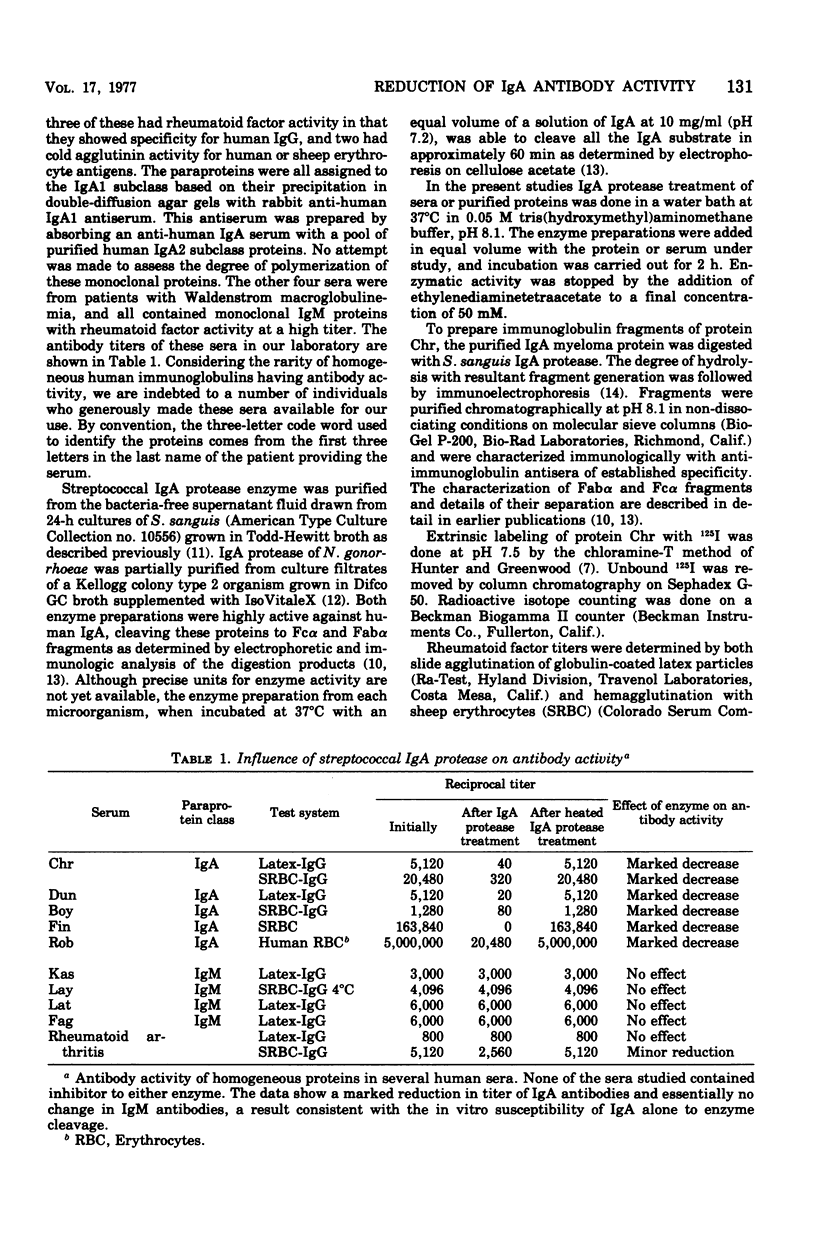
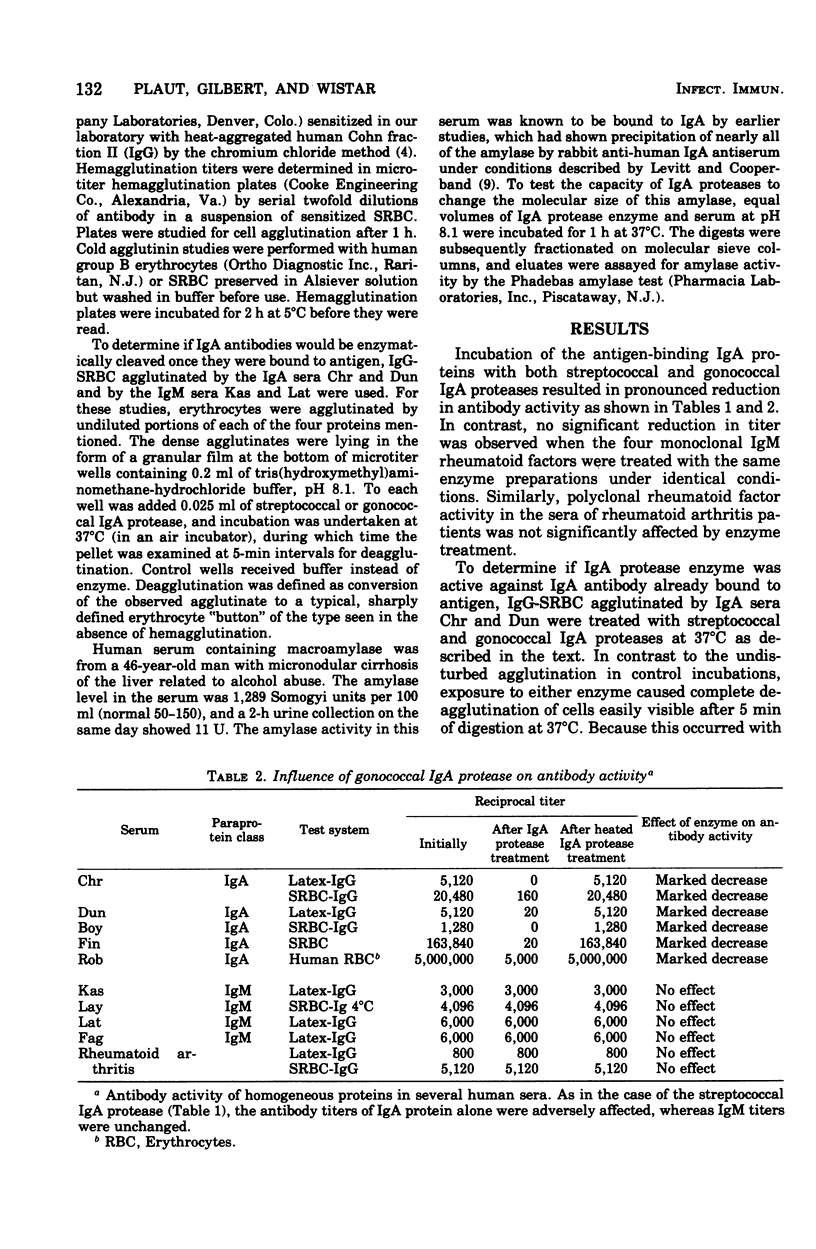
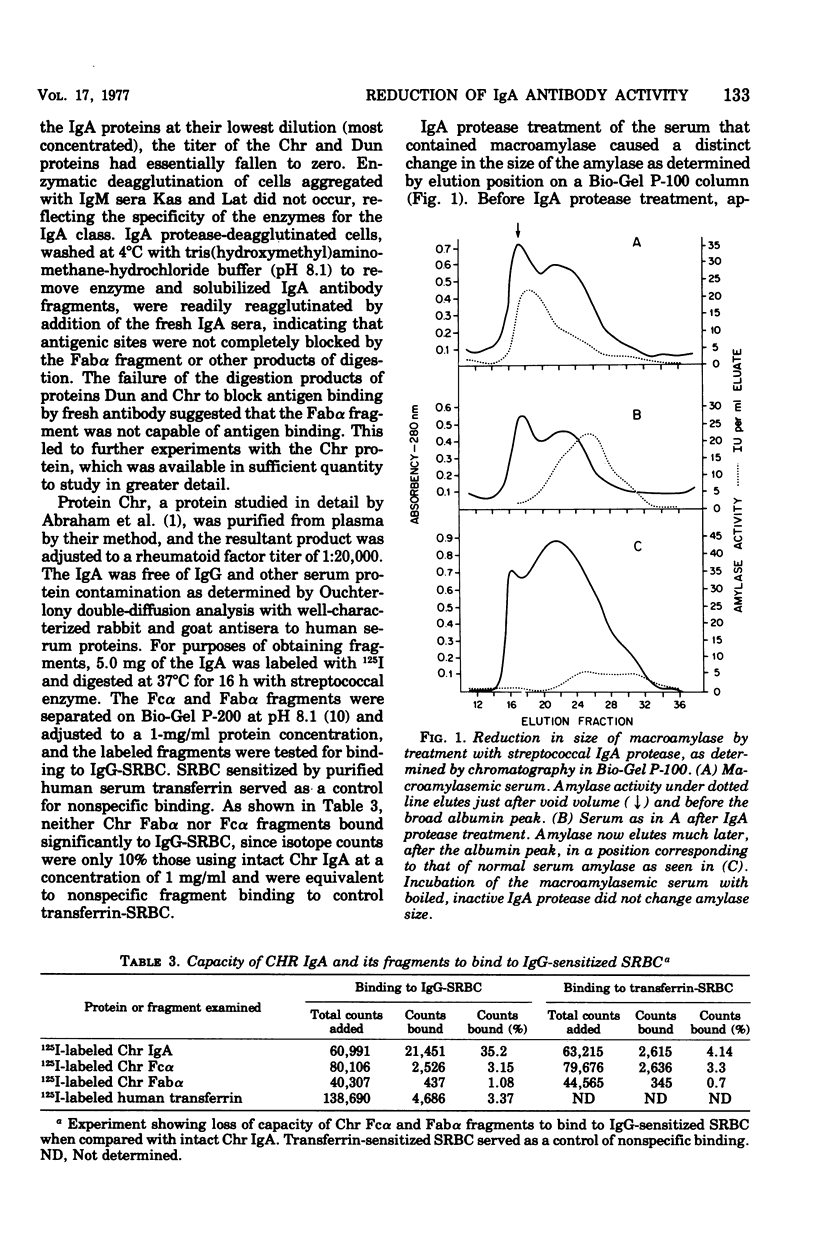
Abstract
Immunoglobulin A (IgA) proteases are extracellular enzymes elaborated by Neisseria gonorrhoeae, N. meningitidis, and Streptococcus sanguis. These enzymes each cleave human IgA1 at a critically situated prolyl-threonyl peptide bond to yield Fab alpha and Fc alpha fragments. To study their effect on the antibody activity of human IgA, we enzymatically digested a group of five human IgA monoclonal immunoglobulins with high-titer rheumatoid factor or cold agglutinin activity and human serum macroamylase, an amylase-IgA complex. In contrast to four control IgM rheumatoid factor monoclonal proteins, whose activity was unaffected by enzyme, gonococcal and streptococcal IgA proteases caused prompt, major reductions of IgA antibody activity to negligible levels and converted macroamylase activity to amylase of normal size, as determined by molecular sieve chromatography. In addition, both enzymes promptly deagglutinated sensitized cells that had been aggregated by IgA rheumatoid factors, indicating that IgA bound to antigen is also susceptible to enzyme cleavage. Fab fragments of Iga protein Chr, a rheumatoid factor, showed essentially no antigen-binding activity despite the high titers observed with the parent protein. These studies emphasize the high degree of specificity of the microbial proteases for IgA and their potential for interfering with antibody activity in the IgA1 subclass.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G. N., Clark R. A., Vaughan J. H. Characterization of an IgA rheumatoid factor: finding properties and reactivity with the subclasses of human G globulin. Immunochemistry. 1972 Mar;9(3):301–315. doi: 10.1016/0019-2791(72)90094-8. [DOI] [PubMed] [Google Scholar]
- Berk J. E., Kizu H., Wilding P., Searcy R. L. Macroamylasemia: a newly recognized cause for elevated serum amylase activity. N Engl J Med. 1967 Nov 2;277(18):941–946. doi: 10.1056/NEJM196711022771801. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Metzger H. The influence of polyvalency on the binding properties of antibodies. Immunochemistry. 1972 Mar;9(3):341–357. doi: 10.1016/0019-2791(72)90097-3. [DOI] [PubMed] [Google Scholar]
- Gold E. R., Fudenberg H. H. Chromic chloride: a coupling reagent for passive hemagglutination reactions. J Immunol. 1967 Nov;99(5):859–866. [PubMed] [Google Scholar]
- Grey H. M., Abel C. A., Yount W. J., Kunkel H. G. A subclass of human gamma-A-globulins (gamma-A2) which lacks the disulfied bonds linking heavy and light chains. J Exp Med. 1968 Dec 1;128(6):1223–1236. doi: 10.1084/jem.128.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hornick C. L., Karuch F. Antibody affinity. 3. The role of multivalance. Immunochemistry. 1972 Mar;9(3):325–340. doi: 10.1016/0019-2791(72)90096-1. [DOI] [PubMed] [Google Scholar]
- Lamm M. E. Cellular aspects of immunoglobulin A. Adv Immunol. 1976;22:223–290. doi: 10.1016/s0065-2776(08)60550-7. [DOI] [PubMed] [Google Scholar]
- Levitt M. D., Cooperband S. R. Hyperamylasemia from the binding of serum amylase by an 11S IgA globulin. N Engl J Med. 1968 Feb 29;278(9):474–479. doi: 10.1056/NEJM196802292780903. [DOI] [PubMed] [Google Scholar]
- Mehta S. K., Plaut A. G., Calvanico N. J., Tomasi T. B., Jr Human immunoglobulin A: production of an Fc fragment by an enteric microbial proteolytic enzyme. J Immunol. 1973 Oct;111(4):1274–1276. [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Artenstein M. S., Capra J. D. Neisseria gonorrhoeae and neisseria meningitidis: extracellular enzyme cleaves human immunoglobulin A. Science. 1975 Dec 12;190(4219):1103–1105. doi: 10.1126/science.810892. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Wistar R., Jr, Capra J. D. Differential susceptibility of human IgA immunoglobulins to streptococcal IgA protease. J Clin Invest. 1974 Dec;54(6):1295–1300. doi: 10.1172/JCI107875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Tomasi T. B., Grey H. M. Structure and function of immunoglobulin A. Prog Allergy. 1972;16:81–213. [PubMed] [Google Scholar]


