Abstract
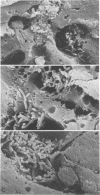
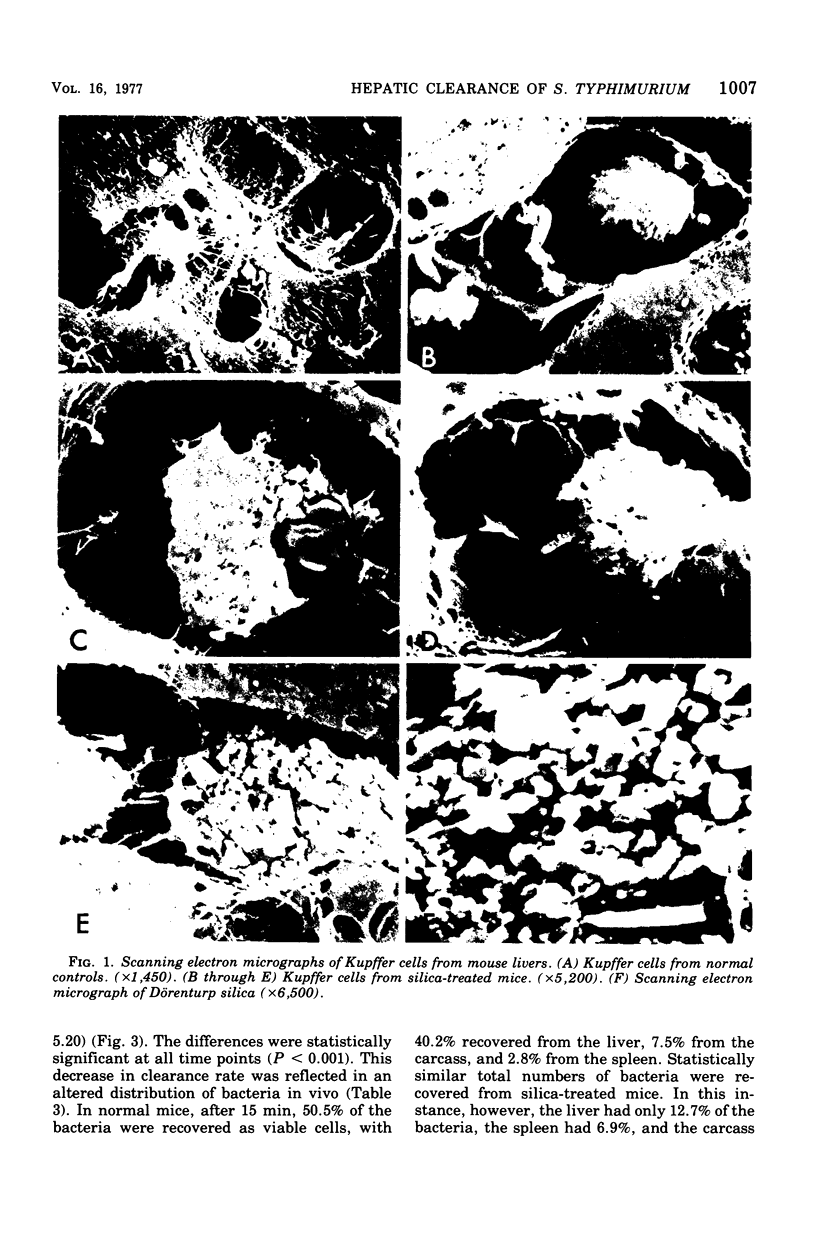
Scanning electron microscopy demonstrates that crystalline silica destroys liver Kupffer cells but has no other obvious deleterious effects on the liver. Silica-treated livers still retain the ability to trap large numbers of bacteria perfused through the portal vein even though the rate of clearance is well below normal. In vivo, silica treatment decreases the rate of bacterial clearance from the blood, alters the in vivo organ distribution of cleared bacteria, and decreases the mean lethal dose of Salmonella typhimurium over 100-fold. Cumulatively, the data indicate that silica treatment enhances susceptibility to gram-negative infection, probably by destruction of macrophages.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C., Hart P. D. Potentiation by silica of the growth of Mycobacterium tuberculosis in macrophage cultures. Br J Exp Pathol. 1968 Oct;49(5):465–476. [PMC free article] [PubMed] [Google Scholar]
- Allison A. C. Lysosomes and the toxicity of particulate pollutants. Arch Intern Med. 1971 Jul;128(1):131–139. [PubMed] [Google Scholar]
- BIOZZI G., BENACERRAF B., HALPERN B. N. Quantitative study of the granulopectic activity of the reticulo-endothelial system. II. A study of the kinetics of the R. E. S. in relation to the dose of carbon injected; relationship between the weight of the organs and their activity. Br J Exp Pathol. 1953 Aug;34(4):441–457. [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Oxman E. Phagocytosis and intracellular disposition of viable bacteria by the isolated perfused rat liver. J Reticuloendothel Soc. 1965 Nov;2(4):313–325. [PubMed] [Google Scholar]
- Bryson G., Bischoff F. Silicate-induced neoplasms. Prog Exp Tumor Res. 1967;9:77–164. [PubMed] [Google Scholar]
- COMOLLI R., PERIN A. In vitro action of silicogenic and non-silicogenic dusts on macrophage metabolism. Proc Soc Exp Biol Med. 1963 Jun;113:289–293. doi: 10.3181/00379727-113-28344. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974 Dec;38(4):371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD J. G. The reticulo-endothelial system and resistance to bacterial infection. Scott Med J. 1961 Feb;6:60–82. doi: 10.1177/003693306100600203. [DOI] [PubMed] [Google Scholar]
- Jeunet F. S., Cain W. A., Good R. A. Reticuloendothelial function in the isolated perfused liver. 3. Phagocytosis of Salmonella typhosa and Brucella melitensis and the blockade of the reticuloendothelial system. J Reticuloendothel Soc. 1969 Aug;6(4):391–410. [PubMed] [Google Scholar]
- Jeunet F. S., Cain W., Good R. A. Differential recognition of Brucella organisms by Kupffer cells: studies with isolated perfused liver. Proc Soc Exp Biol Med. 1968 Oct;129(1):187–190. doi: 10.3181/00379727-129-33280. [DOI] [PubMed] [Google Scholar]
- KESSEL R. W., MONACO L., MARCHISIO M. A. THE SPECIFICITY OF THE CYTOTOXIC ACTION OF SILICA--A STUDY IN VITRO. Br J Exp Pathol. 1963 Aug;44:351–364. [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum F., Knecht E., Budzko D. B., Pizzimenti M. C. Phagocytosis: a defense mechanism against infection with Trypanosoma cruzi. J Immunol. 1974 May;112(5):1839–1844. [PubMed] [Google Scholar]
- Larson C. L., Ushijima R. N., Baker R. E., Baker M. B., Gillespie C. A. Effect of normal serum and antithymocyte serum on Friend disease in mice. J Natl Cancer Inst. 1972 May;48(5):1403–1407. [PubMed] [Google Scholar]
- MARKS J., NAGELSCHMIDT G. Study of the toxicity of dust with use of the in vitro dehydrogenase technique. AMA Arch Ind Health. 1959 Nov;20:383–389. [PubMed] [Google Scholar]
- Moon R. J., Vrable R. A., Broka J. A. In situ separation of bacterial trapping and killing functions of the perfused liver. Infect Immun. 1975 Aug;12(2):411–418. doi: 10.1128/iai.12.2.411-418.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS D. E. Host mechanisms which act to remove bacteria from the blood stream. Bacteriol Rev. 1960 Mar;24(1):50–66. doi: 10.1128/br.24.1.50-66.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. The role of antibody and host cells in the resistance of mice against infection by coxsackie B-3 virus. J Gen Virol. 1973 Jun;19(3):329–338. doi: 10.1099/0022-1317-19-3-329. [DOI] [PubMed] [Google Scholar]
- Robock K. Standard quartz dq12 greater than 5 micro m for experimental pneumoconiosis research projects in the Federal Republic of Germany. Ann Occup Hyg. 1973 Apr;16(1):63–66. doi: 10.1093/annhyg/16.1.63. [DOI] [PubMed] [Google Scholar]
- Saba T. M. Physiology and physiopathology of the reticuloendothelial system. Arch Intern Med. 1970 Dec;126(6):1031–1052. [PubMed] [Google Scholar]
- Sawyer R. T., Moon R. J., Beneke E. S. Hepatic clearance of Candida albicans in rats. Infect Immun. 1976 Dec;14(6):1348–1355. doi: 10.1128/iai.14.6.1348-1355.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisman B., Wheelock E. F., Allison A. C. Role of macrophages and antibody in resistance of mice against yellow fever virus. J Immunol. 1971 Jul;107(1):236–243. [PubMed] [Google Scholar]