which regions might require the smallest and largest care pattern adjustments to meet the vision of the Choosing Wisely initiative. We also assessed changes in rates of DXA testing overall and those done at short interval in association with payment changes to explore the potential for policy and reimbursement initiatives to influence this example of low-value care.
Methods
Data and population
We used a 100 % Medicare administrative dataset from 2006 to 2011 to identify all female beneficiaries over age 66 who received a DXA test (identified with current procedural terminology (CPT) codes for bone density studies (Online Resource 1) in each calendar year. Men were not studied because for men there is no age of universally recommended testing. Testing in men instead is directed by clinical risk, which makes the denominator of men eligible for DXAs and short-interval DXAs difficult to discern in a claims-based study. We excluded beneficiaries not continuously enrolled in fee-for-service Medicare Parts A and B in the 23 months prior to each DXA test identified. We conservatively excluded beneficiaries who were (i) diagnosed with any cancer, except nonmelanoma skin cancer (using the Clinical Classifications Software code) [17] or (ii) diagnosed with fragility fracture in the 23 months prior to the DXA (Online Resource 1). While these conditions do not necessarily change the clinical value of short-interval DXA, they may prompt bone density testing in response to clinical uncertainty or in attempt to comply with quality measures [18, 19]. Fragility fractures were defined as follows: (i) at least one CPT code for hip fracture repair; (ii) at least one International Classification of Diseases, Ninth Edition (ICD-9) diagnosis code for distal radius or proximal humerus fracture, plus at least one upper extremity radiography claim within 7 days (plus or minus) of the ICD-9 diagnosis claim date; or (iii) at least one ICD-9 diagnosis code for vertebral fracture. DXA tests were considered short-interval if they occurred within 23 months of an earlier DXA.
Outcomes
We developed four measures of DXA use for each year, 2008–2011: (i) a DXA population rate, defined as the number of DXAs performed per 100 female beneficiaries; (ii) a short-interval DXA rate, defined as the number of DXAs performed within 23 months of a previous DXA per 100 female beneficiaries; (iii) the proportion of all DXAs done at an inappropriately short interval (occurring within 23 months of a previous DXA); and (iv) the mean inter-test time interval for DXAs done within 23 months of a previous DXA.
Analysis
Based on residential ZIP code, patients were assigned to one of 306 Dartmouth Atlas of Health Care hospital referral regions (HRRs) [20]. We used descriptive statistics to quantify overall and HRR-level DXA testing and inappropriately short-interval DXA testing. We calculated means and coefficients of variation for each of the four measures across the 306 HRRs, weighted by the number of beneficiaries in each HRR. We examined changes in DXA use across the 4 years studied. To estimate spending associated with short-interval DXA use, we multiplied the number of DXAs by the average, national, nonhospital setting payment for bone density studies reported in the Medicare Physician Fee Schedule for each calendar year [14, 13]. Analyses were performed using SAS (version 9.3, SAS Institute Inc., Cary, NC). The study was approved by the institutional review board at Dartmouth College.
Results
We identified four annual cohorts of older female beneficiaries 2008–2011, ranging from 13.0 million beneficiaries in 2008 to 13.8 million beneficiaries in 2011. Among women aged 66 and older, the overall DXA testing rate peaked at 12.4 per 100 beneficiaries in 2008 and was lowest at 11.1 per 100 beneficiaries in 2010. The proportion of DXAs performed within 23 months of a previous DXA (short-interval DXAs) varied modestly from year to year and changed in association with payment adjustments. From 2008 to 2009, after a decrease in payment, short-interval DXA use dropped from 12.4 % to 7.6 % of all DXAs (from 1.6 to 0.9 tests per 100 women); use rose to 10.1 % of all tests in 2011 after a payment increase (to 1.2 tests per 100 women). In contrast, longer-interval DXA use (24 months or more) was constant from 2008 to 2009 (10.8 per 100 beneficiaries) and declined slightly through 2011, when the rate was 10.3 per 100 beneficiaries. The estimated spending associated with short-interval DXA use was US$16 million in 2011.
Across HRRs, in 2011, the overall DXA use rate ranged from 6.3 to 23.0 per 100 female beneficiaries (mean 11.5, coefficient of variation (CV)=0.17) (Table 1). The 2011 HRR-level mean rate of short-interval DXA receipt was 1.2 per 100 female beneficiaries (range 0.3 to 8.0, CV=0.64). The HRR-level mean proportion of DXAs done at an inappropriately short interval was 10.1 % (range 2.7 to 34.8, CV=0.48). Among short-interval DXAs, the mean inter-test time lapse was 15.1 months (standard deviation=0.43).
Table 1.
Variation in use of dual-energy X-ray absorptiometry (DXA) across 306 hospital referral regions, 2011
| Mean (SD)a | Coefficient of variation | Minimum and maximum | Interquartile range | |
|---|---|---|---|---|
| DXAs per 100 beneficiaries | 11.5 (2.1) | 0.17 | 6.3–23.0 | 10.2–12.5 |
| Short-interval DXAs per 100 beneficiaries | 1.2 (0.8) | 0.64 | 0.3–8.0 | 0.7–1.4 |
| Proportion of DXAs administered at short interval | 10.1 % (4.9) | 0.48 | 2.7–34.7 % | 6.6–12.0 % |
| Inter-test interval for short-interval DXAs | 15.1 months (0.43) | 0.03 | 13.8–17.8 months | 14.7–15.4 months |
All results weighted by the number of beneficiaries in each hospital referral region
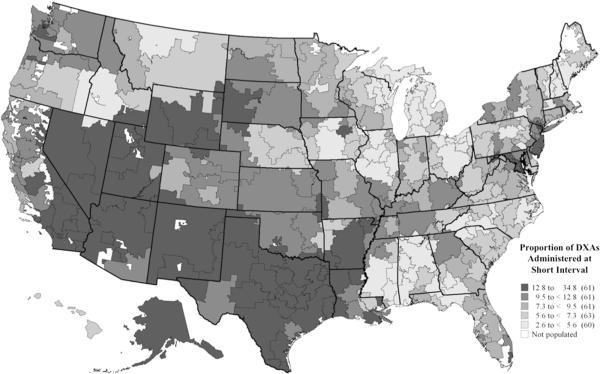
Inappropriate, short-interval DXA use was highest in the south and southwest USA (Fig. 1). The HRRs with the highest use of short-interval DXAs were McAllen, TX; Provo, UT; and Rapid City, SD; HRRs ranking lowest (least use of short-interval testing) were Elyria, OH; Wilkes-Barre, PA; and Lakeland, FL.
Fig. 1.
Map of the proportion of dual-energy X-ray absorptiometry tests administered at short interval across 306 hospital referral regions, 2011
Discussion
Overall, we find approximately 10 % of DXAs reimbursed by Medicare were administered at inappropriately short intervals in this conservatively defined population of women. Most short-interval DXAs were done just over 1 year from the preceding test. Following the Medicare reimbursement decrease in 2009, use of short-interval DXAs dropped substantially more than the use of longer-interval tests; use then rose following a payment increase. These short-interval tests come at substantial cost and most likely have no clinical value.
Our regional analysis of short-interval DXA rates demonstrates the extent to which use of this care varies across the nation. Our work, the first to describe short-interval DXA use geographically, reveals the broad range of practice pattern change needed to achieve the best use of this technology suggested by the Choosing Wisely campaign.
Our finding that short-interval tests declined more in response to payment change than longer-interval tests has important implications. Ideally, policies and payment schedules would achieve optimal rates of health technology use by precisely reducing incentives for marginal procedures and tests. The payment cuts specific to nonhospital settings are a blunt approach to cost containment, but it is reassuring to see these associated with differential declines in short-interval compared to longer-interval DXA tests. A payment policy specific to the short-interval DXA test reimbursement (either denying reimbursement for a too-short a time interval, requiring prior authorization, or establishing a tiered coverage structure) may be the most appropriate approach to reduce low-value testing. Caution must be exercised, however, as more stringent implementation of a minimum-interval policy could be misinterpreted as the recommended testing interval and result in more tests done at intervals only slightly longer than the approved minimum. An ideal policy would curtail low-value short-interval testing while promoting universal initial screening to identify patients at risk of fracture and in need of ongoing attention to bone health.
Our study has limitations common to claims-based analyses. We do not have clinical data on the bone density measurement results, nor do we have data on how the ordering clinician interpreted or responded to the results. For this reason, we do not know the clinical impact of the short-interval tests. Our exclusion of fragility fracture patients and cancer patients is intended to restrict our cohort to patients for whom nearly all short-interval tests are clinically meaningless and less likely prompted by severe disease diagnosis. Our estimates may miss situations in which clinicians and/or patients find short-interval testing clinically meaningful. This could arise when there is concern for accelerated bone loss or when short-interval tests are felt to be a meaningful component of medication monitoring, though such situations should be clinically rare [21]. Additionally, a small number of short interval tests may be repeated due to the poor quality of the original. Less than 1 % of short-interval DXAs, however, were administered within 2 months of a previous DXA, suggesting that the latter situation was uncommon.
While the Choosing Wisely list identifying short-interval DXAs as low-value was published in 2013, we have long known that short-interval DXAs are not clinically valuable in most patients [5, 22]. Medicare policies have reflected this understanding, but enforcement efforts appear insufficient [11]. Recent studies suggest the minimum meaningful intertest interval may be many years for average risk patients [7, 23, 6]. Our results (spanning 2008 to 2011) reflect practice before the time rheumatologists elected to include short-interval DXAs on their Choosing Wisely list and thus portray the starting point from which we hope use of this care will decline in response to the Choosing Wisely initiative. In the near future, Medicare will ideally reduce its spending on short-interval DXAs. This would improve overall care quality and achieve substantial savings that could be invested in efforts aimed at getting all women the universally recommended initial screening after their 65th birthday [4]. Achieving this ideal will depend on meaningful practice change by providers in regions where as much as a third of all DXAs are administered at intervals of less than 23 months. Diverse consumer awareness and education efforts affiliated with the Choosing Wisely campaign may help achieve this goal [24]. Policy and payment changes will likely prove most effective.
Acknowledgments
This study was supported by grants from the National Institute on Aging (P01 AG019783 and K23 AG035030), the Robert Wood Johnson Foundation’s Changes in Health Care Financing and Organization (HCFO) Initiative (©70729), and The Commonwealth Fund (©20130339). We are grateful to Kristen K. Bronner, M.A., of The Dartmouth Institute for Health Policy and Clinical Practice for assistance with mapping. Ms. Bronner received no compensation for this service.
Footnotes
Conflicts of interest None.
Contributor Information
W. L. Schpero, The Dartmouth Institute for Health Policy & Clinical Practice, 35 Centerra Parkway, Lebanon, NH 03766, USA
R. Zaha, The Dartmouth Institute for Health Policy & Clinical Practice, 35 Centerra Parkway, Lebanon, NH 03766, USA
References
- 1.American College of Rheumatology Focus on patient care: choosing wisely. 2012 http://www.rheumatology.org/Practice/FiveThings/Focus_on_Patient_Care__Choosing_Wisely/. Accessed5 Sept 2013.
- 2.ABIM Foundation Choosing wisely: an initiative of the ABIM Foundation. 2013 http://www.choosingwisely.org. Accessed 12 Oct 2013.
- 3.Management of osteoporosis in postmenopausal women 2010 position statement of The North American Menopause Society. Menopause (New York, NY) 2010;17(1):25–54. doi: 10.1097/gme.0b013e3181c617e6. quiz 55–26. doi:10.1097/gme.0b013e3181c617e6. [DOI] [PubMed] [Google Scholar]
- 4.Screening for osteoporosis U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154(5):356–364. doi: 10.7326/0003-4819-154-5-201103010-00307. doi:10.7326/0003-4819-154-5-201103010-00307. [DOI] [PubMed] [Google Scholar]
- 5.Gluer CC. Monitoring skeletal changes by radiological techniques. J Bone Miner Res. 1999;14(11):1952–1962. doi: 10.1359/jbmr.1999.14.11.1952. doi:10.1359/jbmr.1999.14.11.1952. [DOI] [PubMed] [Google Scholar]
- 6.Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, Burckhardt P, Cooper C, Christiansen C, Cummings S, Eisman JA, Fujiwara S, Gluer C, Goltzman D, Hans D, Krieg MA, La Croix A, McCloskey E, Mellstrom D, Melton LJ, 3rd, Pols H, Reeve J, Sanders K, Schott AM, Silman A, Torgerson D, van Staa T, Watts NB, Yoshimura N. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18(8):1033–1046. doi: 10.1007/s00198-007-0343-y. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 7.Berry SD, Samelson EJ, Pencina MJ, McLean RR, Cupples LA, Broe KE, Kiel DP. Repeat bone mineral density screening and prediction of hip and major osteoporotic fracture. JAMA. 2013;310(12):1256–1262. doi: 10.1001/jama.2013.277817. doi:10.1001/jama.2013.277817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillier TA, Stone KL, Bauer DC, Rizzo JH, Pedula KL, Cauley JA, Ensrud KE, Hochberg MC, Cummings SR. Evaluating the value of repeat bone mineral density measurement and prediction of fractures in older women: the study of osteoporotic fractures. Archives of Internal Medicine. 2007;167(2):155–160. doi: 10.1001/archinte.167.2.155. doi:10.1001/archinte.167.2.155. [DOI] [PubMed] [Google Scholar]
- 9.Leslie WD, Morin SN, Lix LM, Manitoba Bone Density P. Rate of bone density change does not enhance fracture prediction in routine clinical practice. J Clin Endocrinol Metab. 2012;97(4):1211–1218. doi: 10.1210/jc.2011-2871. doi:10.1210/jc.2011-2871. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Medicare & Medicaid Services Medicare preventive services, quick reference information. 2012 http://www.cms.gov/Medicare/Prevention/PrevntionGenInfo/Downloads/MPS_ QuickReferenceChart_1.pdf. Accessed 4 Nov 2013.
- 11.Department of Health and Human Services CfMMS Federal Register. 2006;71(162) (Tuesday, August 22, 2006. http://www.gpo.gov/fdsys/pkg/FR-2006-08-22/html/06-6843.htm. Accessed 4 Nov 2013. [Google Scholar]
- 12.McAdam-Marx C, Unni S, Ye X, Nelson S, Nickman NA. Effect of Medicare reimbursement reduction for imaging services on osteoporosis screening rates. Journal of the American Geriatrics Society. 2012;60(3):511–516. doi: 10.1111/j.1532-5415.2011.03837.x. doi:10.1111/j.1532-5415.2011.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The Patient Protection and Affordable Care Act (2010)
- 14.Zhang J, Delzell E, Zhao H, Laster AJ, Saag KG, Kilgore ML, Morrisey MA, Wright NC, Yun H, Curtis JR. Central DXA utilization shifts from office-based to hospital-based settings among medicare beneficiaries in the wake of reimbursement changes. J Bone Miner Res. 2012;27(4):858–864. doi: 10.1002/jbmr.1534. doi:10.1002/jbmr.1534. [DOI] [PubMed] [Google Scholar]
- 15.King AB, Fiorentino DM. Medicare payment cuts for osteoporosis testing reduced use despite Tests’ benefit in reducing fractures. Health affairs (Project Hope) 2011;30(12):2362–2370. doi: 10.1377/hlthaff.2011.0233. doi:10.1377/hlthaff.2011.0233. [DOI] [PubMed] [Google Scholar]
- 16.O’Malley CD, Johnston SS, Lenhart G, Cherkowski G, Palmer L, Morgan SL. Trends in dual-energy X-ray absorptiometry in the United States, 2000–2009. J Clin Densitom. 2011;14(2):100–107. doi: 10.1016/j.jocd.2011.03.003. doi:10.1016/j.jocd.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Clinical Classifications Software (CCS) for ICD-9-CM HCUP. 2013 http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp—overview. Accessed 1 Aug 2013.
- 18.Chien AJ, Goss PE. Aromatase inhibitors and bone health in women with breast cancer. J Clin Oncol. 2006;24(33):5305–5312. doi: 10.1200/JCO.2006.07.5382. doi:10.1200/JCO.2006.07.5382. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Quality Assurance HEDIS and quality measures. 2011 http://www.ncqa.org/HEDISQualityMeasurement/HEDISMeasures.aspx. Accessed 18 Jan 2012.
- 20.The Dartmouth Atlas of Health Care Working Group The Dartmouth Atlas of Health Care. The Dartmouth Institute for Health Policy and Clinical Practice. 2013 Center for Health Policy Research. http://www.dartmouthatlas.org/. Accessed 4 Jan 2013.
- 21.Cawthon PM, Ewing SK, Mackey DC, Fink HA, Cummings SR, Ensrud KE, Stefanick ML, Bauer DC, Cauley JA, Orwoll ES. Change in hip bone mineral density and risk of subsequent fractures in older men. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27(10):2179–2188. doi: 10.1002/jbmr.1671. doi:10.1002/jbmr.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: scientific review. JAMA. 2002;288(15):1889–1897. doi: 10.1001/jama.288.15.1889. [DOI] [PubMed] [Google Scholar]
- 23.U. S. Preventive Services Task Force Screening for osteoporosis: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2011;154(5):356–364. doi: 10.7326/0003-4819-154-5-201103010-00307. doi:10.7326/0003-4819-154-5-201103010-00307. [DOI] [PubMed] [Google Scholar]
- 24.American Board of Internal Medicine Foundation ABIM FOUNDATION FORUM. 2012 [Google Scholar]