Abstract
Objective:
To assess factors at the start of antiretroviral therapy (ART) associated with long-term virological response in children.
Design:
Multicentre national cohort.
Methods:
Factors associated with viral load below 400 copies/ml by 12 months and virologic failure among children starting 3/4-drug ART in the UK/Irish Collaborative HIV Paediatric Study were assessed using Poisson models.
Results:
Nine hundred and ninety-seven children started ART at a median age of 7.7 years (inter-quartile range 2.9–11.7), 251 (25%) below 3 years: 411 (41%) with efavirenz and two nucleoside reverse transcriptase inhibitors (EFV + 2NRTIs), 264 (26%) with nevirapine and two NRTIs (NVP + 2NRTIs), 119 (12%; 106 NVP, 13 EFV) with non-nucleoside reverse transcriptase inhibitor and three NRTIs (NNRTI + 3NRTIs), and 203 (20%) with boosted protease inhibitor-based regimens. Median follow-up after ART initiation was 5.7 (3.0–8.8) years. Viral load was less than 400 copies/ml by 12 months in 92% [95% confidence interval (CI) 91–94%] of the children. Time to suppression was similar across regimens (P = 0.10), but faster over calendar time, with older age and lower baseline viral load. Three hundred and thirty-nine (34%) children experienced virological failure. Although progression to failure varied by regimen (P < 0.001) and was fastest for NVP + 2NRTIs regimens, risk after 2 years on therapy was similar for EFV + 2NRTIs and NVP + 2NRTIs, and lowest for NNRTI + 3NRTIs regimens (P-interaction = 0.03). Older age, earlier calendar periods and maternal ART exposure were associated with increased failure risk. Early treatment discontinuation for toxicity occurred more frequently for NVP-based regimens, but 5-year cumulative incidence was similar: 6.1% (95% CI 3.9–8.9%) NVP, 8.3% (95% CI 5.6–11.6) EFV, and 9.8% (95% CI 5.7–15.3%) protease inhibitor-based regimens (P = 0.48).
Conclusion:
Viral load suppression by 12 months was high with all regimens. NVP + 3NRTIs regimens were particularly efficacious in the longer term and may be a good alternative to protease inhibitor-based ART in young children.
Keywords: antiretroviral therapy, children, HIV, UK/Ireland, virological outcome
Introduction
Maintenance of long-term viral suppression is a particular challenge for HIV-infected children who will likely require antiretroviral therapy (ART) for life, and the foundations of treatment success depend on the effectiveness of first-line regimens [1,2]. A recent European study found risk of triple-class viral load failure was twice as high in children compared to adults [3]. WHO 2013 guidelines recommend lopinavir/ritonavir (LPV/r)-based regimens as the preferred choice for first-line therapy in children aged under 3 years and efavirenz (EFV)-based regimens for 3 years and over; nevirapine (NVP)-based regimens are recommended as an alternative and have the advantage of being available as paediatric fixed-dose combination in most resource-limited settings [4].
Four clinical trials have evaluated first-line ART strategies in children. The International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) 1060 trial cohorts 1 and 2 included mainly African children, all aged below 3 years, with and without NVP exposure for prevention of mother-to-child transmission (pMTCT), respectively [5,6]. Both cohorts reported higher rates of treatment failure (composite endpoint of death, virological failure and regimen-limiting toxicity) in the NVP versus LPV/r-based ART group at 24 weeks, suggesting short-term benefit of LPV/r irrespective of prior NVP exposure for pMTCT. In contrast, the PENPACT-1 [Paediatric European Network for Treatment of AIDS (PENTA) and Pediatric AIDS Clinical Trials Group (PACTG/IMPAACT)] trial with median 5 years follow-up found similar viral load, CD4+, and clinical outcomes among children initiating protease inhibitor compared with non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimens [7]. However, PENPACT-1 enrolled only 26% children aged below 3 years and included EFV and NVP as the NNRTI, and LPV/r and nelfinavir as the protease inhibitor, although virological suppression was similar across initial protease inhibitors and NNRTIs, Finally, the Antiretroviral research for Watoto (ARROW) trial in Uganda and Zimbabwe reported early viral load and CD4+ benefit from four-drug ART induction with NNRTI + 3NRTIs, which was not sustained after stopping the fourth drug at 36 weeks [8]. The NNRTI (NVP or EFV) was chosen by clinicians according to local availability and age, with over a third of the children receiving EFV which was similar across treatment arms. Nearly a third of the children were below 3 years of age, and they had similar rates of viral load suppression less than 400 copies/ml to older children.
Response to different first-line regimens has also been reported from paediatric observational studies. In the European Pregnancy and Paediatric HIV Cohort Collaboration (EPPICC) infant cohort, four-drug NNRTI-based regimens had better 12-month virological and immunological response than other regimens [9]. In several studies from sub-Saharan Africa, longer time to virological failure was observed for EFV versus NVP-based initial ART, consistent with a recent meta-analysis showing superior virologic response for EFV to NVP in both randomized controlled trials and observation studies in adults [10–14]. In addition, randomized trials in adults have shown EFV to have either similar or better efficacy compared to protease inhibitors [15–18].
Further long-term data across all ages in children are therefore required to compare effectiveness of the first-line regimens. We assessed factors associated with virological suppression within 12 months of ART initiation and also virological failure during follow-up among children in the national UK/Ireland Collaborative HIV Paediatric Study (CHIPS), focusing particularly on first-line regimens. Since drug-related adverse events can lead to poorer adherence and regimen changes, complicating clinical management, we also examined drug discontinuation for toxicity by regimen.
Methods
Details of CHIPS were previously described [19]. Briefly, infants born to HIV-infected women and children presenting in the UK/Ireland with HIV are reported to the National Study of HIV in Pregnancy and Childhood. Follow-up data for HIV-infected children are then collected through CHIPS. Both studies have NHS Research Ethics approval.
Analyses included antiretroviral-naïve children aged below 18 years enrolled up to November 2013, who started ART from 1997 with at least three drugs (excluding unboosted-protease inhibitor or triple NRTI regimens, and ART for neonatal prophylaxis), and had at least one viral load measurement within 12 months of initiation.
Statistical methods
Date of virological suppression on ART was estimated as the mid-point between the last viral load measurement above 400 copies/ml before suppression (or ART start date if not available) and the first viral load measurement below 400 copies/ml. Time from starting ART to virological suppression by 12 months was analysed with follow-up censored at the earliest of the following: date of initial suppression, last viral load measurement, and 12 months from initiation.
The definition of virological failure, adapted from a previous study, was the earliest of the following: confirmed rebound above 400 copies/ml (the second of the two consecutive measurements >400 copies/ml within 6 months) after previous suppression; unconfirmed rebound above 400 copies/ml followed by changing of at least two drugs within 6 months (to allow the possibility of the clinician regarding the child to have failed treatment and therefore switched before confirmed rebound, leading to re-suppression); or viral load above 400 copies/ml after 12 months without previous suppression [20]. Censoring for time to virological failure was at the earliest of the following: date of virological failure, last viral load measurement, and the first viral load measurement without a subsequent measurement within 12 months (to allow occasional long gaps between clinic visits). The cumulative proportion of children experiencing each outcome over time from ART initiation was estimated using Kaplan–Meier methods.
Predictors of each virological outcome were assessed using Poisson mixed models, accounting for within-clinic clustering. Analyses ignored treatment changes and interruptions. The effect of covariates were estimated adjusting for time from ART initiation, a priori confounders and all covariates with a P value less than 0.05 in the multivariable models. A priori confounders were age, calendar year, first-line regimen, and also for the comparison of regimen, baseline viral load, CD4% and pre-treatment AIDS diagnosis. Other potential predictors were sex, ethnicity, born in UK/Ireland or abroad, exposure to maternal ART during pregnancy or at delivery, and weight-for-age z-score [21]. Due to the small number of children on EFV + 3NRTIs regimens, they were combined with those on NVP + 3NRTIs in analyses.
The effect of the regimen was compared for all children combined, and by age under and over 3 years, by including an interaction (as EFV was not recommended in <3 years until May 2013) [4,22]. Also, interaction between regimen and time since ART initiation (<24 and ≥24 months) was assessed in the analysis of virological failure.
The following sensitivity analyses were performed: adjusting additionally for NRTI backbones in analyses of 3-drug regimens only; viral load limits of above 1000 and above 5000 copies for rebound in the definition of virological failure, with virologic suppression defined as below 400 copies/ml; restricting analyses to children born in the UK/Ireland without known exposure to maternal NNRTI-based ART, since maternal ART exposure may be under-reported among children born abroad [23]; excluding children ever on once-daily NVP during maintenance dose as twice-daily dosing is currently recommended; and in the analysis of virological failure, follow-up censored at treatment interruption with viral load below 400 copies/ml to exclude viral rebound while off treatment. Missing data for covariates were imputed using Multivariate Imputation by Chained Equations based on 20 cycles [24].
Finally, the cumulative incidence of treatment discontinuation (stopping the initial protease inhibitor or NNRTI drug) for toxicity was estimated by regimen using competing risk methods, with discontinuation for other reasons considered a competing event [25].
Statistical analyses were performed using Stata version 12 (Stata Corporation, College Station, Texas, USA).
Results
Of the 997 children included in this analysis, 97% were perinatally infected, half were female, 82% were of black African ethnic origin, and 42% were born in the UK/Ireland (Table 1). Sixty-eight (7%) were exposed to maternal ART during pregnancy or delivery, of whom 27 were treated with NNRTI-based regimens (including four with single-dose NVP). Median age at ART initiation was 7.7 [inter-quartile range (IQR) 2.9–11.7] years, with 251 (25%) below 3 years of age. Median baseline CD4% was 15% (9–20%). One-fifth of the children had a pre-treatment AIDS-defining diagnosis. Median follow-up after initiation was 5.7 (3.0–8.8) years.
Table 1.
Characteristics at antiretroviral therapy initiation, by type of first-line antiretroviral therapy regimen (n = 997).
| Type of first-line ART regimen | |||||
| NVP + 2NRTIs | EFV + 2NRTIs | Boosted PI | NNRTI + 3NRTIs | Overall | |
| Number (%) or median [IQR] | |||||
| Number of children | 264 | 411 | 203a | 119 [106 NVP, 13 EFV] | 997 |
| Perinatal infection | 253 (97) | 379 (96) | 193 (99) | 117 (99) | 942 (97) |
| Female sex | 136 (52) | 218 (53) | 115 (57) | 61 (51) | 530 (53) |
| Ethnic group | |||||
| White | 22 (9) | 11 (3) | 15 (8) | 9 (8) | 57 (6) |
| Black African | 212 (81) | 352 (87) | 158 (79) | 86 (72) | 808 (82) |
| Other | 27 (10) | 41 (10) | 26 (13) | 24 (20) | 118 (12) |
| Born in the UK/Ireland | 134 (51) | 108 (27) | 86 (43) | 90 (76) | 418 (42) |
| Maternal ART in pregnancy or during labour | |||||
| None | 236 (89) | 407 (99) | 186 (92) | 100 (84) | 929 (93) |
| Single-dose NVP | 3 (1) | 0 (0) | 1 (0.5) | 0 (0) | 4 (0.5) |
| Other NNRTI-based ART | 7 (3) | 2 (0.5) | 7 (3) | 7 (6) | 23 (2)b |
| Other ART | 18 (7) | 2 (0.5) | 10 (5) | 12 (10) | 42 (4) |
| Age (years) | 4.1 [1.6–8.8] | 10.4 [6.8–12.9] | 9.3 [3.4–13.0] | 0.6 [0.3–2.4] | 7.7 [2.9–11.7] |
| <3 years | 104 (39) | 7 (2) | 47 (23) | 93 (78) | 251 (25) |
| Calendar period | |||||
| Before 2002 | 89 (34) | 33 (8) | 3 (1) | 28 (24) | 153 (15) |
| 2002–2004 | 74 (28) | 103 (25) | 49 (24) | 46 (39) | 272 (27) |
| 2005–2007 | 63 (24) | 129 (31) | 39 (19) | 27 (23) | 258 (26) |
| 2008–2013 | 38 (14) | 146 (36) | 112 (55) | 18 (15) | 314 (31) |
| Viral load (log10c/ml) | 5.0 [4.4–5.5] | 4.9 [4.3–5.2] | 4.9 [4.1–5.3] | 5.7 [5.2–5.9] | 5.0 [4.3–5.5] |
| CD4+ per cent | 17 [11–23] | 13 [8–18] | 16 [11–21] | 16 [8–28] | 15 [9–20] |
| Pre-treatment AIDS | 34 (13) | 55 (13) | 39 (19) | 58 (49) | 186 (19) |
| NRTI backbone | |||||
| In three-drug regimens | |||||
| ABC 3TC | 77 (29) | 255 (62) | 111 (59) | – | 443 (51) |
| ZDV 3TC | 114 (43) | 63 (15) | 37 (20) | – | 214 (25) |
| d4T 3TC | 24 (9) | 19 (5) | 6 (3) | – | 49 (6) |
| DDI d4T | 44 (17) | 7 (2) | 4 (2) | – | 55 (6) |
| TDF FTC | 2 (1) | 57 (14) | 20 (11) | – | 79 (9) |
| Other | 3 (1) | 10 (2) | 9 (5) | – | 22 (3) |
| In four-drug regimens | |||||
| ZDV 3TC ABC | – | – | 16 (100) | 116 (98) | 132 (98) |
| Other | – | – | 0 (0) | 3 (2) | 3 (2) |
3TC, lamivudine; ABC, abacavir; d4T, stavudine; ddI, didanosine; FTC, emtricitabine; TDF, tenofovir; ZDV, zidovudine. Number of children with missing data: mode of infection, 30; sex, 1; ethnicity, 14; place of birth, 13; CD4+ percentage, 196; viral load, 199.
a177/203 children in the PI group were on lopinavir/ritonavir, 16 atazanavir and 10 darunavir. 187 were on 2NRTIs and 16 on 3NRTIs.
bTwenty-two were exposed to NVP-based maternal ART (other than single-dose NVP) and 1 to EFV-based maternal ART.
First-line regimen
Types of first-line regimen varied with age and calendar period (Fig. 1). Over three-quarters of the children aged below 3 years were initiated on NVP + 2NRTIs (41%, 104/251) or 3NRTIs (37%, 93/251). Among those aged at least 3 years, 54% (404/746) received EFV + 2NRTIs regimens. In this age group, use of NVP + 2NRTIs regimens decreased over time and NNRTI + 3NRTIs regimens were infrequently prescribed (4%, n = 26; 13 NVP, 13 EFV). Use of boosted protease inhibitor regimens commenced in 2000 and increased to 36% from 2008 onwards, with similar trends across age groups. LPV/r accounted for nearly 90% of the protease inhibitor regimens prescribed (177/203), with the remaining children on atazanavir (8%, 16/203) or darunavir (5%, 10/203). Eight percent (16/203) of the children on protease inhibitor regimens received three NRTIs. Eighty-nine percent (106/119) of the children in the NNRTI + 3NRTIs group received NVP and 11% (13/119) EFV.
Fig. 1.
Distribution of first-line regimen by age at antiretroviral therapy initiation and calendar period.
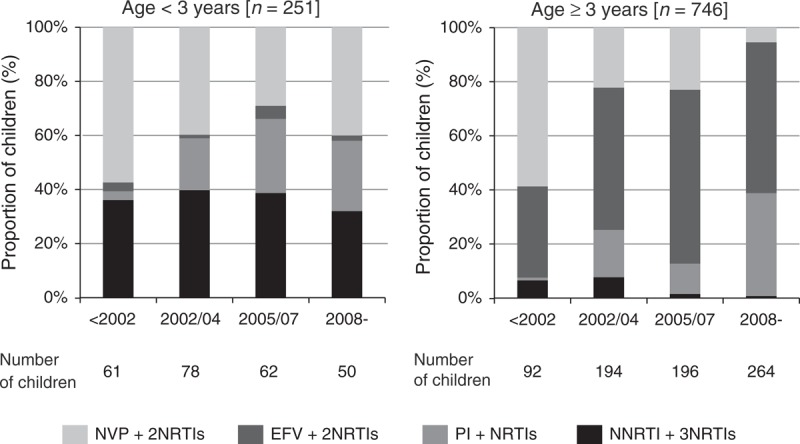
In the NNRTI + 3NRTIs group, all of the 93 children aged below 3 years received NVP and of those aged at least 3 years, 13 were on NVP and 13 on EFV. EFV, efavirenz; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor.
The choice of NRTI backbone differed by regimen (Table 1). Among the children on three-drug regimens, abacavir + lamivudine and tenofovir + emtricitabine were more frequently used with EFV and protease inhibitor-based regimens, and zidovudine + lamivudine and didanosine + stavudine with NVP-based regimens, reflecting ART availability over calendar periods. Nearly all children on four-drug regimens received zidovudine + lamivudine + abacavir.
Compared to those on other regimens, children on NNRTI + 3NRTIs regimens were younger, had lower baseline CD4% (accounting for age), higher viral load and higher proportion with pre-treatment AIDS diagnoses (Table 1). Median time on four drugs before dropping down to three drugs was 21.4 (13.1–36.2) months.
Virological suppression by 12 months
An estimated 92% [95% confidence interval (CI) 91–94%] of the children had viral load suppressed below 400 copies/ml within 12 months; the corresponding proportion was 96% (94–97%) among those starting ART since 2005. Kaplan–Meir curves of time to suppression by regimen and age are shown in Fig. 2a.
Fig. 2.
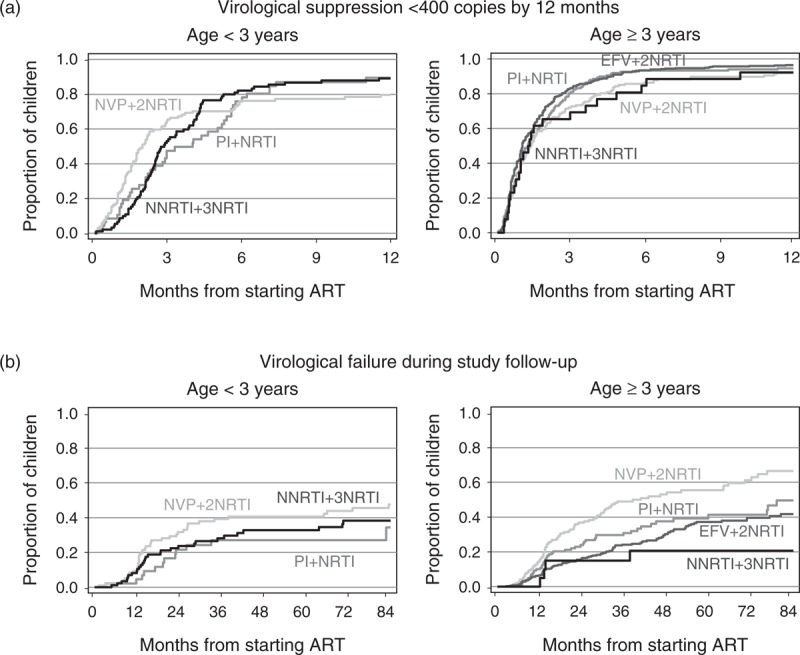
Time to (a) viral load suppression below 400 copies/ml by 12 months and (b) virological failure during study follow-up. ART, antiretroviral therapy; EFV, efavirenz; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor.
In the multivariable analysis, time to suppression was similar by regimen (global P = 0.10; Table 2), which was observed for both age below 3 years and at least 3 years (P-interaction = 0.80, data not shown). Time to suppression improved with later calendar years, older age and lower baseline viral load.
Table 2.
Factors at antiretroviral therapy initiation associated with virological suppression below 400 copies/ml by 12 months and virological failure during study follow-up.
| Factora | Suppression by 12 months | Virological failure during follow-up | ||
| Adjusted RR (95% CI) | P value | Adjusted RR (95% CI) | P value | |
| Type of first-line ART regimen | ||||
| All children | ||||
| NVP + 2NRTIs | 1 | 0.10 | 1 | <0.001 |
| EFV + 2NRTIs | 1.16 (0.95–1.41) | 0.54 (0.40–0.72) | ||
| Boosted PI | 0.92 (0.74–1.15) | 0.71 (0.50–1.00) | ||
| NNRTI + 3NRTIs | 1.03 (0.79–1.36) | 0.63 (0.41–0.97) | ||
| Comparing regimen, by time from ART initiation | ||||
| During first 2 years | ||||
| NVP + 2NRTIs | 1 | |||
| EFV + 2NRTIs | 0.46 (0.32–0.66) | |||
| Boosted PI | 0.78 (0.51–1.17) | |||
| NNRTI + 3NRTIs | 0.66 (0.40–1.07) | |||
| From 2 years onwards | ||||
| NVP + 2NRTIs | 1 | |||
| EFV + 2NRTIs | 0.86 (0.57–1.31) | |||
| Boosted PI | 0.74 (0.43–1.26) | |||
| NNRTI + 3NRTIs | 0.51 (0.28–0.92) | |||
| P for interaction between regimen and time from initiation = 0.03 | ||||
| Other factorsb | ||||
| Calendar year of ART initiation | ||||
| Before 2002 | 0.61 (0.47–0.79) | 0.001 | 2.54 (1.75–3.68) | <0.001 |
| 2002–2004 | 0.86 (0.71–1.04) | 1.46 (1.04–2.04) | ||
| 2005–2007 | 0.85 (0.70–1.01) | 1.03 (0.73–1.46) | ||
| 2008–2013 | 1 | 1 | ||
| Age at ART initiation (years) | ||||
| <1 | 0.61 (0.47–0.80) | <0.001 | 0.72 (0.48–1.06) | <0.001 |
| 1–2 | 0.72 (0.55–0.94) | 0.59 (0.39–0.89) | ||
| 3–4 | 0.93 (0.73–1.18) | 0.79 (0.55–1.15) | ||
| 5–9 | 1 | 1 | ||
| ≥10 | 1.05 (0.88–1.24) | 1.68 (1.26–2.24) | ||
| Viral load at ART initiation | ||||
| Per log10 increase | 0.88 (0.81–0.95) | 0.001 | ||
| Maternal ART during pregnancy/at delivery | ||||
| No | 1 | <0.001 | ||
| NVP-based regimen | 2.51 (1.38–4.57) | |||
| Other ART | 2.06 (1.26–3.38) | |||
ART, antiretroviral therapy; CI, confidence interval; EFV, efavirenz; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; NVP, nevirapine; RR, rate ratio.
aResults are only presented for factors with P value less than 0.05 associated with each outcome. Both models were adjusted for a priori confounders (previously described in the Methods section) and other predictors, with corresponding P value was less than 0.05.
bThe effect of a given factor was estimated based on data from all children combined.
Results were similar in sensitivity analyses which included only children starting ART since 2005 (n = 572), were restricted to children born in the UK/Ireland without maternal NNRTI-based ART exposure, and adjusted for NRTI backbones (data not shown).
Virological failure
Three hundred and thirty-nine (34%) of the 997 children experienced virological failure during follow-up: 268 (79% of 339) had confirmed rebound above 400 copies/ml; 13 (4%) had unconfirmed rebound followed by a change of at least two drugs within 6 months; and 58 (17%) had viral load above 400 copies/ml after 12 months without having previously suppressed. The estimated proportion experiencing virological failure by 2 and 5 years was 23.6% (95% CI 20.9–26.6%) and 39.4% (95% CI 35.8–43.2%), respectively. The Kaplan–Meier curves of time to virological failure are shown in Fig. 2b.
In adjusted analysis, progression to virological failure differed by regimen, being slowest for EFV + 2NRTIs and NNRTI + 3NRTIs regimens and fastest for NVP + 2NRTIs regimens (global P < 0.001; Table 2). Differences between regimens did not vary by age (<3 versus ≥3 years; P-interaction = 0.52), but depended on the time on treatment (P-interaction = 0.03; Table 2). Lower risk of failure with EFV + 2NRTIs regimens compared to NVP + 2NRTIs regimens was mainly observed during the first 2 years on treatment, with only a modest difference thereafter. From 2 years onwards, the risk was lowest for 4-drug NNRTI-based regimens, though CIs were wide due to sparse data. The benefit of the 4-drug NVP-based regimens remained when children on EFV + 3NRTIs regimens (n = 13) were excluded (data not shown). Among children starting ART since 2005, time to virological failure still differed between regimens (global P = 0.04), with adjusted rate ratio for EFV + 2NRTIs versus NVP + 2NRTIs regimens being 0.52 (0.32–0.85), NNRTI + 3NRTIs 0.54 (0.23–1.28) and protease inhibitor-based regimens 0.77 (0.47–1.27).
Earlier calendar years and older age (particularly ≥10 years) were independently associated with increased risk of virological failure (Table 2). In addition, children exposed to maternal ART, either with NNRTI-based regimens [adjusted rate ratio 2.51 (1.38–4.57)] or other regimens [2.06 (1.26–3.38)], were more likely to fail virologically compared to those without exposure (global P < 0.001).
Results remained similar in sensitivity analyses, including after adjusting for NRTI backbones and when restricted to children born in the UK/Ireland without maternal NNRTI-based ART exposure.
Discontinuation of initial regimen for toxicity
Seventy-seven (8%) of the 997 children discontinued the initial NNRTI (27 NVP, 32 EFV) or protease inhibitor (18) drug for toxicity. At 6 months, the cumulative incidence of toxicity-related treatment discontinuation was 4.6% (95% CI 2.8–7.1%) for NVP-based regimens, which is higher than that observed with EFV (2.4%, 95% CI 1.2–4.2) or protease inhibitor-based regimens (1.5%, 95% CI 0.4–4.1%; P = 0.09) (Fig. 3). Thereafter, treatment discontinuation for toxicity occurred less frequently for NVP-based regimens than for both EFV and protease inhibitor-based regimens (P = 0.006). By 5 years, the cumulative incidence was 6.1% (95% CI 3.9–8.9%) for NVP, 8.3% (5.6–11.6%) for EFV and 9.8% (5.7–15.3%) for protease inhibitor-based regimens (P = 0.48).
Fig. 3.
Time to discontinuation of initial non-nucleoside reverse transcriptase inhibitor or protease inhibitor for toxicity.
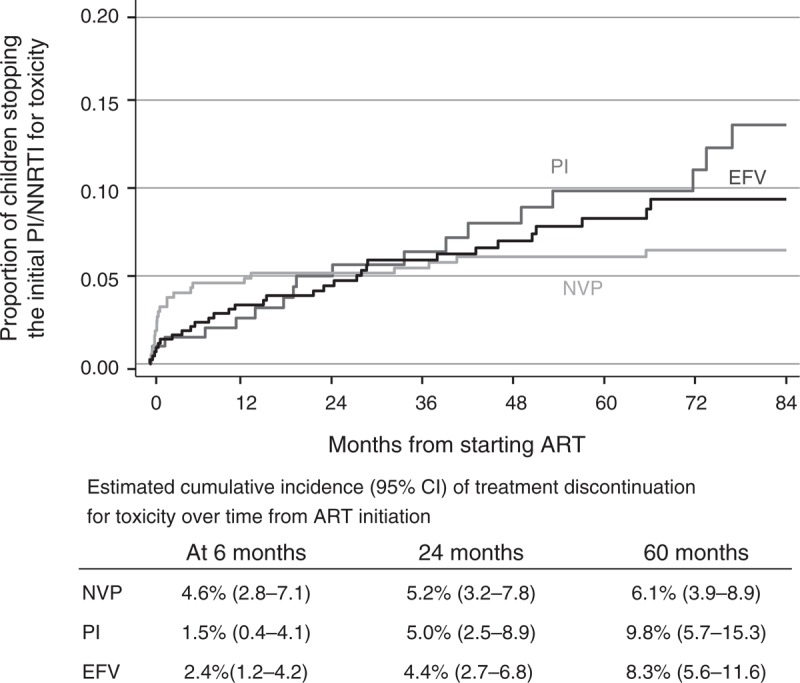
Children on 2 and 3 NRTIs were combined within each regimen group. Estimated cumulative incidence presented up to 7 years from ART initiation only due to sparse data thereafter. ART, antiretroviral therapy; EFV, efavirenz; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor.
Discussion
Among HIV-infected children in the UK and Ireland, rates of viral load suppression by 12 months were similarly high for different first-line regimens, whereas time to virological failure varied by regimen. Three-drug NVP-based regimens were associated with faster progression to failure than EFV-based regimens, mainly during the first 2 years on therapy, but similar thereafter. Four-drug NNRTI-based regimens (89% with NVP) had the lowest risk of failure in the longer term, though CIs were wide. There was a moderate benefit for the three-drug protease inhibitor over the NVP-based regimens, but this was offset by toxicity-related treatment discontinuation occurring more frequently for protease inhibitors with longer time on therapy.
Our study has several important limitations. Firstly, unmeasured confounders relating to regimen choice could have distorted findings. Boosted protease inhibitor regimens are preferred to NNRTI regimens for patients perceived by clinicians to be poor adherers. Also, children with underlying neurocognitive/psychological conditions (and therefore potentially poorer adherers) are less likely to be prescribed EFV [22]. Secondly, this is a historical cohort (6% started ART before 2000), although differences between regimens persisted among children initiating since 2005. In relation to this, only a small proportion of the children received tenofovir + emtricitabine, the preferred first-line NRTI combination in adolescents and adults, but used with caution in children because of concerns about long-term bone toxicity [4,26]. Thirdly, the choice of NRTI backbone, which can impact on treatment efficacy, varied substantially with the regimen type [16,27–30], with 76, 70 and 30% of the children on three-drug EFV, protease inhibitor and NVP-based regimens, respectively, receiving either lamivudine + abacavir or tenofovir + emtricitabine; these combinations are generally more potent compared to zidovudine + lamivudine (the most commonly prescribed backbone with NVP), including marginally so among children in the recently reported CHAPAS-3 trial (DM Gibb, personal communication). Whereas results remained similar after adjustment for NRTI backbones, it may have been difficult to disentangle regimen type and individual drug effects. Finally, differences between regimen type may vary by NRTI backbone, though our study did not have sufficient power to evaluate this [31].
Despite WHO recommendations of LPV/r-based regimens for children under 3 years and EFV-based for 3 years and over, most children worldwide currently initiate on NVP-based regimens due to the low cost of NVP and its wide availability in paediatric fixed-dose combination tablets [4,32]. LPV/r is only available for young children as a liquid which is unpalatable and has cold-chain requirements, making it much more challenging to use [33]. NVP also has a well characterized toxicity profile (which is low in young children), whereas long-term safety data for EFV (including central nervous system side effects) and LPV/r are more limited in children [4,8]. In our cohort, toxicity-related treatment discontinuation was more frequent for NVP during the initial ART period, but relatively uncommon thereafter, with higher risks observed for other regimens in the longer term, particularly protease inhibitors.
The decrease in the relative benefit of the three-drug EFV over NVP-based regimens with longer time on therapy observed in our study could partly be due to a ‘survivor bias’ effect, as children who had not experienced early virological failure were more likely to be good adherers. However, a similar trend was also seen in the ARROW trial, although interestingly, long-term virological suppression was better for EFV than NVP among children starting ART aged below 10 years, whereas the opposite was true at age at least 10 years (AS Walker, personal communication); neuropsychological side effects of EFV among older children may play a part [34].
Four-drug induction with NNRTI + 3NRTIs, decreasing after 36 weeks to three drugs, improved short-term response in the ARROW trial and our findings suggest long-term benefit of four-drug regimens (predominantly with NVP), as previously observed in the EPPICC infants study [8,9,35]. However, the number of children on EFV + 3NRTIs regimens was too small (n = 13) to allow direct comparison of the four-drug vs. the three-drug EFV-based regimens. Four-drug NNRTI-based regimens were also superior to the three-drug protease inhibitor-based regimens in the infant analysis, and we found a trend towards this, though CIs were wide. These results suggest that NNRTI + 3NRTIs long-term ‘induction maintenance’, which preserves protease inhibitor-based therapy for the second-line treatment, may be an alternative option, especially when ART is initiated during infancy or early childhood when viral load levels are particularly high and LPV/r more challenging for use than NVP [36].
The IMPACT 1060 trial found inferior response for the three-drug NVP versus LPV/r-based regimens by 24 weeks, with virological failure accounting for a greater proportion of the primary endpoints in the NVP than in the LPV/r group, irrespective of pMTCT exposure to NVP [5,6]. Consistent with this, we observed a moderately lower risk of virological failure for the three-drug protease inhibitor compared to the three-drug NVP-based regimens during the longer-term follow-up. In contrast, the EPPICC infants study and PENPACT-1 trial reported similar virological outcomes between the three-drug NNRTI and the protease inhibitor regimens at 12 months and 4 years, respectively [7,9]. In PENPACT-1, the initial regimen within randomized groups was chosen by the treating clinician, with LPV/r and nelfinavir (no longer recommended as sub-optimal) equally prescribed in the protease inhibitor group and approximately 60 and 40% of the NNRTI group on EFV and NVP, respectively; however, this is unlikely to account for the trial's findings given that virological suppression was similar across initial protease inhibitors and NNRTIs, Of note, long-term virological outcome was comparable between infants on LPV/r + zidovudine + lamivudine in the Children with HIV Early antiretroviral (CHER) trial and children on NVP + abacavir + lamivudine in the ARROW trial (for both age <3 and ≥3 years), with 84% suppressing below 400 copies/ml in both studies at 144 weeks and at 5 years, respectively [8,37].
Several factors may explain the increased risk of virological failure associated with the three-drug NVP compared to the EFV and LPV/r-based regimens observed in our study. Firstly, drug discontinuation for toxicity was higher for NVP during the initial period on ART; adverse drug events may lead to poor adherence and failure of viral load suppression [38]. In keeping with this, the difference in risk of virological failure between NVP and EFV was mainly observed within the first 2 years on ART in our study (and was apparent even during the first 12 months with children on NVP more likely to fail early following viral load suppression, data not shown). Secondly, under-dosing of NVP in children may play a role. The CHAPAS-1 trial in Uganda showed the lead-in dosing for NVP (half-target dose during the initial 2 weeks) lowers NVP levels at 4 weeks, especially in younger children who metabolize NVP faster, potentially increasing risk of NNRTI resistance and virological failure [39]. Also, under-dosing was more common for NVP than EFV among children in the UK/Ireland before 2007 because initial US Food and Drug Administration (FDA) paediatric dosing recommendation for NVP based on weight was too low [40]. Thirdly, NVP has a lower genetic barrier to resistance than LPV/r [2,34]; in the P1060 study, initial NVP dosing was low and over half of the children in the NVP group with virological failure had NVP resistance at the time of failure [6]. Finally, EFV has once-daily dosing, which may be advantageous to adherence than twice-daily dosing for NVP. Whereas NVP resistance after exposure to failed pMTCT ART may lead to impaired virological response in subsequent NVP-based treatment in children, this is unlikely to have an important impact on our findings for various reasons [23,41]: few children were reported to have been exposed to single-dose NVP, maternal ART exposure had been adjusted for in analyses, and in addition, results remained similar when only children born in the UK/Ireland without maternal NNRTI-based ART exposure were included in sensitivity analyses.
Consistent with other European findings, we observed risk of virological failure was higher when ART was started during adolescence, with little age effect among younger children [3]. Poorer response in young adults compared to older adults has been reported, likely due to adherence issues [42]. We also found children exposed to maternal ART were more likely to experience virological failure, though the study had insufficient power to evaluate this in more depth. Given single-dose NVP exposure was uncommon in our cohort; this association (also reported in previous studies) could reflect a worse HIV prognosis among children who become HIV-infected despite maternal ART [9,11].
In conclusion, whereas there are complexities associated with comparing studies of children starting treatment across different ages, calendar years and regions, observational cohort data allow the effectiveness of different regimens within real-life clinical settings to be evaluated. Improved understanding of the reasons why three-drug NVP-based regimens are associated with higher risk of virological failure, including management-related and/or dosing issues, could help optimize the therapeutic effect of NVP. Nevertheless, with worldwide ART coverage lagging significantly in children compared to adults (34 vs. 64% in 2012) and the majority of children worldwide currently initiating NVP-based regimens, NVP remains an important treatment option for children [4,32]. Furthermore, it may have a better toxicity profile than EFV and LPV/r with longer time on therapy. For children with viraemia controlled on NVP-based ART, there is no strong case for changing to EFV, given the two NNRTIs are similarly effective after 2 years on ART. Four-drug NVP-based regimens may also be a good alternative to protease inhibitor-based three-drug ART in young children. The availability of more potent drugs, including integrase inhibitors, and once-daily protease inhibitors for adolescents offering low pill burden regimens could improve viral load suppression in children further in future [22,43].
Acknowledgements
CHIPS Steering Committee: K. Butler, K. Doerholt, S. Donaghy, C. Foster, D.M. Gibb, A. Judd, J. Kenny, N. Klein, E.G.H. Lyall, E. Menson, V. Novelli, A. Riordan, F. Shackley, M. Sharland, D. Shingadia, P.A. Tookey, G. Tudor-Williams, S. Welch.
MRC Clinical Trials Unit: K. Bellenger, T. Childs, I.J. Collins, D. Dobson, K. Doerholt, D.M. Gibb, D. Johnson, A. Judd, A. Tostevin, A.S. Walker, L. Walker-Nthenda.
National Study of HIV in Pregnancy & Childhood, UCL Institute of Child Health: P.A. Tookey.
We thank the staff, families and children from the following hospitals who participate in CHIPS (in alphabetical order):
Republic of Ireland: Our Lady's Children's Hospital Crumlin, Dublin: K. Butler, A. Walsh. UK: Birmingham Heartlands Hospital, Birmingham: S. Scott, Y. Vaughan, S. Welch; Blackpool Victoria Hospital, Blackpool: N. Laycock; Bristol Royal Hospital for Children, Bristol: J. Bernatoniene, A. Finn, L. Hutchison; Calderdale Royal Hospital, Halifax: G. Sharpe; Central Middlesex Hospital, London: A. Williams; Chelsea and Westminster Hospital, London: EGH Lyall, P. Seery; Coventry & Warwickshire University Hospital, Coventry: P. Lewis, K. Miles; Derbyshire Children's Hospital, Derby: B. Subramaniam; Derriford Hospital, Plymouth: L. Hutchinson, P. Ward; Ealing Hospital, Middlesex: K. Sloper; Eastbourne District General Hospital, Eastbourne: G. Gopal; Glasgow Royal Hospital for Sick Children, Glasgow: C. Doherty, R. Hague, V. Price; Great Ormond St Hospital for Children, London: H. Bundy, M. Clapson, J. Flynn, D.M. Gibb, N. Klein, V. Novelli, D. Shingadia; Halliwell Children's Centre, Bolton: P. Ainsley-Walker; Harrogate District Hospital, Harrogate: P. Tovey; Homerton University Hospital, London: D. Gurtin; Huddersfield Royal Infirmary, Huddersfield: J.P. Garside; James Cook Hospital, Middlesbrough: A. Fall; John Radcliffe Hospital, Oxford: D. Porter, S. Segal; King's College Hospital, London: C. Ball, S. Hawkins; Leeds General Infirmary, Leeds: P. Chetcuti, M. Dowie; Leicester Royal Infirmary, Leicester: S. Bandi, A. McCabe; Luton and Dunstable Hospital, Luton: M. Eisenhut; Mayday University Hospital, Croydon: J. Handforth; Milton Keynes General Hospital, Milton Keynes: P.K. Roy; Newcastle General Hospital, Newcastle: T. Flood, A. Pickering; Newham General Hospital, London: S. Liebeschuetz; Norfolk & Norwich Hospital, Norwich: C. Kavanagh; North Manchester General Hospital, Manchester: C. Murphy, K. Rowson, T. Tan; North Middlesex Hospital, London: J. Daniels, Y. Lees; Northampton General Hospital, Northampton: E. Kerr, F. Thompson; Northwick Park Hospital Middlesex; M. Le Provost, A. Williams; Nottingham City Hospital, Nottingham: L. Cliffe, A. Smyth, S. Stafford; Queen Alexandra Hospital, Portsmouth: A. Freeman; Raigmore Hospital, Inverness: T. Reddy; Royal Alexandra Hospital, Brighton: K. Fidler; Royal Belfast Hospital for Sick Children, Belfast: S. Christie; Royal Berkshire Hospital, Reading: A. Gordon; Royal Children's Hospital, Aberdeen: D. Rogahn; Royal Cornwall Hospital, Truro: S. Harris, L. Hutchinson; Royal Devon and Exeter Hospital, Exeter: A. Collinson, L. Hutchinson; Royal Edinburgh Hospital for Sick Children, Edinburgh: L. Jones, B. Offerman; Royal Free Hospital, London: V. Van Someren; Royal Liverpool Children's Hospital, Liverpool: C. Benson, A. Riordan; Royal London Hospital, London: A. Riddell; Royal Preston Hospital, Preston: R. O’Connor; Salisbury District General Hospital, Salisbury: N. Brown; Sheffield Children's Hospital, Sheffield: L. Ibberson, F. Shackley; Southampton General Hospital, Southampton: S.N. Faust, J. Hancock; St George's Hospital, London: K. Doerholt, S. Donaghy, K. Prime, M. Sharland, S. Storey; St Luke's Hospital, Bradford: S. Gorman; St Mary's Hospital, London: E.G.H. Lyall, C. Monrose, P. Seery, G. Tudor-Williams, S. Walters; St Thomas’ Hospital (Evelina Children's Hospital), London: R. Cross, E. Menson; Torbay Hospital, Torquay: J. Broomhall, L. Hutchinson; University Hospital Lewisham, London: D. Scott, J. Stroobant; University Hospital of North Staffordshire, Stoke On Trent: A. Bridgwood, P. McMaster; University Hospital of Wales, Cardiff: J. Evans, T. Gardiner; Wexham Park, Slough: R. Jones; Whipps Cross Hospital, London: K. Gardiner.
Authors contributions: T.D., D.D., A.J., I.C. and D.G. were responsible for the study concept and design. T.D. carried out the statistical analyses. T.D., A.J., I.C. and D.G. drafted the manuscript. K.D., H.L., C.F., K.B., D.S., E.M., and D.G. collected the data. All co-authors participated in discussions about the design of the study, interpretation of the findings, and critically reviewed the manuscript.
Financial support: The National Study of HIV in Pregnancy and Childhood is funded by Public Health England (formerly the Health Protection Agency) and has received additional support from the Welton Foundation, the National Screening Committee and AbbVie. The Collaborative HIV Paediatric Study is funded by the NHS (London Specialised Commissioning Group) and has received additional support from the PENTA Foundation as well as Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen and Roche. The views expressed in the publication are those of the authors and not necessarily those of Public Health England or the London NHS Specialised Commissioning Group, or any of the additional funders.
Funding: NHS England (London Specialised Commissioning Group).
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Fitzgerald F, Penazzato M, Gibb D. Development of antiretroviral resistance in children with HIV in low- and middle-income countries. J Infect Dis 2013; 207 Suppl 2:S85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sigaloff KC, Calis JC, Geelen SP, van Vugt M, de Wit TF. HIV-1-resistance-associated mutations after failure of first-line antiretroviral treatment among children in resource-poor regions: a systematic review. Lancet Infect Dis 2011; 11:769–779 [DOI] [PubMed] [Google Scholar]
- 3.Castro H, Judd A, Gibb DM, Butler K, Lodwick RK, van Sighem A, et al. Risk of triple-class virological failure in children with HIV: a retrospective cohort study. Lancet 2011; 377:1580–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. June 2013. Geneva: World Health Organization. http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [Accessed August 2013] [PubMed] [Google Scholar]
- 5.Palumbo P, Lindsey JC, Hughes MD, Cotton MF, Bobat R, Meyers T, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med 2010; 363:1510–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Violari A, Lindsey JC, Hughes MD, Mujuru HA, Barlow-Mosha L, Kamthunzi P, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med 2012; 366:2380–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The PENPACT-1 Study Team. First-line antiretroviral therapy with a protease inhibitor versus nonnucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. Lancet Infect Dis 2011; 11:273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ARROW Trial Team. Routine versus clinically driven laboratory monitoring and first-line antiretroviral therapy strategies in African children with HIV (ARROW): a 5-year open-label randomised factorial trial. Lancet 2013; 381:1391–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Judd A. The European Pregnancy and Paediatric HIV Cohort Collaboration (EPPICC) Study Group in EuroCoord. Early antiretroviral therapy in HIV-1-infected infants, 1996-2008: treatment response and duration of first-line regimens. AIDS 2011; 25:2279–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pursuing Later Treatment Options (Plato II) Project Team for the Collaboration of Observational HIV Epidemiological Research Europe (COHERE). Triple-class virologic failure in HIV-infected patients undergoing antiretroviral therapy for up to 10 years. Arch Intern Med 2010; 170:410–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies MA, Moultrie H, Eley B, Rabie H, Van Cutsem G, Giddy J, et al. Virologic failure and second-line antiretroviral therapy in children in South Africa: the IeDEA Southern Africa collaboration. J Acquir Immune Defic Syndr 2011; 56:270–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowenthal ED, Ellenberg JH, Machine E, Sagdeo A, Boiditswe S, Steenhoff AP, et al. Association between efavirenz-based compared with nevirapine-based antiretroviral regimens and virological failure in HIV-infected children. JAMA 2013; 309:1803–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sebunya R, Musiime V, Kitaka SB, Ndeezi G. Incidence and risk factors for first line anti retroviral treatment failure among Ugandan children attending an urban HIV clinic. AIDS Res Ther 2013; 10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillay P, Ford N, Shubber Z, Ferrand RA. Outcomes for efavirenz versus nevirapine-containing regimens for treatment of HIV-1 infection: a systematic review and meta-analysis. PLoS One 2013; 8:e68995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daar ES, Tierney C, Fischl MA, Sax PE, Mollan K, Budhathoki C, et al. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med 2011; 154:445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratsela A, Polis M, Dhlomo S, Emery S, Grandits G, Khabo P, et al. A randomized factorial trial comparing 4 treatment regimens in treatment-naive HIV-infected persons with AIDS and/or a CD4 cell count <200 cells/muL in South Africa. J Infect Dis 2010; 202:1529–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riddler SA, Haubrich R, DiRienzo AG, Peeples L, Powderly WG, Klingman KL, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med 2008; 358:2095–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sierra-Madero J, Villasis-Keever A, Mendez P, Mosqueda-Gomez JL, Torres-Escobar I, Gutierrez-Escolano F, et al. Prospective, randomized, open label trial of Efavirenz vs Lopinavir/Ritonavir in HIV+ treatment-naive subjects with CD4+<200 cell/mm3 in Mexico. J Acquir Immune Defic Syndr 2010; 53:582–588 [DOI] [PubMed] [Google Scholar]
- 19.Gibb DM, Duong T, Tookey PA, Sharland M, Tudor-Williams G, Novelli V, et al. Decline in mortality, AIDS, and hospital admissions in perinatally HIV-1 infected children in the United Kingdom and Ireland. Br Med J 2003; 327:1019–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scherrer AU, Ledergerber B, von Wyl V, Boni J, Yerly S, Klimkait T, et al. Improved virological outcome in White patients infected with HIV-1 non-B subtypes compared to subtype B. Clin Infect Dis 2011; 53:1143–1152 [DOI] [PubMed] [Google Scholar]
- 21.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 1998; 17:407–429 [PubMed] [Google Scholar]
- 22.PENTA 2009 guidelines for the use of antiretroviral therapy in paediatric HIV-1 infection. HIV Med 2009; 10:591–613 [DOI] [PubMed] [Google Scholar]
- 23.Arrive E, Newell ML, Ekouevi DK, Chaix ML, Thiebaut R, Masquelier B, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol 2007; 36:1009–1021 [DOI] [PubMed] [Google Scholar]
- 24.Royston P. Multiple imputation of missing data. Stata J 2004; 4:227–241 [Google Scholar]
- 25.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509 [Google Scholar]
- 26.Grigsby IF, Pham L, Mansky LM, Gopalakrishnan R, Mansky KC. Tenofovir-associated bone density loss. Ther Clin Risk Manag 2010; 6:41–47 [PMC free article] [PubMed] [Google Scholar]
- 27.Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med 2006; 354:251–260 [DOI] [PubMed] [Google Scholar]
- 28.Green H, Gibb DM, Walker AS, Pillay D, Butler K, Candeias F, et al. Lamivudine/abacavir maintains virological superiority over zidovudine/lamivudine and zidovudine/abacavir beyond 5 years in children. AIDS 2007; 21:947–955 [DOI] [PubMed] [Google Scholar]
- 29.Post FA, Moyle GJ, Stellbrink HJ, Domingo P, Podzamczer D, Fisher M, et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr 2010; 55:49–57 [DOI] [PubMed] [Google Scholar]
- 30.Sax PE, Tierney C, Collier AC, Daar ES, Mollan K, Budhathoki C, et al. Abacavir/lamivudine versus tenofovir DF/emtricitabine as part of combination regimens for initial treatment of HIV: final results. J Infect Dis 2011; 204:1191–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang MW, Kanki PJ, Shafer RW. A review of the virological efficacy of the 4 World Health Organization-recommended tenofovir-containing regimens for initial HIV therapy. Clin Infect Dis 2012; 54:862–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Global report: UNAIDS report on the global AIDS epidemic 2013. Geneva: World Health Organization. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. [Accessed January 2014] [Google Scholar]
- 33.Prendergast AJ, Penazzato M, Cotton M, Musoke P, Mulenga V, Abrams EJ, et al. Treatment of young children with HIV infection: using evidence to inform policymakers. PLoS Med 2012; 9:e1001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penazzato M, Giaquinto C. Role of nonnucleoside reverse transcriptase inhibitors in treating HIV-infected children. Drugs 2011; 71:2131–2149 [DOI] [PubMed] [Google Scholar]
- 35.Picat MQ, Lewis J, Musiime V, Prendergast A, Nathoo K, Kekitiinwa A, et al. Predicting patterns of long-term CD4 reconstitution in HIV-infected children starting antiretroviral therapy in sub-Saharan Africa: a cohort-based modelling study. PLoS Med 2013; 10:e1001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abrams EJ, Weedon J, Steketee RW, Lambert G, Bamji M, Brown T, et al. Association of human immunodeficiency virus (HIV) load early in life with disease progression among HIV-infected infants. New York City Perinatal HIV Transmission Collaborative Study Group. J Infect Dis 1998; 178:101–108 [DOI] [PubMed] [Google Scholar]
- 37.Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet 2013; 382:1555–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Dakkak I, Patel S, McCann E, Gadkari A, Prajapati G, Maiese EM. The impact of specific HIV treatment-related adverse events on adherence to antiretroviral therapy: a systematic review and meta-analysis. AIDS Care 2013; 25:400–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fillekes Q, Mulenga V, Kabamba D, Kankasa C, Thomason MJ, Cook A, et al. Is nevirapine dose escalation appropriate in young, african, HIV-infected children?. AIDS 2013; 27:2111–2115 [DOI] [PubMed] [Google Scholar]
- 40.Menson EN, Walker AS, Sharland M, Wells C, Tudor-Williams G, Riordan FA, et al. Underdosing of antiretrovirals in UK and Irish children with HIV as an example of problems in prescribing medicines to children, 1997-2005: cohort study. BMJ 2006; 332:1183–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lockman S, Shapiro RL, Smeaton LM, Wester C, Thior I, Stevens L, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med 2007; 356:135–147 [DOI] [PubMed] [Google Scholar]
- 42.Lodwick R, Costagliola D, Reiss P, Torti C, Teira R, Dorrucci M, et al. Triple-class virologic failure in HIV-infected patients undergoing antiretroviral therapy for up to 10 years. Arch Intern Med 2010; 170:410–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riordan A, Bugembe T. Update on antiretroviral therapy. Arch Dis Child 2009; 94:70–74 [DOI] [PubMed] [Google Scholar]


