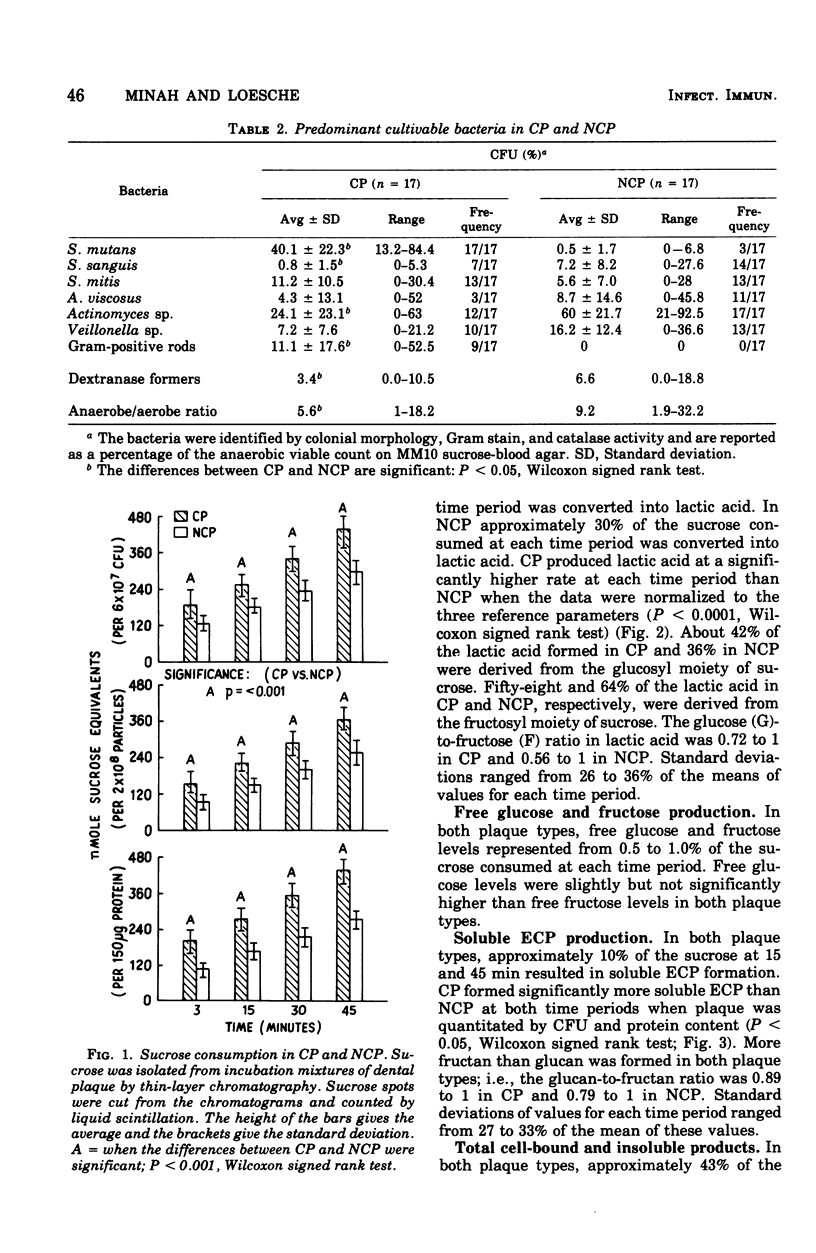
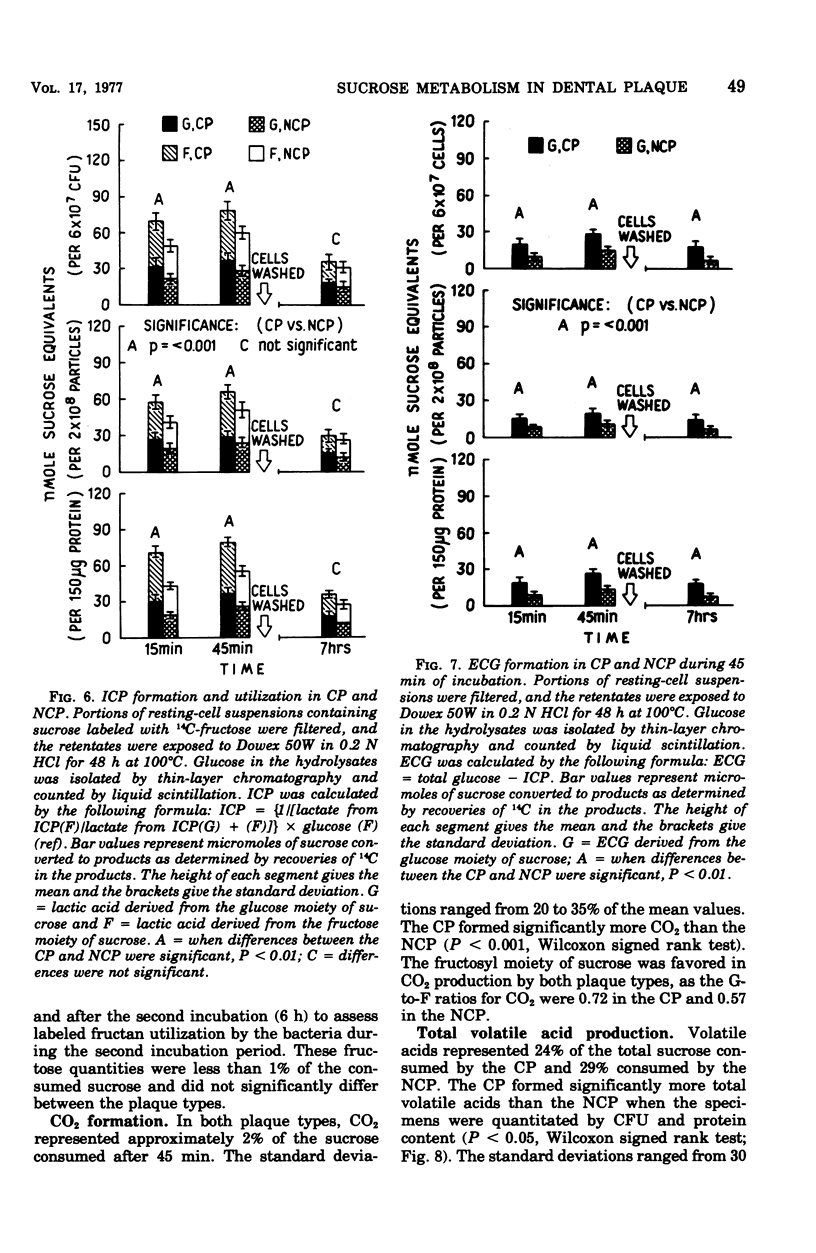
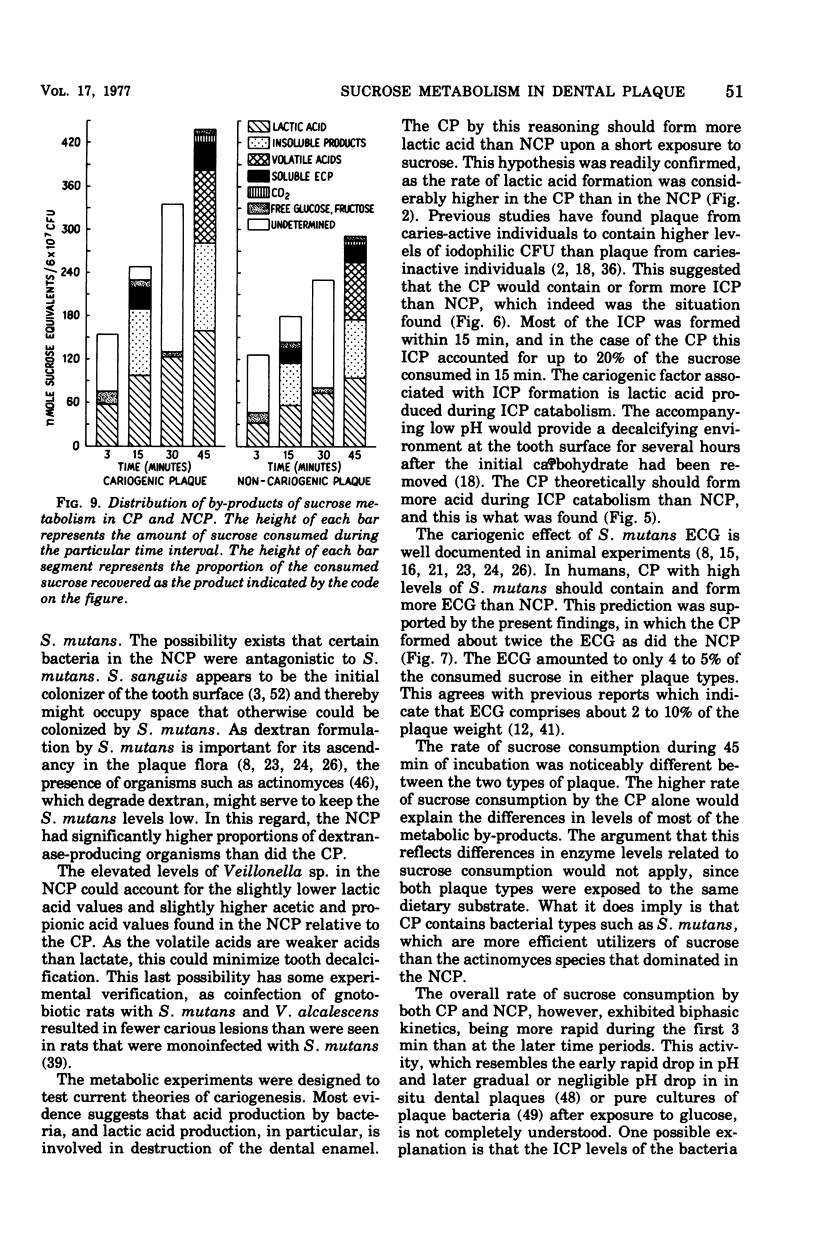
Abstract
Small specimens of cariogenic plaque (CP) and non-cariogenic plaque (NCP) from the same tooth were individually dispersed in buffer, divided equally, and incubated for 45 min with [14C]sucrose uniformly labeled either in the glucosyl moiety or the fructosyl moiety. Sucrose metabolism was analyzed periodically during an anaerobic incubation at 37°C. Radiochemical techniques were devised to analyze formation of lactic acid, soluble extracellular polysaccharide, total cell-bound and insoluble products, intracellular polysaccharide, lactic acid from intracellular polysaccharide catabolism, insoluble extracellular glucan, CO2, total volatile acids, individual volatile acids, and rates of sucrose consumption. The contribution of the glucosyl and fructosyl moieties of sucrose to each metabolic by-product was determined. All of the metabolic data were adjusted to the size of the plaque specimens as determined by colony-forming units, Coulter counter particle counts, and fluorometric protein analyses. Both types of dental plaque transformed from 70 to 80% of the consumed sucrose into lactic acid and cell-bound and insoluble products, primarily intracellular polysaccharide and extracellular glucan. Volatile acids accounted for most of the remaining by-products. CP metabolized significantly more sucrose than NCP and consequently produced significantly higher levels of each metabolic by-product. High levels of Streptococcus mutans were found in CP (averaging 40% of colony-forming units), whereas it was virtually absent in NCP. Actinomyces and S. sanguis levels were distinctly higher in NCP. NCP harbored more anaerobes and dextranase-forming microorganisms than CP.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman K. S., Gibbons R. J. Iodophilic polysaccharide synthesis by human and rodent oral bacteria. Arch Oral Biol. 1966 May;11(5):533–542. doi: 10.1016/0003-9969(66)90160-9. [DOI] [PubMed] [Google Scholar]
- FITZGERALD R. J., JORDAN H. V., STANLEY H. R. Experimental caries and gingival pathologic changes in the gnotobiotic rat. J Dent Res. 1960 Sep-Oct;39:923–935. doi: 10.1177/00220345600390052701. [DOI] [PubMed] [Google Scholar]
- FITZGERALD R. J., KEYES P. H. Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J Am Dent Assoc. 1960 Jul;61:9–19. doi: 10.14219/jada.archive.1960.0138. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. J., SOCRANSKY S. S. Intracellular polysaccharide storage by organisms in dental plaques. Its relation to dental caries and microbial ecology of the oral cavity. Arch Oral Biol. 1962 Jan-Feb;7:73–79. doi: 10.1016/0003-9969(62)90050-x. [DOI] [PubMed] [Google Scholar]
- Geddes D. A. Acids produced by human dental plaque metabolism in situ. Caries Res. 1975;9(2):98–109. doi: 10.1159/000260149. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F., Chludzinski A. M. Rapid filter paper assay for the dextransucrase activity from Streptococcus mutans. J Dent Res. 1974 Nov-Dec;53(6):1355–1360. doi: 10.1177/00220345740530061101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. B. Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol. 1967 Jan;12(1):11–23. doi: 10.1016/0003-9969(67)90137-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Berman K. S., Knoettner P., Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966 Jun;11(6):549–560. doi: 10.1016/0003-9969(66)90220-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Depaola P. F., Spinell D. M., Skobe Z. Interdental localization of Streptococcus mutans as related to dental caries experience. Infect Immun. 1974 Mar;9(3):481–488. doi: 10.1128/iai.9.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Gilmour M. N., Poole A. E. The fermentative capabilities of dental plaque. Caries Res. 1967;1(3):246–260. [PubMed] [Google Scholar]
- Gold O. G., Jordan H. V., Van Houte J. A selective medium for Streptococcus mutans. Arch Oral Biol. 1973 Nov;18(11):1357–1364. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- Guggenheim B. Enzymatic hydrolysis and structure of water-insoluble glucan produced by glucosyltransferases from a strain of streptococcus mutans. Helv Odontol Acta. 1970 Nov;14(Suppl):89+–89+. [PubMed] [Google Scholar]
- Guggenheim B., König K. G., Mühlemann H. R. Modifications of the oral bacterial flora and their influence on dental caries in the rat. I. The effects of inoculating 4 labelled strains of streptococci. Helv Odontol Acta. 1965 Oct;9(2):121–129. [PubMed] [Google Scholar]
- Guggenheim B., König K. G., Mühlemann H. R., Regolati B. Effect of dextranase on caries in rats harbouring an indigenous cariogenic bacterial flora. Arch Oral Biol. 1969 May;14(5):555–558. doi: 10.1016/0003-9969(69)90149-6. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Regolati B., Mühlemann H. R. Caries and plaque inhibition by mutanase in rats. Caries Res. 1972;6(4):289–297. doi: 10.1159/000259808. [DOI] [PubMed] [Google Scholar]
- Gästrin B., Kallings L. O., Marcetic A. The survival time for different bacteria in various transport media. Acta Pathol Microbiol Scand. 1968;74(3):371–380. doi: 10.1111/j.1699-0463.1968.tb03490.x. [DOI] [PubMed] [Google Scholar]
- Hayashi J. A., Shklair I. L., Bahn A. N. Immunization with dextransucrases and glycosidic hydrolases. J Dent Res. 1972 Mar-Apr;51(2):436–442. doi: 10.1177/00220345720510023201. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Sandham H. J., Bradley E. L., Jr Changes in Streptococcus mutans and lactobacilli in plaque in relation to the initiation of dental caries in Negro children. Arch Oral Biol. 1973 Apr;18(4):555–566. doi: 10.1016/0003-9969(73)90076-9. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Englander H. R., Lim S. Potentially cariogenic streptococci in selected population groups in the western hemisphere. J Am Dent Assoc. 1969 Jun;78(6):1331–1335. doi: 10.14219/jada.archive.1969.0194. [DOI] [PubMed] [Google Scholar]
- KEYES P. H. The infectious and transmissible nature of experimental dental caries. Findings and implications. Arch Oral Biol. 1960 Mar;1:304–320. doi: 10.1016/0003-9969(60)90091-1. [DOI] [PubMed] [Google Scholar]
- Keene H. J., Shklair I. L. Relationship of Streptococcus mutans carrier status to the development of carious lesions in initially cariesfree recruits. J Dent Res. 1974 Sep-Oct;53(5):1295–1295. doi: 10.1177/00220345740530053801. [DOI] [PubMed] [Google Scholar]
- Krasse B. Human streptococci and experimental caries in hamsters. Arch Oral Biol. 1966 Apr;11(4):429–436. doi: 10.1016/0003-9969(66)90107-5. [DOI] [PubMed] [Google Scholar]
- Krasse B., Jordan H. V., Edwardsson S., Svensson I., Trell L. The occurrence of certain "caries-inducing" streptococci in human dental plaque material with special reference to frequency and activity of caries. Arch Oral Biol. 1968 Aug;13(8):911–918. doi: 10.1016/0003-9969(68)90006-x. [DOI] [PubMed] [Google Scholar]
- Littleton N. W., Kakehashi S., Fitzgerald R. J. Recovery of specific "caries-inducing" streptococci from carious lesions in the teeth of children. Arch Oral Biol. 1970 May;15(5):461–463. doi: 10.1016/0003-9969(70)90073-7. [DOI] [PubMed] [Google Scholar]
- Llory H., Dammron A., Gioanni M., Frank R. M. Some population changes in oral anaerobic microorganisms, Streptococcus mutans and yeasts following irradiation of the salivry glands. Caries Res. 1972;6(4):298–311. doi: 10.1159/000259809. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Henry C. A. Intracellular microbial polysaccharide production and dental caries in a Guatemalan Indian Village. Arch Oral Biol. 1967 Feb;12(2):189–194. doi: 10.1016/0003-9969(67)90037-4. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Hockett R. N., Syed S. A. The predominant cultivable flora of tooth surface plaque removed from institutionalized subjects. Arch Oral Biol. 1972 Sep;17(9):1311–1325. doi: 10.1016/0003-9969(72)90164-1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Rowan J., Straffon L. H., Loos P. J. Association of Streptococcus mutants with human dental decay. Infect Immun. 1975 Jun;11(6):1252–1260. doi: 10.1128/iai.11.6.1252-1260.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Walenga A., Loos P. Recovery of Streptococcus mutans and Streptococcus sanguis from a dental explorer after clinical examination of single human teeth. Arch Oral Biol. 1973 Apr;18(4):571–575. doi: 10.1016/0003-9969(73)90078-2. [DOI] [PubMed] [Google Scholar]
- Mikx F. H., van der Hoeven J. S., König K. G., Plasschaert A. J., Guggenheim B. Establishment of defined microbial ecosystems in germ-free rats. I. The effect of the interactions of streptococcus mutans or Streptococcus sanguis with Veillonella alcalescens on plaque formation and caries activity. Caries Res. 1972;6(3):211–223. doi: 10.1159/000259801. [DOI] [PubMed] [Google Scholar]
- Minah G. E., Loesche W. J., Dziewiatkowski D. D. The in-vitro effect of fungal dextranase on human dental plaque. Arch Oral Biol. 1972 Jan;17(1):35–42. doi: 10.1016/0003-9969(72)90131-8. [DOI] [PubMed] [Google Scholar]
- ORLAND F. J., BLAYNEY J. R., HARRISON R. W., REYNIERS J. A., TREXLER P. C., ERVIN R. F., GORDON H. A., WAGNER M. Experimental caries in germfree rats inoculated with enterococci. J Am Dent Assoc. 1955 Mar;50(3):259–272. doi: 10.14219/jada.archive.1955.0061. [DOI] [PubMed] [Google Scholar]
- Shklair I. L., Keene H. J., Cullen P. The distribution of Streptococcus mutans on the teeth of two groups of naval recruits. Arch Oral Biol. 1974 Feb;19(2):199–202. doi: 10.1016/0003-9969(74)90214-3. [DOI] [PubMed] [Google Scholar]
- Shklair I. L., Keene H. J., Simonson L. G. Distribution and frequency of streptococcus mutants in caries-active individuals. J Dent Res. 1972 May-Jun;51(3):882–882. doi: 10.1177/00220345720510034201. [DOI] [PubMed] [Google Scholar]
- Staat R. H., Gawronski T. H., Schachtele C. F. Detection and preliminary studies on dextranase-producing microorganisms from human dental plaque. Infect Immun. 1973 Dec;8(6):1009–1016. doi: 10.1128/iai.8.6.1009-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Chassy B. M., Krichevsky M. I. Sucrose metabolism by Streptococcus mutans, SL-I. Biochim Biophys Acta. 1971 Feb 28;261(2):379–387. doi: 10.1016/0304-4165(72)90062-1. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Banghart S. B. Adherence as a determinant of the presence of Streptococcus salivarius and Streptococcus sanguis on the human tooth surface. Arch Oral Biol. 1970 Nov;15(11):1025–1034. doi: 10.1016/0003-9969(70)90115-9. [DOI] [PubMed] [Google Scholar]
- Weigele M., DeBernardo S., Leimgruber W. Fluorometric assay of secondary amino acids. Biochem Biophys Res Commun. 1973 Jan 23;50(2):352–356. doi: 10.1016/0006-291x(73)90847-4. [DOI] [PubMed] [Google Scholar]
- Woods R. A dental caries susceptibility test based on the occurrence of Streptococcus mutans in plaque material. Aust Dent J. 1971 Apr;16(2):116–121. doi: 10.1111/j.1834-7819.1971.tb02314.x. [DOI] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. J Bacteriol. 1975 Oct;124(1):55–61. doi: 10.1128/jb.124.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachrisson B. U. Mast cells of the human gingiva. I. Investigations concerning the preservation and demonstration of mast cells in the gingival area. Odontol Revy. 1968;19(1):1–22. [PubMed] [Google Scholar]


