Abstract
Many behaviors and substances have been purported to induce labor. Using data from the Third Pregnancy, Infection, and Nutrition cohort, we focus on 663 women who experienced spontaneous labor. Of the women who reported a specific labor trigger, 32% reported physical activity (usually walking), 24% a clinician-mediated trigger, 19% a natural phenomenon, 14% some other physical trigger (including sexual activity), 12% reported ingesting something, 12% an emotional trigger, and 7% maternal illness. With the exceptions of walking and sexual intercourse, few women reported any one specific trigger, although various foods/substances were listed in the “ingesting something” category. Discussion of potential risks associated with “old wives’ tale” ways to induce labor may be warranted as women approach term.
Keywords: obstetric, birth, trigger, labor
Medical induction of labor, although a vital tool in the management of some pregnancies, nonetheless carries risks, including uterine hyperstimulation, placental abruption, uterine infection, iatrogenic preterm birth, fetal distress, operative vaginal birth, and cesarean surgery (American College of Obstetricians and Gynecologists, 1999; Engle, 2006; Mealing, Roberts, Ford, Simpson, & Morris, 2009; Moleti, 2009; Simpson, 2010; Simpson & Knox, 2009; Simpson & Thorman, 2005). The proportion of labors that are induced has been rising (Curtin & Park, 1999; Declercq, Sakala, Corry, & Applebaum, 2006; Mealing et al., 2009; Simpson, 2010) and now stands at 23% (Martin et al., 2012)—not a trivial number given the potential drawbacks. Rumors of “conventional” ways of inducing labor abound in the popular literature and include nipple stimulation, acupuncture, acupressure, massage, sexual intercourse, raspberry leaf tea, spicy food, balsamic vinegar, walking, castor or cod liver oil, enema, black or blue cohosh, heavy exertion, dehydration, starvation, stress, fear, and mechanical agitation such as riding in a car along a bumpy road (Curtis & Schuler, 2008; Douglas & Sussman, 2004; Evans & Aronson, 2005; Gaskin, 2002, 2003; Goer, 1999; Iovine, 2007; Kimes & Tisherman, 2004; Murkoff & Mazel, 2008; “Ways to Bring on Labor,” n.d.). If any of these methods are truly effective, then perhaps some medical inductions could be avoided.
Rumors of “conventional” ways of inducing labor abound in the popular literature and include nipple stimulation, acupuncture, acupressure, massage, sexual intercourse, raspberry leaf tea, spicy food, balsamic vinegar, walking, castor or cod liver oil, enema, black or blue cohosh, heavy exertion, dehydration, starvation, stress, fear, and mechanical agitation such as riding in a car along a bumpy road.
Indeed, in recent years, numerous articles have been published evaluating some of these traditional induction alternatives. Vaginal intercourse may help ripen the cervix because semen contains prostaglandins, but studies of association with onset of labor have reported mixed results (Kavanagh, Kelly, & Thomas, 2005; Petridou et al., 2001; Summers, 1997; Tan, Yow, & Omar, 2007; Tan, Yow, & Omar, 2009). The role of acupuncture is also uncertain, with two studies reporting success (Dunn, Rogers, & Halford, 1989; Rabl, Ahner, Bitschnau, Zeisler, & Husslein, 2001) and one reporting no effect (Smith, Crowther, Collins, & Coyle, 2008). A Cochrane review concluded that breast stimulation appears to be somewhat effective and safe for low-risk women at term (Kavanagh et al., 2005). Castor oil may work, although it has potential serious side effects such as meconium-stained amniotic fluid and maternal nausea (Azhari, Pirdadeh, Lotfalizadeh, & Shakeri, 2006; Boel et al., 2009; Davis, 1984; Garry, Figueroa, Guillaume, & Cucco, 2000; Harris & Nye, 1994; Holmes, 1934; Kelly, Kavanagh, & Thomas, 2001). Petridou et al. (2001) reported that physical exertion is related to preterm birth. The other methods described earlier for starting one’s own labor have not, to our knowledge, been evaluated for safety or efficacy in a systematic manner. Taken together, these largely mixed results suggest the need for large-scale randomized controlled trials to evaluate the efficacy of traditional labor induction methods. Most appear to be safe, but efficacy remains uncertain. Given the potential benefits of avoiding medical inductions, further scientific inquiry may be warranted.
However, if no women actually engage in these practices, then studying them is of little use. The Listening to Mothers II (LTMII) study reported that 22% of mothers attempted to self-induce their labors (Declercq et al., 2006), although the questionnaire used in LTMII had semi–close-ended questions about induction, thus potentially limiting the answers that women would give (Bowling, 2002; Childbirth Connection, n.d.). In this study, our aim was to describe, using less restrictive open-ended questions, women’s self-reported labor triggers.
METHOD
This study used data collected as part of the third Pregnancy, Infection, and Nutrition (PIN3) cohort, a large ongoing study of pregnancy in central North Carolina. The PIN3 Study recruited women from January 2001 to June 2005. Participants were recruited by a female study staff member from prenatal clinics affiliated with the University of North Carolina (UNC) Hospitals if they presented for prenatal care at less than 20 weeks’ gestation, intended to deliver at a UNC hospital, were carrying a singleton fetus, were 16 years of age or older, read and spoke English, and had access to a telephone. Women were followed through postpartum. Complete details about the data collection protocols can be found at the PIN3 website (http://www.cpc.unc.edu/projects/pin). The PIN3 protocol was approved by the institutional review board at the UNC at Chapel Hill; all women provided written informed consent.
This analysis focused on data collected as part of an in-person interview, administered before the mother was discharged from the hospital following her birth. The questions on labor triggers were added to the postpartum interview in late November 2001, 6 months after the study began. There were 1,437 women who answered the labor trigger questions between November 2001 and December 2005, of whom 663 reported spontaneous labor and are included in this analysis (see Figure 1).
FIGURE 1.
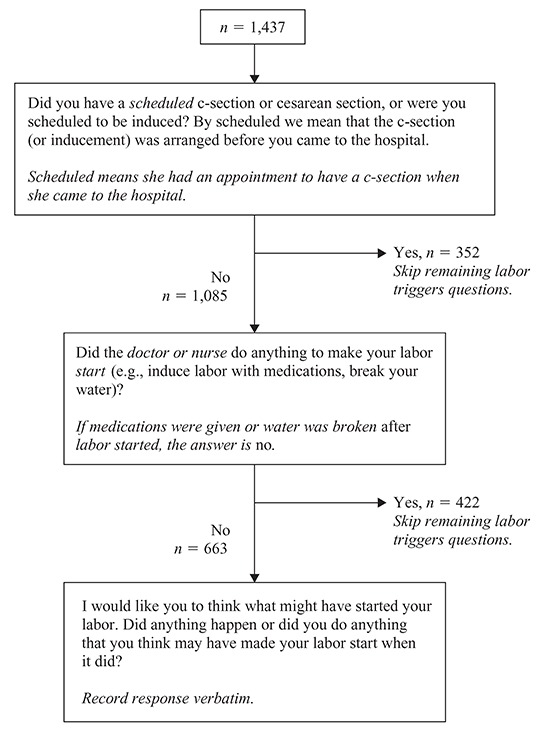
Labor triggers questions from the postpartum interview, third Pregnancy, Infection, and Nutrition (PIN3) Study. Questions are in plain text; instructions intended for the interviewers (but not read aloud to participants) are shown in italics following the questions.
Covariables
During a telephone interview administered at 17–22 weeks’ gestation, women self-reported their race and ethnicity, marital status, household information, and obstetrical history. Based on frequencies in the race/ethnicity variable, we collapsed categories into four distinct levels: White, Black, Asian, and Other (includes women identifying as Latina and Native American).
For marital status, women self-identified as single (never married), currently married, separated, divorced, or widowed. For this analysis, the latter three categories were combined. Maternal age at conception and years of completed education were also collected during the telephone interview. This analysis grouped women’s attained education into three categories: 12 years or less of completed education, 13–16 years, and 17 years or more.
Women also reported household income and household composition (number of adults and number of children). From these data, we calculated the percentage of the 2001 poverty level (Proctor & Dalaker, 2002): A score of 100 indicated a household living exactly at the poverty line. We interpreted poverty percentage as a marker of socioeconomic status.
Women reported previous pregnancies, including both live births and stillbirths, which were combined to define parity. Study staff measured maternal height on recruitment; pregravid weight was self-reported. Pregravid body mass index was calculated from these values. Weight gain during pregnancy was calculated by subtracting self-reported pregravid weight from the measured weight at the last prenatal visit (obtained via medical record abstraction following birth). Adequacy of weight gain is calculated from the Institute of Medicine’s gestational weight gain recommendations; dividing actual weight gain by expected (recommended) weight gain yields the adequacy of weight gain ratio. A ratio higher than about 1.2 indicates excessive weight gain; a ratio lower than about 0.8 indicates insufficient weight gain. Precise boundaries for interpretation of this ratio vary slightly depending on pregravid body mass index.
Gestational age was determined preferentially by a usual-care clinical ultrasound performed prior to 22 weeks’ completed gestation. If no ultrasound was available, or if the ultrasound was performed after 22 weeks’ gestation (less than 8% of the sample), then the pregnancy was dated using the self-reported first day of the last menstrual period. Preterm birth was defined as giving birth prior to 37 completed weeks. Birthweight was abstracted from the medical record following birth.
Data Analysis
One researcher (MLB) examined the free-text responses to the what started your labor question (Figure 1), identified themes, and assigned codes to the various themes. Codes were not specified a priori. Many women gave more than one response (e.g., “I did a lot of walking the day before and I took an herb that was supposed to prepare the uterus for birth”) and thus were assigned more than one code. Responses and coding were checked by a different researcher (KRE) and disagreements resolved by consensus. Codes were then collapsed into broader categories (Figure 2). These categories were not prespecified; rather, they were created based on the tabulation of recorded responses.
FIGURE 2.
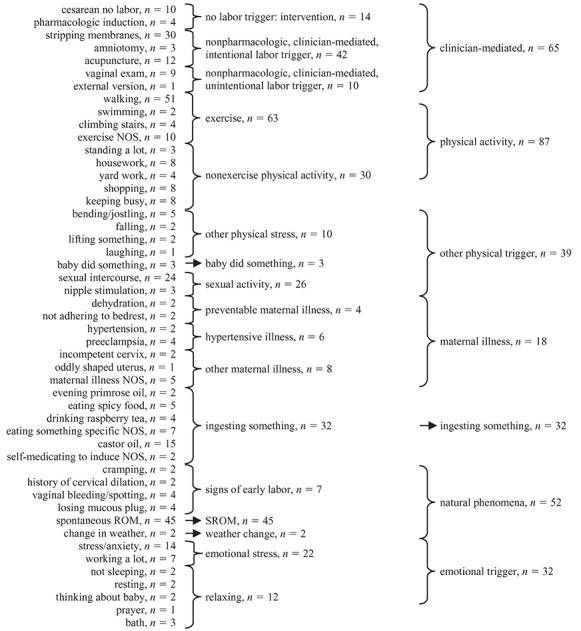
Categorization scheme for free-text responses to the question, “Did anything happen or did you do anything that you think may have made your labor start when it did?” NOS = not otherwise specified; ROM = rupture of membranes; SROM = spontaneous rupture of membranes. Numbers shown are total numbers of women, and women were allowed more than one answer. Therefore, a woman who reported both walking and swimming would appear in each of those categories in the far left column but only once in the “exercise” category in the middle column.
Finally, we examined differences in response categories (Figure 2) across covariables. First, we conducted simple bivariable analyses to determine whether women who reported at least one labor trigger differed from those who did not. Next, we tested globally whether any of the broad categories of labor triggers (far right hand column, Figure 2) differed from others. These analyses used chi-square tests (or Fisher’s exact test if any cell counts were less than 10) for categorical variables; one-way analysis of variance for continuous variables, which we expected to be normally distributed (maternal age at conception, maternal height, weight gain during pregnancy, birthweight); and the Kruskal-Wallis rank sum test for skewed continuous variables (maternal pregravid weight, percentage of 2001 poverty index, maternal pregravid body mass index, adequacy of weight gain, gestational age). All analyses were conducted using S-PLUS version 7.0 (Insightful Corporation, Seattle, WA); statistical significance was set at α < .05.
RESULTS
Women in our sample were largely White, non-Hispanic, married, well-educated, and from a reasonably wealthy household. They tended to gain more weight during pregnancy than recommended by the Institute of Medicine; 13% delivered preterm. The median gestational age was 39 weeks; the median birthweight was 3,348 g (7.38 lbs). Additional details about the sample are presented in Table 1.
TABLE 1. Characteristics of Women (N = 633) From the Third Pregnancy, Infection, and Nutrition (PIN3) Cohort Who Delivered Following Spontaneous Onset of Labor Between November 2001 and December 2005.
| Number | Percentage | Missing | Mean (SD) | Median | Range | |
| Race/ethnicity | 0 | |||||
| White non-Hispanic | 476 | 72 | — | — | — | — |
| Black | 109 | 16 | — | — | — | — |
| Asian or Pacific Islander | 21 | 3 | — | — | — | — |
| Other (includes Hispanic) | 57 | 9 | — | — | — | — |
| Marital status | 0 | |||||
| Single (never married) | 133 | 20 | — | — | — | — |
| Married | 512 | 77 | — | — | — | — |
| Separated/divorced/widowed | 18 | 3 | — | — | — | — |
| Education (years) | 0 | |||||
| ≤ 12 | 106 | 16 | — | — | — | — |
| 13–16 | 317 | 48 | — | — | — | — |
| ≥ 17 | 240 | 36 | — | — | — | — |
| Parity | 0 | |||||
| 1 | 336 | 51 | — | — | — | — |
| 2 | 225 | 34 | — | — | — | — |
| 3+ | 102 | 15 | — | — | — | — |
| Pregravid IOM BMI categorya | 10 | |||||
| Underweight (< 19.8 kg/m2) | 107 | 16 | — | — | — | — |
| Normal weight (19.8–26.0 kg/m2) | 368 | 56 | — | — | — | — |
| Overweight (26.1–29.0 kg/m2) | 64 | 10 | — | — | — | — |
| Obese (> 29.0 kg/m2) | 114 | 17 | — | — | — | — |
| Preterm birthb | 0 | |||||
| No | 89 | 13 | — | — | — | — |
| Yes | 574 | 87 | — | — | — | — |
| Gestational age (weeks) | — | — | 0 | 38.4 (2.5) | 39.0 | 19.0–42.0 |
| Birthweight (g) | — | — | 3 | 3,256 (628.7) | 3,348 | 170–5,027 |
| % 2001 poverty levelc | — | — | 24 | 422 (223) | 464 | 11–923 |
| Age at conception (years) | — | — | 0 | 29.0 (5.5) | 29.0 | 16.0–47.0 |
| Weight gain (kg) | — | — | 14 | 15.1 (5.8) | 15.0 | −7.7–34.5 |
| Adequacy of weight gain indexd | — | — | 14 | 1.44 (0.71) | 1.33 | −1.23–5.32 |
aIOM = Institute of Medicine; BMI = body mass index. These are the boundaries specified by the IOM in their recommendations for weight gain during pregnancy.
bBirth prior to 37 weeks completed gestation.
cCalculated based on income, number of adults in household, and number of children in household.
dThis number is calculated as actual weight gain/IOM-recommended weight gain. Generally speaking, a score of 1.0 means that the woman gained sufficient weight to fall in the middle of the range of recommended weight gain; less than 0.8 roughly corresponds to inadequate weight gain, whereas greater than 1.2 roughly corresponds to excess weight gain.
Of the 663 women who answered the question, “Did anything happen or did you do anything that you think may have made your labor start when it did?,” 60% (n = 393) answered “no,” “nothing,” or some variant thereof; 30% (n = 200) listed one specific trigger; 7% (n = 46) listed two specific triggers; 3% (n = 18) listed three specific triggers; and 2 women listed four specific triggers. Four women were missing data for this question.
The 60% of women who did not report a specific trigger were older (mean age at conception 29.4 years vs. 28.4 years, p = .02) and gained less weight during their pregnancy (mean weight gain 14.6 kg vs. 15.8 kg, p < .01) when compared to women reporting at least one specific trigger. The women reporting no triggers may also have had a higher socioeconomic status (433% vs. 404% of the 2001 poverty level, p = .08). These two groups did not differ with respect to gestational age at birth (median 39 weeks for each, p = 1.00), race/ethnicity (72.5% White vs. 70.3%, p = .80), marital status (79.4% married vs. 73.7%, p = .20), maternal education (14.0% 12 years or less vs. 18.8%, p = .20), parity (47.8% primiparas vs. 54.9%, p = .20), birth weight (median 3,330 g vs. 3,366 g; p = .30), or maternal pregravid body mass index (median 22.8 vs. 23.8 kg/m2, p = .50).
There were 50 specific triggers mentioned in the original free-text responses (n = 266); the most commonly reported triggers were walking, spontaneous rupture of membranes, and sexual intercourse (see Figure 2). The 50 specific triggers were collapsed into 17 categories containing related items and then further collapsed into 7 broad categories. Of note, 2 of these categories were not conventional labor triggers per se (“signs of early labor,” “clinician-mediated”). For comparisons of trigger categories by demographic variables, we dropped women in both of these categories because we were focusing on potentially modifiable labor triggers. We also dropped the two women in the change in weather category for the same reason (as well as to maintain adequate power).
We found no differences among the remaining five broad trigger categories (far right column, Figure 2) regarding race/ethnicity, marital status, education, parity, socioeconomic status, maternal age at conception, maternal height, weight gain, or adequacy of weight gain (see Table 2). We did, however, observe differences by gestational age. Women in the “maternal illness” category had shorter gestations (median 35.5 weeks) than did women in other categories; in addition, women in the “ingested something” category had slightly longer gestations (median 40 weeks; all other categories were 39 weeks, p < .001 across all categories).
TABLE 2. Comparison of Demographics Across Categories of Labor Trigger.
| Physical Activity | Other Physical Trigger | Maternal Illness | Ingesting Something | Emotional Trigger | p Valuea | |
| Race/ethnicity | ||||||
| White | 54 | 30 | 13 | 23 | 23 | .80 |
| Black | 19 | 6 | 4 | 5 | 4 | — |
| Asian/Pacific Islander | 2 | 1 | 0 | 1 | 0 | — |
| Other | 12 | 2 | 1 | 3 | 5 | — |
| Marital status | ||||||
| SNM | 20 | 11 | 5 | 5 | 7 | .70 |
| Married | 61 | 27 | 13 | 26 | 23 | — |
| DWS | 6 | 1 | 0 | 1 | 2 | — |
| Maternal education (years) | ||||||
| ≤ 12 | 17 | 8 | 3 | 5 | 9 | .17 |
| 13−16 | 35 | 13 | 11 | 21 | 14 | — |
| 17+ | 40 | 46 | 22 | 19 | 28 | — |
| Parity | ||||||
| 1 | 53 | 24 | 8 | 21 | 18 | .60 |
| 2 | 25 | 12 | 6 | 7 | 7 | — |
| 3+ | 9 | 3 | 4 | 4 | 7 | — |
| Median SESb (%) | 353 | 379 | 379 | 379 | 473 | .70 |
| Median maternal age at conception (years) | 29.0 | 28.0 | 27.0 | 30.0 | 29.5 | .30 |
| Median maternal pregravid BMIc (kg/m2) | 22.62 | 22.74 | 25.42 | 22.28 | 23.10 | .30 |
| Median adequacy of weight gain indexd | 1.4 | 1.4 | 1.6 | 1.3 | 1.4 | .60 |
Note. SNM = single (never married); DWS = divorced/widowed/separated; SES = socioeconomic status.
ap values are from chi-square tests for categorical variables and from Kruskal-Wallis tests for continuous variables.
bCalculated based on income, number of adults in household, and number of children in household.
cBMI = body mass index.
dThis number is calculated as actual weight gain/IOM-recommended weight gain. Generally speaking, a score of 1.0 means that the woman gained sufficient weight to fall in the middle of the range of recommended weight gain; less than 0.8 roughly corresponds to inadequate weight gain, whereas greater than 1.2 roughly corresponds to excess weight gain.
DISCUSSION
Our main findings were that most women reporting spontaneous labor did not recall a specific labor trigger and that among those who did, many reported something which was not, in fact, a conventional “labor trigger” but rather was either a sign of early labor or an intervention initiated by a clinician.
When exploring women who reported at least one modifiable (potentially genuine) labor trigger, we found that gestations were shorter (median 35.5 weeks) among those women reporting maternal illness as a labor trigger. Although perhaps not surprising, this cannot be explained by clinicians inducing or operating for preeclampsia or other medical cause because our sample was limited to women experiencing spontaneous labor. It still, however, is not unexpected that a maternal illness might trigger an earlier labor. Exact causes of labor are as yet unknown, but it is widely believed that the fetus is at least partially responsible (Blackburn, 2003). Maternal illness could alter the uterine environment sufficiently to cause a fetus to initiate labor.
The longest gestations were found among those women who believed that ingesting something started their labor (median 40 weeks). This pattern could be explained by thinking in terms of women attempting to self-induce their labors. We cannot say with certainty, based on the wording of our questions, that all women were attempting to self-induce in this manner, but some clearly were. For example, one woman said, “I was walking a lot that day.” It is unclear whether or not she was purposefully trying to trigger her own labor or whether she just thinks that the unusual amount of walking she did (for some other reason) was an unintentional trigger. However, a different woman said, “I tried to walk the baby out of me this week.” She clearly was trying to start her labor. Ingesting castor oil, large quantities of raspberry tea, or black/blue cohosh would likely not be a normal behavior for a late-term pregnant woman, unless she was trying to start her labor. This assumption is supported by our result that women who reported that ingesting something started their labor had longer median gestational ages than did women in other trigger categories or women who reported no specific labor trigger.
As shown in Figure 1, women who did not have a scheduled cesarean or induction were asked, “Did the doctor or nurse do anything to make your labor start (e.g., induce labor with medications, break your water)?” Only women who answered no to this question were asked the follow-up question about specific labor triggers. Fifty-five women in our sample answered no to the first question but then named a clinician-mediated specific labor trigger (acupuncture, amniotomy, external version, vaginal exam, membrane stripping, or pharmacologic induction—although it is possible that acupuncture was administered by someone other than a doctor or nurse). Although the questions were pilot-tested, clearly some women still did not understand the question sequence. This could happen if, for example, a woman is unaware that membrane stripping is usually performed with the intent of causing labor onset. This woman may very well answer no to the question about the doctor/nurse doing something but then reply, “The nurse stripped my membranes at my last prenatal visit,” to the triggers question—in other words, perhaps she interpreted the first question (on doctors/nurses) to mean “intentionally” but then reported the membrane stripping as a trigger, thinking it was accidentally associated with labor onset. This suggests that perhaps the intentions behind procedures, as well as associated risks and benefits, need to be explained more clearly to obstetric patients during the informed consent process. This also might be an area for focus during childbirth education classes.
Childbirth educators encountering pregnant women approaching term should consider discussing the old wives’ tale labor induction methods with their clients, including what is known about efficacy and safety.
Our series of labor triggers questions did not account for nonscheduled cesarean surgeries without labor, as evidenced by the 10 women who reported things such as, “No labor. Came for ultrasound appointment and was admitted to the hospital because of high blood pressure, had emergency cesarean section,” or “I never went into labor. I had too low amniotic fluid so they sectioned me.” This was an oversight during questionnaire development. Eliminating these 10 women from the analysis did not noticeably change the results (data not shown).
Twelve women reported that acupuncture caused their labor to start. This probably represents a higher than normal proportion of pregnant women because of a concurrent study at our institution examining whether or not acupuncture can be used to induce women with term pregnancies. Women were allowed to enroll concurrently in both the PIN3 Study and the acupuncture study, and most of those reporting acupuncture as their trigger mentioned the other study specifically.
Our study had two main limitations. First, our study population, being largely White, well-educated, and married, is not representative of the U.S. child-bearing population as a whole. It is possible that women from other cultural backgrounds would not have heard of the same “old wives’ tale” labor triggers and that similar studies in other populations would yield substantially different results. Second, only 266 women reported any labor trigger, and of these, only 149 reported a conventional, potentially modifiable labor trigger. Repeating the study with a larger, more diverse sample may well yield additional interesting results.
Practice Implications
Our study suggests that some fraction of women are indeed attempting to induce their own labors, often with methods that are probably not harmful (e.g., walking, sexual intercourse), but at times with methods that are either known to have risks or that have unknown risk profiles in pregnant women (e.g., castor oil, evening primrose oil). Not surprisingly, this practice seems to be more common in women who are still pregnant after their due date has passed. Childbirth educators encountering pregnant women approaching term should consider discussing the old wives’ tale labor induction methods with their clients, including what is known about efficacy and safety. In addition, some women in our sample appeared not to understand that procedures such as membrane stripping are often employed by obstetricians and midwives with the express intent of causing labor to start. A discussion during childbirth education may be warranted for these practices as well.
The LTMII survey found that 22% of women tried to self-induce their labors and that the most common methods were walking/physical activity, sexual intercourse, and nipple stimulation.
CONCLUSION
Our study confirms the findings of the only other study of which we are aware that looked at conventional labor triggers in a U.S. population: The LTMII survey found that 22% of women tried to self-induce their labors and that the most common methods were walking/physical activity, sexual intercourse, and nipple stimulation (Declercq et al., 2006). Although we cannot comment, in our study, about the proportion of all women who may have attempted self-induction with a conventional method, in our sample, women also reported walking, other physical activity, and sexual activity (including nipple stimulation) more often than other triggers. Further study of physical activity (including walking) and sexual intercourse using randomized controlled trials is warranted to determine whether or not these activities can in fact trigger labor because they may allow clinicians and pregnant women to avoid pharmacologic inductions.
ACKNOWLEDGMENTS
The third Pregnancy, Infection, and Nutrition (PIN3) Study is a joint effort of many investigators and staff members whose work is gratefully acknowledged. Funding for this study was provided by National Institutes of Health (NIH; Bethesda, Maryland)/National Institute of Child Health and Human Development (#HD37584, #HD052468-01A2), NIH/National Cancer Institute (#CA109804-01), and NIH General Clinical Research Center (#RR00046). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. None of the authors have conflicts of interest to disclose.
Biographies
MARIT L. BOVBJERG, PhD, MS, is a research associate and instructor at Oregon State University. Her research interests include evidence-based maternity care and physical activity during pregnancy.
KELLY R. EVENSON, PhD, is a research professor in the Gillings School of Global Public Health at the University of North Carolina. Her research focuses primarily on physical activity, with a secondary interest in lifestyle behaviors during pregnancy.
CHYRISE BRADLEY, MA, is a clinical research coordinator at the University of North Carolina. She was the lead study coordinator for the PIN project.
JOHN M. THORP, JR., MD, is the Hugh McAllister Distinguished Professor of Obstetrics and Gynecology at the University of North Carolina School of Medicine. He is interested in substance abuse during pregnancy and preterm prevention trials.
REFERENCES
- American College of Obstetricians and Gynecologists. (1999). Induction of Labor (Practice Bulletin #10). Washington, DC: Author [Google Scholar]
- Azhari S., Pirdadeh S., Lotfalizadeh M., & Shakeri M. T. (2006). Evaluation of the effect of castor oil on initiating labor in term pregnancy. Saudi Medical Journal, 27(7), 1011–1014 [PubMed] [Google Scholar]
- Blackburn S. T. (2003). Maternal, fetal, & neonatal physiology: A clinical perspective (2nd ed.). St. Louis, MO: Saunders [Google Scholar]
- Boel M. E., Lee S. J., Rijken M. J., Paw M. K., Pimanpanarak M., Tan S. O., . . . McGready R. (2009). Castor oil for induction of labour: Not harmful, not helpful. The Australian & New Zealand Journal of Obstetrics & Gynaecology, 49(5), 499–503. 10.1111/j.1479-828X.2009.01055.x [DOI] [PubMed] [Google Scholar]
- Bowling A. (2002). Research methods in health (2nd ed.). Berkshire, United Kingdom: Open University Press [Google Scholar]
- Childbirth Connection. (n.d.). Listening to Mothers II Questionnaire. Retrieved from http://www.childbirthconnection.org/pdfs/LTMII_questionnaire.pdf
- Curtin S. C., & Park M. M. (1999). Trends in the attendant, place, and timing of births, and in the use of obstetric interventions: United States, 1989-97. National Vital Statistics Reports, 47(27), 1–13 [PubMed] [Google Scholar]
- Curtis G. B., & Schuler J. (2008). Your pregnancy week by week (6th ed.). Philadelphia, PA: Da Capo Press [Google Scholar]
- Davis L. (1984). The use of castor oil to stimulate labor in patients with premature rupture of membranes. Journal of Nurse-Midwifery, 29(6), 366–370 [DOI] [PubMed] [Google Scholar]
- Declercq E., Sakala C., Corry M., & Applebaum S. (2006). Listening to mothers II: Report of the second national U.S. survey of women’s childbearing experiences. Retrieved from www.childbirthconnection.com [DOI] [PMC free article] [PubMed]
- Douglas A., & Sussman J. (2004). The unofficial guide to having a baby (2nd ed.). Hoboken, NJ: Wiley Publishing [Google Scholar]
- Dunn P. A., Rogers D., & Halford K. (1989). Transcutaneous electrical nerve stimulation at acupuncture points in the induction of uterine contractions. Obstetrics and Gynecology, 73(2), 286–290 [PubMed] [Google Scholar]
- Engle W. A. (2006). A recommendation for the definition of “late preterm” (near-term) and the birth weight-gestational age classification system. Seminars in Perinatology, 30(1), 2–7. 10.1053/j.semperi.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Evans J., & Aronson R. (2005). The whole pregnancy handbook: An obstetrician’s guide to integrating conventional and alternative medicine before, during, and after pregnancy. New York, NY: Gotham Books [Google Scholar]
- Garry D., Figueroa R., Guillaume J., & Cucco V. (2000). Use of castor oil in pregnancies at term. Alternative Therapies in Health and Medicine, 6(1), 77–79 [PubMed] [Google Scholar]
- Gaskin I. M. (2002). Spiritual midwifery (4th ed.). Summertown, TN: Book Publishing Company [Google Scholar]
- Gaskin I. M. (2003). Ina May’s guide to childbirth. New York, NY: Bantam Dell [Google Scholar]
- Goer H. (1999). The thinking woman’s guide to a better birth. New York, NY: The Berkley Publishing Group [Google Scholar]
- Harris M., & Nye M. (1994). Self-administration of castor oil. Modern Midwife, 4(6), 29–30 [PubMed] [Google Scholar]
- Holmes O. M. (1934). Induction of labor: Using quinin, castor oil, rupture of membranes, and nasal pituitrin. California and Western Medicine, 41(4), 241–244 [PMC free article] [PubMed] [Google Scholar]
- Iovine V. (2007). The girlfriend’s guide to pregnancy (2nd ed.). New York, NY: Pocket Books [Google Scholar]
- Kavanagh J., Kelly A. J., & Thomas J. (2005). Breast stimulation for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews, (3), CD003392. http://dx.doi.org10.1002/14651858.CD003392.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A. J., Kavanagh J., & Thomas J. (2001). Castor oil, bath and/or enema for cervical priming and induction of labour. Cochrane Database of Systematic Reviews, (2), CD003099. 10.1002/14651858.CD003099 [DOI] [PubMed] [Google Scholar]
- Kimes J., & Tisherman S. (2004). Pregnancy sucks: What to do when your miracle makes you miserable. Avon, MA: Adams Media [Google Scholar]
- Martin J. A., Hamilton B. E., Ventura S. J., Osterman M. J. K., Wilson E. C., & Mathews T. (2012). Births: Final data for 2010. National Vital Statistics Reports, 61(1), 1–72 [PubMed] [Google Scholar]
- Mealing N. M., Roberts C. L., Ford J. B., Simpson J. M., & Morris J. M. (2009). Trends in induction of labour, 1998-2007: A population-based study. The Australian & New Zealand Journal of Obstetrics & Gynaecology, 49(6), 599–605. 10.1111/j.1479-828X.2009.01086.x [DOI] [PubMed] [Google Scholar]
- Moleti C. A. (2009). Trends and controversies in labor induction. MCN. The American Journal of Maternal Child Nursing, 34(1), 40–47; quiz 48–49.10.1097/01.NMC.0000343864.49366.66 [DOI] [PubMed] [Google Scholar]
- Murkoff H., & Mazel S. (2008). What to expect when you’re expecting (4th ed.). New York, NY: Workman Publishing [Google Scholar]
- Petridou E., Salvanos H., Skalkidou A., Dessypris N., Moustaki M., & Trichopoulos D. (2001). Are there common triggers of preterm deliveries? BJOG: An International Journal of Obstetrics and Gynaecology, 108(6), 598–604 [DOI] [PubMed] [Google Scholar]
- Proctor B. D., & Dalaker J. (2002). Poverty in the United States: 2001 (Publication No. P60-219). Washington, DC: U.S. Government Printing Office [Google Scholar]
- Rabl M., Ahner R., Bitschnau M., Zeisler H., & Husslein P. (2001). Acupuncture for cervical ripening and induction of labor at term—A randomized controlled trial. Wiener Klinische Wochenschrift, 113(23–24), 942–946 [PubMed] [Google Scholar]
- Simpson K. R. (2010). Reconsideration of the costs of convenience: Quality, operational, and fiscal strategies to minimize elective labor induction. The Journal of Perinatal & Neonatal Nursing, 24(1), 43–52; quiz 53–54.10.1097/JPN.0b013e3181c6abe3 [DOI] [PubMed] [Google Scholar]
- Simpson K. R., & Knox G. E. (2009). Oxytocin as a high-alert medication: Implications for perinatal patient safety. MCN. The American Journal of Maternal Child Nursing, 34(1), 8–15; quiz 16–17.10.1097/01.NMC.0000343859.62828.ee [DOI] [PubMed] [Google Scholar]
- Simpson K. R., & Thorman K. E. (2005). Obstetric “conveniences”: Elective induction of labor, cesarean birth on demand, and other potentially unnecessary interventions. The Journal of Perinatal & Neonatal Nursing, 19(2), 134–144 [DOI] [PubMed] [Google Scholar]
- Smith C. A., Crowther C. A., Collins C. T., & Coyle M. E. (2008). Acupuncture to induce labor: A randomized controlled trial. Obstetrics and Gynecology, 112(5), 1067–1074. 10.1097/AOG.0b013e31818b46bb [DOI] [PubMed] [Google Scholar]
- Summers L. (1997). Methods of cervical ripening and labor induction. Journal of Nurse-Midwifery, 42(2), 71–85 [DOI] [PubMed] [Google Scholar]
- Tan P. C., Yow C. M., & Omar S. Z. (2007). Effect of coital activity on onset of labor in women scheduled for labor induction: A randomized controlled trial. Obstetrics and Gynecology, 110(4), 820–826. 10.1097/01.AOG.0000267201.70965.ec [DOI] [PubMed] [Google Scholar]
- Tan P. C., Yow C. M., & Omar S. Z. (2009). Coitus and orgasm at term: Effect on spontaneous labour and pregnancy outcome. Singapore Medical Journal, 50(11), 1062–1067 [PubMed] [Google Scholar]
- Ways to Bring on Labor. (n.d.). Retrieved from http://www.whattoexpect.com/pregnancy/ask-heidi/week-41/bring-on-labor.aspx


