Abstract
Granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting tumor vaccines are bioactive, but limited by disease burden and immune tolerance. Cyclophosphamide (CY) augments vaccine activity in tolerant neu mice and metastatic breast cancer (MBC) patients. HER-2-specific monoclonal antibodies (MAb) enhance vaccine activity in neu mice. We hypothesized that CY-modulated vaccination with HER-2-specific MAb safely induces relevant HER-2-specific immunity in neu mice and HER-2+ MBC patients. Adding both CY and the HER-2-specific MAb 7.16.4 to vaccination maximized HER-2-specific CD8+ T-cell immunity and tumor-free survival in neu transgenic mice. We therefore conducted a single arm feasibility study of CY, an allogeneic HER-2+ GM-CSF-secreting breast tumor vaccine, and weekly trastuzumab in 20 HER-2+ MBC patients. Primary clinical trial objectives were safety and clinical benefit (CB), in which CB represents complete response+partial response+stable disease. Secondary study objectives were to assess HER-2-specific T-cell responses by delayed type hypersensitivity (DTH) and intracellular cytokine staining. Subjects received three monthly vaccinations, with a boost 6-8 months from trial entry. This combination immunotherapy was safe, with CB rates at 6 months and 1 year of 55% (95% CI:32-77%, p=0.013) and 40% (95% CI:19-64%) respectively. Median progression-free survival (PFS) and overall survival (OS) were 7 (95% CI:4-16) and 42 months (95% CI:22-70) respectively. Increased HER-2-specific DTH developed in 7/20 subjects (of whom 4 had CB (95% CI:18-90)), with a trend toward longer PFS and OS in DTH responders. Polyfunctional HER-2-specific CD8+ T cells progressively expanded across vaccination cycles. Further investigation of CY-modulated vaccination with trastuzumab is warranted. (Clinicaltrials.gov identifier: NCT00399529)
Keywords: vaccine, trastuzumab, cyclophosphamide, HER-2+ breast cancer, immune response
INTRODUCTION
The availability of multiple drugs specific for HER-2 (trastuzumab, lapatinib, pertuzumab, and ado-trastuzumab emtansine) has revolutionized the treatment of HER-2+ metastatic breast cancer (MBC) (1, 2). However, not all patients benefit and survival gains could be further enhanced. Innovative therapies that complement and synergize with these drugs are urgently needed to improve disease outcomes.
Immune-based therapies, including vaccines and immune checkpoint blockade, have several unique properties (3). First, immune-based therapies recruit the host tumor-specific immune response rather than targeting the tumor directly. Second, the host immune system can recognize an unlimited number of overexpressed and/or mutated targets differentially expressed by tumor cells relative to normal tissue. Third, immune-based therapies confer a durable treatment response due to immunologic memory. Although the evaluation of immune checkpoint blockade has been limited in breast cancer patients, multiple vaccines have been tested. Despite a good safety profile, immune responses have been modest and inconsistently associated with clinical benefit. Most of these vaccination regimens were limited by some combination of immune tolerance, established disease burdens, or the use of suboptimal therapeutic targets and/or vaccination regimens.
Cytokine-modified tumor cell vaccines that secrete granulocyte-macrophage colony-stimulating factor (GM-CSF) can induce robust T cell-dependent immunity that clears established tumors in preclinical models (4). Multiple clinical studies have demonstrated their single agent safety and bioactivity in cancer patients, but clinical benefit remains unproven (5, 6). This lack of success is probably because vaccination alone is not potent enough to induce immune responses sufficiently robust to overcome immune tolerance and suppression, and lyse bulky disease. More recently, clinical trials combining GM-CSF-secreting vaccines with the immune checkpoint agent ipilimumab demonstrated enhanced T-cell activation associated with survival benefit in prostate and pancreas cancer (7, 8). These trials provide clinical proof of principle for combination immunotherapies.
Standard cancer drugs may also augment immune-based therapies, either at approved doses or at an immune-modulating dose and schedule (9). We showed that low dose cyclophosphamide (CY) enhances a HER-2+ GM-CSF-secreting vaccine in both immune tolerant neu transgenic mice (10), and in MBC patients (11). Low dose CY relieves the suppressive influence of CD4+CD25+ regulatory T cells (Treg) in neu mice, allowing the recruitment of potent, tumor-specific CD8+ T cells (12, 13). The monoclonal antibody (MAb) trastuzumab also has immunomodulatory activity (14). We showed that HER-2-specific MAbs can augment HER-2-specific CD8+ T-cell responses and tumor-free survival in vaccinated, tumor-bearing neu mice (15); further, the trastuzumab-like MAb 7.16.4 enhances immune priming, augmenting primary and memory CD8+ T-cell responses in vaccinated neu mice (16). In breast cancer patients, trastuzumab-based chemotherapy induces T-cell responses within both the peripheral blood and tumor microenvironment (17, 18).
We therefore evaluated the immunologic and antitumor activity of a GM-CSF-secreting, HER-2+ whole cell breast cancer vaccine given in sequence with immune-modulating doses of CY and weekly HER-2-specific MAb in tolerant neu mice, and in MBC patients. The clinical study was designed to assess the safety, clinical benefit, and immunologic activity of this combination immunotherapy regimen in patients with measurable or evaluable HER-2+ MBC.
METHODS
Preclinical Study Design
Mice
Neu-N (neu) mice were derived from the colony of Muller (19), bred to homozygosity as verified by Southern blot (20), and maintained at Johns Hopkins University. Experiments were performed with 8-10 week old mice using AAALAC-compliant protocols approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
Cell lines and media
The GM-CSF-secreting vaccine cell lines GM (mock) and neuGM (neu-specific), the NT2.5 neu-expressing tumor cell line, and the NT2.5B7.1 antigen-presenting cell line were derived and grown as previously described (4, 10). GM-CSF levels were verified by ELISA, and neu and B7.1 levels were verified by flow cytometry biannually. All cell lines used were regularly tested and validated to be mycoplasma-free; no other authentication assay was performed.
Antibody purification
Hybridoma cells secreting the anti-rat neu MAb 7.16.4 (21) were grown in athymic nude mice, and ascites collected (Harlan Bioproducts for Science). 7.16.4 was purified over a T-Gel column (Pierce Biotechnology) using the Biologic LP purification system (Bio-Rad), dialyzed into PBS, and >90% purity determined by SDS-PAGE analysis using Criterion 4-15% Tris-HCl Pre-cast Gels and the Criterion Cell (Bio-Rad). Nonspecific isotype-matched antibody served as the control. Antibodies were confirmed endotoxin-free by the Limulus amebocyte lystate test (Bio Whittaker).
Murine immunotherapy experiments
Neu mice were challenged with 5×104 NT2.5 tumor cells, and vaccinated 3 days later. Vaccine cells were irradiated prior to subcutaneous (SQ) injection in both hind limbs and the left forelimb. CY (Bristol-Myers Squibb) at 100 mg/kg and 7.16.4 at 100 μg were injected intraperitoneally (IP) 1 day prior to vaccination; 7.16.4 was then given IP weekly. The tumor and vaccine cell doses, and the CY and MAb dose and schedule have been optimized previously (10, 20, 22). Mice were monitored for tumor onset and growth twice weekly, and tumors were measured in 2 perpendicular dimensions with calipers.
ELISPOTS
CD8+ splenic lymphocytes were purified by negative selection (Dynal Biotech, Invitrogen). 105 CD8+ T cells were incubated in duplicate with 104 target cells (NT2.5B7.1 cells stimulated with IFNγ for 2 days) at 37°C overnight (22) on pre-coated IFNγ-specific ELISPOT plates and developed according to the manufacturer’s protocols (R&D Systems). IFNγ-secreting CD8+ T cells were enumerated using the Immunospot counter (Cellular Technology, LTD). The number of spots in control wells was averaged and subtracted from the number of spots in each well containing both CD8+ T cells and targets.
Statistical analysis
A Student’s t test was applied to determine the statistical significance of differences between treatment groups, with P<0.05 being significant. Analyses were performed using GraphPad Prism, version 3.0a for Macintosh (GraphPad Software). All experiments were repeated at least twice, with 5-10 mice per group.
Clinical Study Design
The clinical study was an open label, single arm feasibility study to evaluate the safety and clinical benefit associated with the administration of a fixed dose of allogeneic GM-CSF-secreting breast tumor vaccine cells given in sequence with low dose CY and weekly trastuzumab. A Simon two-stage design (23) was used to evaluate 20 vaccinated research subjects.
The clinical study was conducted in accordance with the principles of Good Clinical Practice and the ethical principles stated in the Declaration of Helsinki. It was approved by the Johns Hopkins University School of Medicine Institutional Review Board, the National Institutes of Health Recombinant DNA Advisory Committee, and the US Food and Drug Administration Center for Biologics Evaluation and Research.
Patient Selection
Twenty-two patients with HER-2+ MBC were enrolled at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center between November 14, 2006 and July 13, 2009; 20 were treated on study. Eligible patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, and a histologic diagnosis of HER-2+ breast cancer by IHC 3+ staining or gene amplification >2.0 by FISH. Prior chemotherapy must have been completed ≥28 days before vaccination; concurrent endocrine and/or bisphosphonate therapy was allowed. Other requirements included a cardiac ejection fraction ≥45%, adequate end-organ function, and negative HIV and pregnancy tests. Stable treated central nervous system disease was allowed. Key exclusion criteria included past/current autoimmune disease, non-protocol-specific treatment or parenteral steroids within 28 days of vaccination, and past/current second malignancy (except superficial non-melanoma skin cancer, bladder cancer, tamoxifen-related endometrial cancer cured by hysterectomy, and cervical carcinoma in situ).
Study Plan and Intervention
Eligibility Determination
Written informed consent was obtained from each research participant. Baseline studies included computed tomography of the chest, abdomen, and pelvis, bone scans, complete blood count with differential, chemistry profile, absolute eosinophil count, and echocardiogram or multiple gated acquisition scan.
Treatment Plan
Subjects received three monthly vaccinations, with a boost 6-8 months from trial entry. During active vaccination cycles, patients received CY 300 mg/m2 on D−1 and 5×108 vaccine cells on D0, on a backbone of weekly trastuzumab given at the standard dose (2 mg/kg, with a 4 mg/kg loading dose as necessary); trastuzumab was given on D−1 the week of vaccination. During the interim period between cycles 3 and 4, trastuzumab could be given weekly (2 mg/kg) or every 3 weeks (6 mg/kg). CY-modulated vaccination was given every 4-6 weeks for three cycles, with a fourth cycle 6-8 months after the first cycle. Patients with disease progression were taken off study.
Vaccinations
Vaccine development and manufacturing has been published (24). Briefly, the parent cell lines T47D (HER2low) and SKBR3 (HER2high) were genetically modified by plasmid DNA transfection to secrete GM-CSF. Clinical lots were prepared from two subcloned cell lines secreting bioactive levels of GM-CSF, 2T47D-V and 3SKBR3-7. On D0, serum-free, cryopreserved, irradiated vaccine cells were thawed and mixed to create a HER2+ vaccine that secreted GM-CSF levels of about 300 ng/106 cells /24 hours. Vaccine cells were injected intradermally, evenly distributed over 3 lymph node areas. Anesthetic lidocaine cream was applied to the injection sites before vaccination.
End Points
Toxicity assessment
Toxicities were graded using the National Cancer Institute’s Clinical Trials Common Terminology Criteria for Adverse Events Version 3.0 (CTCAE v.3.0). Toxicity monitoring included clinical assessment and complete blood counts on days 3 and 7; chemistry profiles were measured before and after each cycle and on day 7.
Clinical Benefit Rate and Survival Outcomes
The primary endpoint of clinical benefit (CB) was defined as complete response+partial response+stable disease (CR+PR+SD) at 6 months; CB at 1 year was also determined. Exploratory endpoints of progression-free survival (PFS) and overall survival (OS) were defined as time to first disease progression or death from the eligibility date.
Analysis of serum cytokine levels
Serum was collected at baseline and after each vaccination, and on days 0, 1, 2, 3, 4, 7, and 14 of each cycle. Serum was separated from whole blood by centrifugation, and frozen in 1-mL aliquots at −80°C. Serum GM-CSF levels were measured as described previously (11). Serum TGF-β was measured by Luminex pre- and post-vaccination according to the manufacturer’s protocols.
Assessment of delayed-type hypersensitivity (DTH) using HER-2 peptides
One hundred ug each of two MHC class II HER-2 epitopes (p369 and p776) (25), with mutated k-ras as a negative control, were injected intradermally on the back as previously described (11). Erythema and induration were assessed 2-3 days after injection.
DTH was considered positive if induration increased by >5mm from baseline.
Collection, preparation and cryopreservation of peripheral blood mononuclear cells (PBMC)
200cc whole blood was collected in heparin tubes pre- and post-vaccination; 20cc whole blood was collected on days 0, 1, 2, 3, 4, 7, and 14. PBMCs were isolated by Ficoll-Hypaque gradient centrifugation; interface cells were harvested and washed twice. PBMCs were resuspended in AIM-V medium (Invitrogen) with 10% dimethylsulfoxide, frozen at − 80°C for 1-2 days, and stored in LN2 until analysis (8).
Assessment of Treg and myeloid-derived suppressor cells (MDSC)
Treg and MDSC analyses were performed with modifications to published methods (26-28). Briefly, 106 PBMCs were stained with for 30 minutes at 4°C with: Pacific blue-CD4 (BD Pharmingen), FITC-CD25 (BD Pharmingen), PE-Cy7-CD45RA (eBioscience), PerCP-Cy5.5-CCR7 (BD Pharmingen), APC-CTLA-4 (BD Pharmingen), FITC-Lineage cocktail (Biolegend), APC-HLA-DR (BD Pharmingen), PE-Cy7-CD33 (eBioscience) and/or PE-CD11b (eBioscience). For Treg analysis, cells were fixed for 40 minutes at 4°C and permeabilized with Transcription Factor Buffer Set (BD Pharmingen), followed by staining with PE-FoxP3 (BD Pharmingen) for 30 minutes at 4°C. Data were acquired by a Gallios cytometer (Beckman Coulter) and analyzed using FlowJo V7.6.5 software (TreeStar, Inc.).
Assessment of peripheral HER-2-specific CD8+ T cells
T-cell analyses were conducted with modifications to published methods (29, 30). Monocytes were isolated using the MACS CD14 isolation kit (Miltenyi Biotec), and cultured in 6-well plates (2×106 cell/mL) in RPMI-1640 medium containing 5% pooled human serum (PHS), 50 ng/mL GM-CSF (Miltenyi Biotech) and 50 ng/mL IL-4 (Miltenyi Biotech). Cells were cultured for 48 hours and subsequently incubated for 18 hours with 10 ng/mL IL-1β (Miltenyi Biotech), 10 ng/mL IL-6 (Miltenyi Biotech), 10 ng/mL TNF-α (Miltenyi Biotech), and 0.5-1 μg/mL PGE2 (Sigma-Aldrich). The quality of monocytic dendritic cells (MoDC) was assessed by staining with PE-Cy7-CD80 (Biolegend), FITC-CD83 (Biolegend), Pacific blue-CD86 (Biolegend), APC-HLA-DR (BD Pharmingen), and/or APC-IL-12 (BD Pharmingen). Overlapping peptide mixtures were used to stimulate CD8+ T cells (JPT Peptide Technologies). PepMix HER-2 (extracellular and intracellular domains) is a pool of 15-mer peptides that span the entire HER-2 protein and overlap by 11 amino acids; PepMix CEF Pool and PepMix HIV served as positive and negative controls, respectively. Peptides were resuspended in DMSO, stored at −80°C, thawed the day of assay and diluted to the required concentration.
Isolated, activated MoDCs were mixed with peptides at 1 μg/ml in RPMI-1640 medium containing 5% PHS and incubated at 37°C for 2 hours. CD8+ T cells were purified from PBMCs using the Dynabeads Untouched CD8 T cell kit (Invitrogen). HER-2-specific CD8+ T cells were indistinguishable from background by flow cytometry directly ex vivo, and were therefore stimulated in vitro by co-culturing peptide-loaded MoDCs with autologous CD8+ T cells at a ratio of 1:3 in the presence of IL-7 (R&D Systems) and IL-2 (R&D Systems) at concentrations of 10 ng/ml and 10 IU/ml, respectively. On days 8 and 15, T cells were re-stimulated with MoDCs. The cells were re-fed with IL-7 and IL-2 every 3 days. The cells were harvested on day 15 after 4 hours of re-stimulation and assayed by ICS using FITC-CD8 (eBioscience), PE-IFN-γ (Biolegend), APC-TNF-α (Biolegend), and PE-Cy7-IL-2 (BD Pharmingen). Sample data were acquired by a Gallios cytometer (Beckman Coulter) and analyzed using FlowJo Version 7.6.5 software. The proportions of polyfunctional CD8+ effector T cell subsets were classified by cytokine secretion.
Statistical Considerations and Data Analysis
The trial database was closed on October 16, 2013. A Simon two-stage design was used (23). Nine research subjects were to be enrolled and treated in the first stage. At the end of the first stage, if 2 or more research subjects demonstrated CB, 11 additional research subjects were to be enrolled and treated. This design had an alpha level of 0.08 with a power of 0.86 to detect a clinical benefit (CR+PR+SD) of 0.45 from a null hypothesis rate of 0.20. At the end of the second stage, if 7 or more patients demonstrated CB, the null hypothesis was rejected. Based on this study design, there was a 44% chance of early stopping if the true response rate was 0.20, and a 4% chance of early stopping if the true response rate was 0.45.
The study met criteria for full accrual, and data from 20 patients were used in the analysis. Tumor responses (CR, PR, and SD) were determined by RECIST 1.0. CB was estimated using the binomial distribution along with the 95% confidence interval (CI). Continuous data were summarized using mean and standard deviation. Categorical data were represented using percent or proportion. Survival probability was estimated using the Kaplan-Meier method (31). The CI of median survival time was constructed by the method of Brookmeyer-Crowley (32). Paired and unpaired Student’s t tests were used for pre- and post-vaccine comparisons, and between group comparisons. All P values are reported as two-sided, and all analyses were conducted using SAS software (version 9.2 SAS Institute, Cary, NC).
RESULTS
Preclinical Modeling
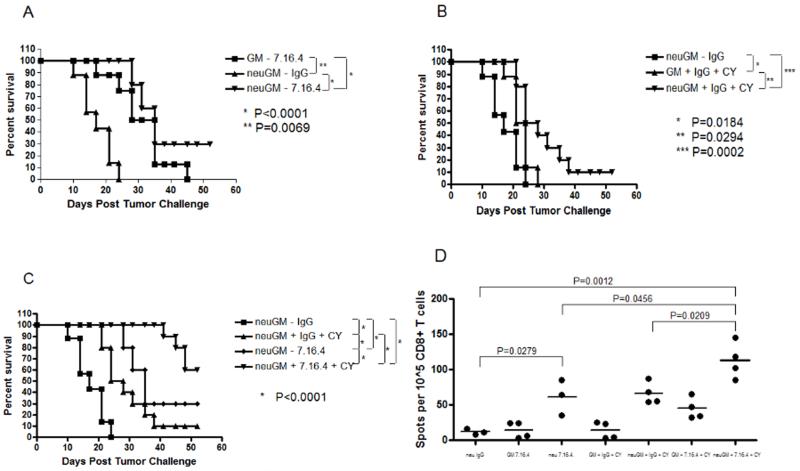
Neu mice exhibit pre-existing immune tolerance specific for rat HER-2 (neu), and are a stringent model for developing clinically relevant cancer immunotherapies (10, 20, 22). We first evaluated whether the trastuzumab-like MAb 7.16.4 could augment the activity of neu-specific vaccination alone or sequenced with immune modulating doses of CY in these mice. Treating tumor-bearing neu mice with 7.16.4 and mock vaccination delayed tumor growth relative to neu-targeted vaccination alone, which is ineffective in this model (Figure 1). Adding CY or 7.16.4 to neu-specific vaccination also delayed tumor outgrowth, increasing tumor-free survival from 0% (vaccine or 7.16.4 alone) to 10% (vaccine + CY) and 30% (vaccine + 7.16.4) (Figure 1A and 1B). Adding both CY and 7.16.4 to neu-targeted vaccination increased tumor-free survival to about 60% (Figure 1C). This relative antitumor efficacy was reflected in the numbers of tumor-specific IFNγ-secreting CD8+ T cells by ELISPOT, where the highest levels were detected in the setting of CY + 7.16.4 + vaccine (Figure 1D).
Figure 1. HER-2-specific MAbs combined with HER-2-specific vaccination augment tumor-free survival and HER-2-specific CD8+ T cell levels in neu mice.
Neu mice (10 per group) were challenged with NT-2.5 tumor cells, and vaccinated 3 days later. A single dose of cyclophosphamide (CY) was given one day prior to vaccination in selected mice; weekly HER-2-specific MAb (7.16.4) or nonspecific IgG was also started one day prior to vaccination. (A) Vaccination + 7.16.4. (B) Vaccination + CY. (C) Vaccination + CY + 7.16.4. (D) Splenic T cells were isolated from neu mice at the end of treatment (3-5 per group). CD8+ T cells were isolated and incubated with NT2.5B7.1 target cells for IFNγ ELISPOT analysis. Circles represent individual mice; bars represent the average value. neuGM=3T3 cells that secrete granulocyte-macrophage colony-stimulating factor (GM-CSF) and express rat HER-2 (neu vaccine); GM=3T3 cells that secrete GM-CSF (control vaccine).
Clinical Trial
Patient Characteristics
Based on this preclinical modeling, we conducted a clinical trial testing CY-modulated vaccination with trastuzumab in patients with HER-2+ MBC. The intervention and data collection schedules are shown in Figure 2. Twenty eligible patients were vaccinated, with an age range of 34 to 69 years (Table 1). All had HER-2+ metastatic disease, 13 had ER-positive and/or PR-positive disease. The mean disease-free interval to relapse from first diagnosis was 32 months (range, 0 to 130 months); 7 (35%) patients presented with MBC at diagnosis. Eighteen patients (90%) received prior trastuzumab for MBC, while 3 received prior trastuzumab as adjuvant therapy. Eleven (55%) were on concurrent endocrine therapy, and the majority (70%) received concurrent bisphosphonate therapy for skeletal metastasis. The mean and median sums of the longest tumor diameter at study entry were 40.7 mm and 24.5 mm, respectively.
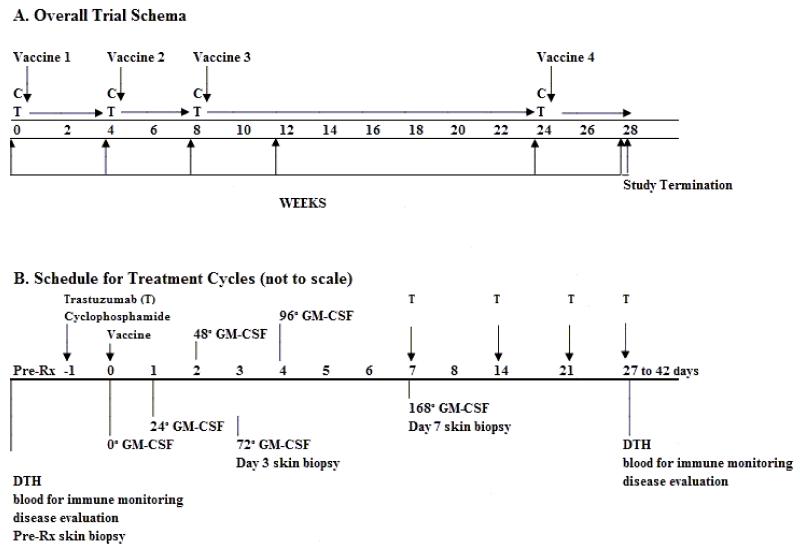
Figure 2. Study schema.
Patients received up to four vaccination cycles. Cyclophosphamide (CY) was given on day −1, and vaccine on day 0. Trastuzumab (T) was given weekly during active vaccine cycles, and timed to coincide with CY administration on day −1. During the interim period between cycles 3 and 4, trastuzumab could be given weekly or every 3 weeks as per the standard of care. GM-CSF levels and immunity were measured as indicated. CY=cyclophosphamide; T=trastuzumab; DTH=delayed-type hypersensitivity to HER-2-derived peptides. Skin biopsies on days +3 and +7 were from the largest vaccine site.
Table 1. Patient Characteristics.
| Characteristic | Number of Patients | % |
|---|---|---|
|
| ||
| Total number of patients | 22 enrolled | |
| 20 vaccinated | ||
| 20 evaluable | ||
|
| ||
| Age, years | ||
| Median | 52 | |
| Range | 34-69 | |
|
| ||
| ER-positive or PR-positive tumor | 13 | 65 |
|
| ||
| HER-2-positive tumor | 20 | 100 |
|
| ||
| Metastatic disease at diagnosis | 7 | 35 |
|
| ||
| DFI to relapse (months)* | ||
| Mean | 32 | |
| Median | 28 | |
| Range | 0-130 | |
|
| ||
| Sites of distant metastasis | ||
| Bone | 14 | 70 |
| Liver | 5 | 25 |
| Lung | 4 | 20 |
| Lymph node (distant) | 3 | 15 |
| Brain | 1 | 5 |
|
| ||
| Sum of the longest tumor diameter at study entry |
||
| Mean | 40.7 mm | |
| Median | 24.5 mm | |
|
| ||
| Prior lines of chemotherapy for metastatic disease |
||
| Median | 1 | |
| Range | 0-6 | |
| Mean | 1.25 | |
|
| ||
| Prior trastuzumab therapy | ||
| Adjuvant | 3 | 15 |
| Metastatic | 18 | 90 |
|
| ||
| Prior lapatinib therapy | 2 | 10 |
|
| ||
| Concurrent endocrine therapy | 11 | 55 |
|
| ||
| Concurrent bisphosphonate therapy | 14 | 70 |
Abbreviations: ER=estrogen receptor; PR=progesterone receptor; HER-2=human epidermal growth factor receptor-2; DFI=disease-free interval
13 patients who did not present with metastatic disease at diagnosis
All patients (100%) received at least one vaccination, 15 (75%) received at least three vaccinations, and 8 (40%) received all four vaccinations. Two patients came off-study for reasons unrelated to disease progression, one after 3 vaccine cycles due to re-location, and another after 2 vaccine cycles due to study demands. A third off-study event was related to both a vaccine-related serious adverse event and simultaneous disease progression. All other off-study events before cycle 4 were due to breast cancer progression.
Toxicity
The most common adverse events were local vaccine site reactions, including erythema, induration, pruritus, and/or discomfort (Table 2). These self-limited local reactions occurred in all individuals, and increased in intensity but not duration with subsequent vaccination cycles. The most common systemic adverse events were fatigue, urticaria, pruritus, and fever. Seven patients (35%) developed urticaria distant from the vaccine site; of these, 3 (15%) developed Grade 2-3 urticaria that required parenteral intervention with diphenhydramine and/or steroids. Of these, one was a vaccine-related serious adverse event requiring overnight hospitalization and steroid treatment. No evidence of clinically significant autoimmunity was detected. Despite the use of trastuzumab, no decrement in cardiac function was detected (data not shown).
Table 2. Summary of Treatment-Related Adverse Events.
| Adverse Event | All Grades | Grade 3 or 4 | ||
|---|---|---|---|---|
| # Patients | % | # Patients | % | |
| Local vaccine site reactions | ||||
| Erythema/induration | 20 | 100 | 0 | 0 |
| Pruritus | 20 | 100 | 0 | 0 |
| Pain/soreness/tenderness | 17 | 85 | 0 | 0 |
| Local rash at/near vaccine site | 7 | 35 | 1 | 5 |
| Blister formation | 5 | 25 | 0 | 0 |
| Hyperpigmentation | 4 | 20 | 0 | 0 |
| Ecchymosis (bruising) | 3 | 15 | 0 | 0 |
| Local edema | 2 | 10 | 0 | 0 |
| Vaccine site flare | 2 | 10 | 0 | 0 |
| Groin tightness | 1 | 5 | 0 | 0 |
| Systemic toxicities | ||||
| Fatigue | 8 | 40 | ||
| Urticaria | 7 | 35 | 2 | 10 |
| Pruritus (distant from vaccine site) | 6 | 30 | 1 | 5 |
| Fever | 5 | 25 | ||
| Flu-like symptoms/myalgia | 4 | 20 | ||
| Lymphadenopathy | 4 | 20 | ||
| Abdominal pain | 3 | 15 | 1 | 5 |
| Rash (distant from vaccine site) | 3 | 15 | 1 | 5 |
| Achiness/malaise | 3 | 15 | ||
| Chills | 3 | 15 | ||
| Dizziness | 2 | 10 | ||
| Anorexia | 1 | 5 | ||
| Erythema (distant from vaccine site) | 1 | 5 | ||
| Headache | 1 | 5 | ||
| Nausea | 1 | 5 | ||
| Arm pain | 1 | 5 | ||
| Cancer site pain | 1 | 5 | ||
| Leg tenderness | 1 | 5 | ||
NOTE: Data are given as any incident per patient, for a maximum of 20 counts per event.
Serum GM-CSF Pharmacokinetics
Serum GM-CSF levels were measured as an indicator of the vaccine’s life span post-injection. Serum GM-CSF levels peaked at 48 hours, with peak levels persisting across all four cycles as previously observed (11). Trastuzumab did not alter the timing of the peak or the kinetics of GM-CSF decay (data not shown).
Clinical Outcomes
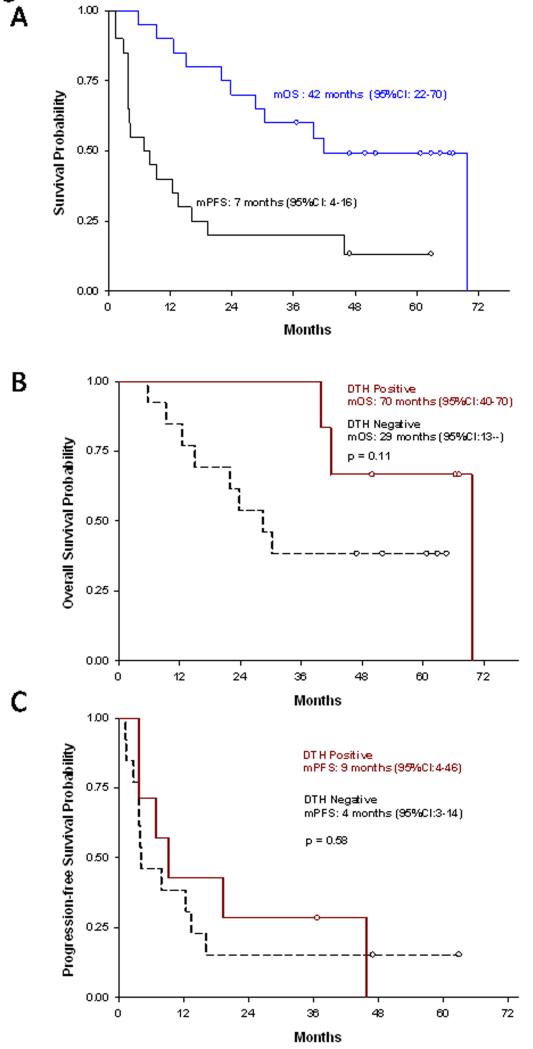
The CB rates of weekly trastuzumab with CY-modulated vaccination at 6 months and 1 year were 11/20 (55%, 95% CI:32-77%, p=0.013) and 8/20 (40%, 95% CI:19-64%), respectively. One PR and no CRs were observed. Overall median PFS and OS were 7 months (95% CI:4-16) and 42 months (95% CI: 22-70), respectively (Figure 3A). The 5-year survival rate was 6/20 (30%, 95% CI:12-54).
Figure 3. Progression-free survival (PFS) and overall survival (OS) of vaccinated patients.
(A) Kaplan-Meier curves of the percent PFS and OS in months from the time of eligibility determination (n=20). (B) Kaplan-Meier curves of PFS stratified by DTH-positive and DTH-negative research subjects. (C) Kaplan-Meier curves of OS stratified by DTH-positive and DTH-negative research subjects. DTH=delayed-type hypersensitivity.
Immunologic Analyses
We previously demonstrated the feasibility of using DTH responses specific for HER-2-derived peptides and HER-2-specific antibody responses by ELISA as immune response biomarkers for this cell-based vaccine (11). Both because our preclinical data demonstrate a correlation of HER-2-specific CD8+ T cells with tumor-free survival in vaccinated neu mice, and because trastuzumab confounds the assessment of HER-2-specific antibody responses, we assessed HER-2-specific DTH and measured HER-2-specific CD8+ T cells by ICS as biomarkers of vaccine-induced immunity in this study. HER-2-specific DTH developed in 7/20 subjects (35%, 95% CI:15-59%); 4 of these (57%, 95% CI:18-90%) had CB. There was a trend toward longer PFS and OS for patients who developed HER-2-specific DTH post-vaccination (Figure 3B and 3C), though this was not statistically significant due to the small sample size of this study. Median PFS for DTH positive versus DTH negative subjects was 9 (95% CI:4-46) versus 4 months (95% CI:3-14, p=0.58). Median OS for DTH positive versus DTH negative subjects was 70 (95% CI:40-70) versus 29 months (95% CI:13—not reached, p=0.11). Larger randomized studies are required to rigorously evaluate the possible relationship between DTH and survival.
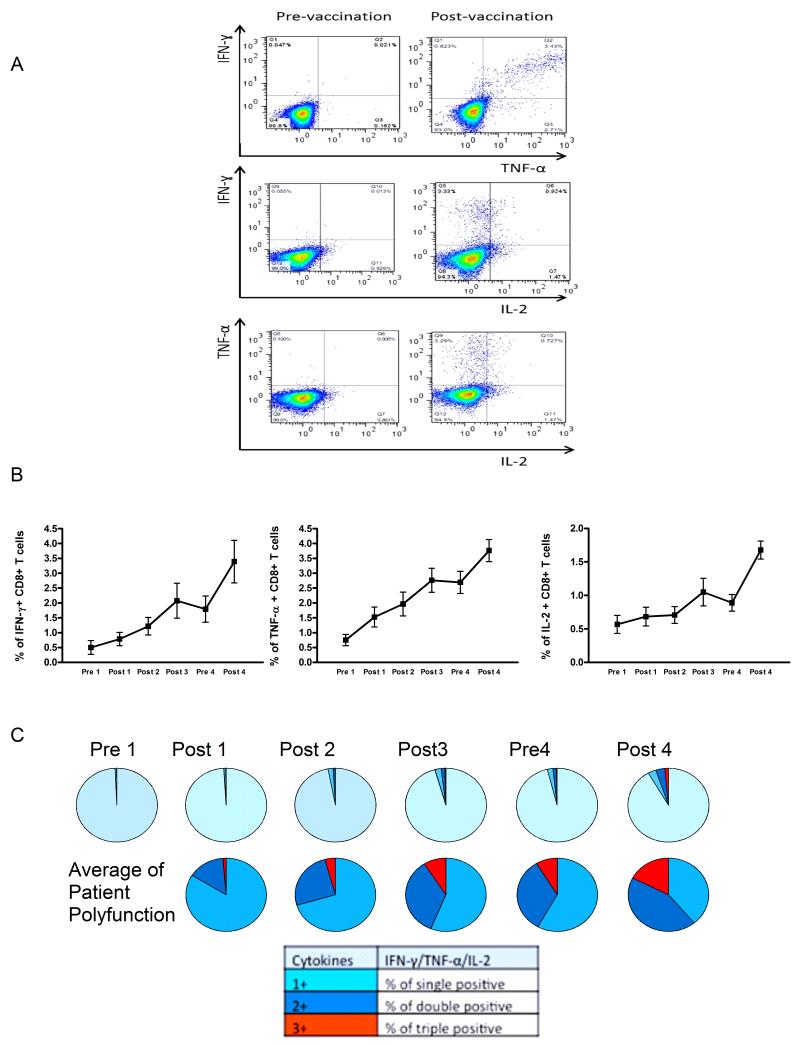
HER-2-specific CD8+ T cell function pre- and post-vaccination was assessed in vitro by ICS using commercially available peptide mixtures containing overlapping epitopes spanning the entire HER-2 protein, a representative analysis is shown in Figure 4A. Both the average level of CD8+ T cells secreting IFNγ 0.501% versus 0.785%, p<0.0001), interleukin-2 (IL-2) (0.567% versus 0.683%, p=0.0006) and tumor necrosis factor-TNF-0.751% versus 1.525%, p<0.0001), and the proportion of polyfunctional CD8+ T cells secreting more than one cytokine (0.023% versus 0.735%, p<0.0001) increased across the vaccination cycles (Figure 4B and 4C). In exploratory analyses, increased polyfunctional CD8+ T cells were possibly associated with longer PFS (HR=0.44, 95% CI:0.2-0.98, p=0.04) and OS (HR=0.43, 95% CI:0.13-1.36, p=0.15).
Figure 4. Vaccination elicits HER-2-specific CD8+ T-cell responses in MBC patients as measured by intracellular cytokine staining (ICS).
(A) HER-2-specific CD8+ T cells that secrete IFNγ, tumor necrosis factor-α(TNF-α), or interleukin-2 (IL-2) are detectable by ICS in vaccinated patients. A representative patient is shown. (B) The percentage of HER-2-specific CD8+ T cells that secrete IFNγ, TNF-α, or IL-2 increase across the vaccination cycles. The connected points represent the mean and standard deviation of values across the patient population, with three replicates per patient. (C) The proportion of polyfunctional HER-2-specific CD8+ T cells that secrete more than one cytokine increases across the vaccination cycles.
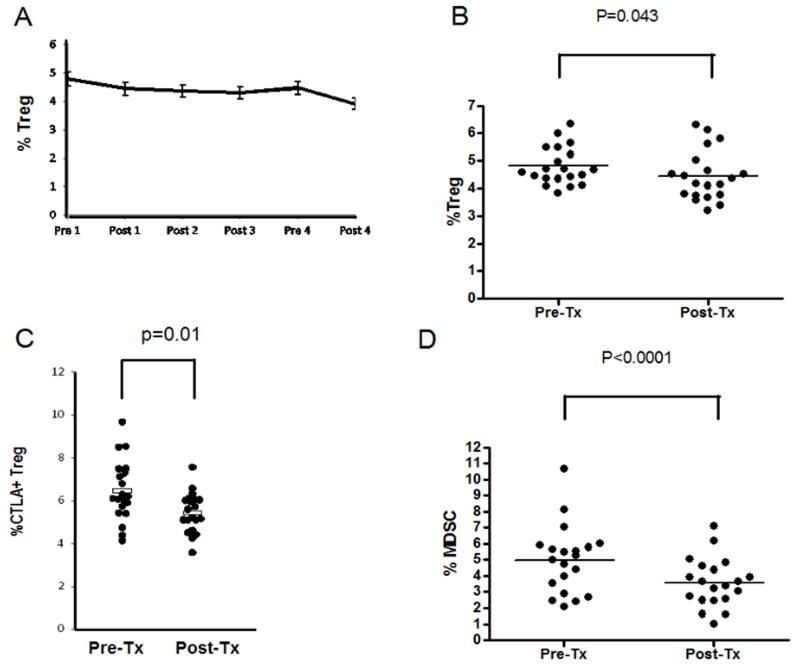
We also assessed peripheral markers of immune suppression by FACS (Treg and MDSC) and Luminex (TGF-β). Though an association between decreased TGF-β levels and immunity in patients treated with a HER-2 peptide vaccine and concurrent trastuzumab was reported previously (33), we found no difference in serum TGF-β levels pre- and post-vaccination (43,125 versus 46,936 pg/ml, p=0.21). Tregs decreased across all vaccine cycles, and both overall and CTLA-4+ Treg decreased from baseline to post-vaccine cycle 1; MDSCs also decreased from baseline to post-vaccine cycle 1 (Figure 5). Exploratory analyses suggested that neither baseline nor decreased levels of peripheral Tregs were associated with survival benefit. However, decreased numbers of peripheral MDSC were possibly associated with both longer PFS (HR=0.5, 95% CI:0.29-0.85, p=0.010) and OS (HR=0.52, 95% CI:0.31-0.89, p=0.016) baseline levels of MDSC were not. To further explore possible associations between immunologic biomarkers and survival, a subgroup analysis of 12 patients was performed comparing 6 individuals with OS≤24 months to 6 individuals with OS≥60 months. Both decreased MDSCs and increased polyfunctional T cells post-vaccination were possibly associated with OS, with p values of 0.012 and 0.028, respectively.
Figure 5. Markers of immune suppression in the peripheral blood decrease after vaccination.
(A) CD4+FoxP3+ Tregs decrease across the vaccination cycles. (B) CD4+ FoxP3+ Tregs decrease from baseline after one vaccination cycle, p=0.043. (C) CD4+FoxP3+ CTLA-4+ Tregs decrease from baseline after one vaccination cycle, p=0.01. (D) HLA-DRneg/linlo/CD33+/CD11b+ MDSCs, p<0.0001.
DISCUSSION
This study of a HER-2+ GM-CSF-secreting breast tumor vaccine with low dose CY and weekly trastuzumab supports the following seven conclusions. First, in the clinically relevant neu transgenic mouse, adding both CY and 7.16.4 (a trastuzumab-like MAb) to vaccination increases tumor-free survival and IFNγ-secreting tumor-specific CD8+ T cells relative to adding either CY or 7.16.4 alone to vaccination. Second, up to four sequential CY-modulated vaccinations are safe and well tolerated in patients with HER-2+ MBC receiving weekly trastuzumab. Third, this immunotherapy was associated with a 6-month CB rate of 55%, and median PFS and OS of 7 and 42 months. This study met its primary endpoint, as the target therapeutic outcome was a 6-month CB rate of 45%. Fourth, this combination immunotherapy induced new or increased HER-2-specific T-cell immunity in HER-2+ MBC patients as measured by HER-2-specific DTH and CD8+ T cells by ICS. There was a trend toward longer PFS and OS in patients who developed HER-2-specific DTH relative to those who did not. Fifth, polyfunctional HER-2-specific CD8+ T cells progressively expanded across the vaccination cycles. Sixth, both Tregs and MDSCs decreased across the vaccination cycles. Finally, hypothesis-generating analyses suggest that both decreased MDSCs and a greater proportion of polyfunctional HER-2-specific CD8+ T cells may be associated with longer PFS and OS. A comparative trial powered for survival will be required to rigorously evaluate these associations.
Trastuzumab has revolutionized the management of HER-2+ breast cancer, and remains the major backbone of therapy for early and late stage disease (1, 2, 14). However, intrinsic and acquired trastuzumab resistance remains a significant limitation to its clinical utility. In patients, trastuzumab-based chemotherapy is associated with increased levels of HER-2-specific antibodies and T cells (17), and intratumoral lymphoid nodules develop in the setting of neoadjuvant trastuzumab-based chemotherapy (18). These observations suggest that leveraging the ability of trastuzumab to activate adaptive immunity could increase its efficacy. One clinical study combined a peptide vaccine with weekly trastuzumab, demonstrating increased levels of HER-2-specific T cells, epitope spreading, and decreased serum levels of the immune suppressive cytokine TGF-β (33). We showed that HER-2-specific MAbs alone induce low levels of neu-specific T cells in neu transgenic mice. The addition of HER-2-specific MAbs to neu-specific GM-CSF-secreting vaccination more effectively augments neu-specific CD8+ effector T-cell function and prolongs tumor-free survival in these mice (15). We further showed that the trastuzumab-like MAb 7.16.4 augments locoregional immune priming through binding the Fc receptor of dendritic cells in similarly vaccinated neu mice, augmenting CD8+ T-cell proliferation, cytokine secretion, and central memory development (16). Here we build on these prior studies to show that the combination of low dose CY, trastuzumab, and a GM-CSF-secreting HER-2+ allogeneic cell-based vaccine is safe, bioactive, and associated with CB in patients with HER-2+ MBC.
A significant challenge in optimizing combination tumor cell vaccine-based immunotherapy is the lack of clear biomarkers for assessing immune response and drug-drug interactions. We previously demonstrated the feasibility of using DTH responses specific for two MHC Class II HER-2-derived peptides and HER-2-specific antibody responses by ELISA pre- and post-vaccination as immune response biomarkers for this cell-based vaccine (11). Because the use of trastuzumab here confounds the assessment of HER-2-specific antibody responses, we measured HER-2-specific DTH and peripheral HER-2-specific CD8+ T cells by flow cytometry. We found that HER-2-specific DTH developed in 7/20 subjects (35%); 4 of these patients had CB. In addition, there was a trend toward longer PFS and OS in patients who developed HER-2-specific DTH relative to those who did not. These data are consistent with other small clinical trials that have shown an association between enhanced tumor-specific DTH and survival (34-37). Cytokine-secreting CD8+ T cells also increased with vaccination in MBC patients. While vaccine-induced CD8+ T cells from neu mice could be detected by ELISPOT directly ex vivo, vaccine-induced CD8+ T cells from MBC patients were detected by flow cytometry only after in vitro stimulation. This differential sensitivity in detecting vaccine-induced CD8+ T cells is likely explained by differences in the host (transgenic neu mice are genetically homogenous whereas MBC patients are genetically and biologically heterogeneous), the vaccine (the murine vaccine specifically targets HER-2, whereas the human vaccine delivers multiple tumor antigens where responses to HER-2 are used as a sentinel measure of vaccine activity), the T cells (assayed fresh from mice as opposed to frozen from MBC patients), and the assays (ELISPOT is more sensitive than flow cytometry). We used ICS by flow cytometry to rapidly evaluate HER-2-specific CD8+ T cells that secrete one or more cytokines in MBC patients.
Polyfunctional antigen-specific T cells may be indicators of disease-control and/or survival in patients with chronic infections and cancer (38-40). Polyfunctional T cells secrete multiple relevant cytokines (IFNγ, TNF-α, IL-2) that reflect the quality of the immune response as a more informative correlate of sustained protective immunity than T cells that secrete only a single cytokine (IFNγ or TNF-α). Durable T-cell polyfunctionality correlates with long-term disease control in HIV (41) and malaria patients (42), but has been less studied in vaccinated cancer patients. A telomerase peptide vaccine given with temezolomide to metastatic melanoma patients induced polyfunctional T cells (43). Here we demonstrate the progressive expansion of polyfunctional HER-2-specific T cells across vaccination cycles, where a greater proportion of polyfunctional CD8+ T cells may be associated with longer PFS and OS. A comparative clinical study powered for survival is required to formally evaluate this hypothesis.
Tregs and MDSCs are significant mediators of immune tolerance and suppression in cancer patients. We showed previously that low dose CY depletes Tregs in neu mice, facilitating the vaccine-mediated recruitment and activation of latent, high avidity tumor-specific T cells and tumor rejection (10, 12, 13). Here, we demonstrate a decline in peripheral Tregs and MDSCs in vaccinated MBC patients. Several clinical trials have explored depleting Tregs in the setting of vaccination for cancer, with enhancement of antigen-specific immune responses to vaccination (11, 44-46). Notably, a randomized Phase II study evaluating a multi-peptide renal cell carcinoma vaccine alone or sequenced with low dose CY demonstrated that CY-modulated vaccination reduced Tregs and induced T-cell responses specific for multiple peptide antigens, increasing the OS of vaccinated patients (46). An association between levels of circulating MDSCs and immune response or clinical outcomes has been reported in several immunotherapy trials, and these results are consistent with our findings (46-49).
This combination immunotherapy was safe. Given the known cardiac toxicity of trastuzumab (14), cardiac function was assessed every 3 months on study. No patient developed a significant drop in left ventricular ejection fraction. These data suggest that the addition of CY-modulated vaccination to weekly trastuzumab did not increase cardiac toxicity, though the sample size is limited. Notably, urticaria was observed in a significant number of patients in this study; three patients required parenteral diphenhydramine and/or steroids. It remains unclear if urticaria may be associated with higher levels of immunity or clinical benefit. We observed CB rates of 55% at 6 months and 40% at one year. The median PFS in this study was 7 months, the median OS was 42 months, and the 5-year survival rate was 30%. Although the sample size is small, these clinical results compare favorably to other trials of HER-2-directed therapy for metastatic HER-2+ breast cancer that do not include cytotoxic chemotherapy. The seminal trials of single agent trastuzumab for the first line and second/third line treatment of patients with metastatic disease showed CB rates of 48% and 56% at 6 months (50, 51); median OS in these seminal trials was 24 and 13 months. More recently, novel MAb-based HER-2-directed therapies were approved for metastatic HER-2+ breast cancer progressing on prior trastuzumab therapy (2). Concurrent pertuzumab and trastuzumab therapy is associated with a CBR of 50%, and a median PFS of 5.5 months (52) and ado-trastuzumab emtansine therapy is associated with a median PFS and OS of 9.6 months and 30.9 months (53).
In conclusion, this allogeneic GM-CSF-secreting breast tumor vaccine is safe, bioactive, and confers clinical benefit when sequenced with low-dose CY and standard trastuzumab in HER-2+ MBC patients. It induces HER-2-specific immunity as measured by DTH and polyfunctional CD8+ T cells, two measures of a high quality immune response. We observed a CB rate of 55% at 6 months, with PFS and OS of 7 and 42 months. Since this study began, two additional MAb-based therapies with survival benefit were approved for metastatic HER-2+ breast cancer (pertuzumab and ado-trastuzumab emtansine). The data presented here are encouraging relative to results with other HER-2-directed combination therapies, including these new agents. Further investigation of GM-CSF-secreting vaccines in combination with low dose CY and HER-2-specific MAb therapies is warranted.
ACKNOWLEDGEMENTS
We thank Mark Greene at the University of Pennsylvannia for providing the 7.16.4 hybridoma. Presented in part at the 47th annual meeting of the American Society of Clinical Oncology, June 3-7, 2011.
Financial Support: This work was supported by Department of Defense Clinical Translational Research Award W81XWH-07-1-0485 (LE), Genentech, Incorporated (LE), and in part by American Cancer Society RSG CCE 112685 (LE), the Specialized Programs in Research Excellence (SPORE) in Breast Cancer P50CA88843 (ND, EJ, and LE), the Rapid Access to Investigational Drugs (RAID) Program of the National Cancer Institute/National Institutes of Health (LE). It was also supported by the Gateway Foundation (LE), the Safeway Foundation (LE), Avon Foundation (EJ and LE), and in part by the Johns Hopkins University Institute for Clinical and Translational Research, Climb for Hope, and the Josephine A. Peiser Foundation. Dr. Elizabeth Jaffee is the Dana and Albert “Cubby” Professor of Oncology.
Footnotes
Conflict of interest: Dr. Emens receives research funding from Genentech, Incorporated. Dr. Emens and Dr. Jaffee receive research funding from Roche, Incorporated. Under a licensing agreement between Aduro, Incorporated and the Johns Hopkins University, the University and Dr. Jaffee are entitled to milestone payments and royalty on sales of the GM-CSF-secreting breast cancer vaccine. The terms of these arrangements are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
REFERENCES
- 1.Davidson NE. HER2-targeted therapies: how far we’ve come--and where we’re headed. Oncology (Williston Park) 2011;25:425–6. [PubMed] [Google Scholar]
- 2.Jelovac D, Emens LA. HER-2-directed therapy for metastatic breast cancer. Oncology (Williston Park) 2013;27:1091–100. [PubMed] [Google Scholar]
- 3.Emens LA. Breast cancer immunobiology driving immunotherapy: vaccines and immune checkpoint blockade. Expert Rev Anticancer Ther. 2012;12:1597–611. doi: 10.1586/era.12.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dranoff G, Jaffee E, Lazenby A, Glumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta R, Emens LA. GM-CSF-secreting vaccines for solid tumors: moving forward. Disc Med. 2010;10:52–60. [PMC free article] [PubMed] [Google Scholar]
- 6.van den Eertwigh AJ, Versluis A, van den Berg HP, Santegoets S, van Moorselaar RJ, van der Sluis TM, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase I dose escalation trial. Lancet Oncol. 2012;13:509–17. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 7.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–9. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, et al. A lethally irradiated allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma: a phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–35. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emens LA. Re-purposing cancer therapeutics for breast cancer immunotherapy. Cancer Immunol Immunother. 2012;61:1299–305. doi: 10.1007/s00262-012-1247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of a granulocyte-macrophage colony-stimulating factor-secreting whole cell vaccine in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–97. [PubMed] [Google Scholar]
- 11.Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–8. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, et al. Recruitment of latent pools of high-avidity CD8+ T cells to the antitumor immune response. J Exp Med. 2005;201:1591–602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss VL, Lee TH, Song H, Kouo TS, Black CM, Sqouros G, et al. Trafficking of high avidity HER-2/neu-specific T cells into HER-2-expressing tumors after depletion of effector/memory-like regulatory T cells. PLoS One. 2012;7:e31962. doi: 10.1371/journal.pone.0031962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emens LA, Davidson NE. Trastuzumab in breast cancer. Oncology (Williston Park) 2004;18:1117–28. [PubMed] [Google Scholar]
- 15.Wolpoe ME, Lutz ER, Ercolini AM, Murata S, Ivie SE, Garrett ES, et al. HER-2/neu-specific monoclonal antibodies collaborate with HER-2/neu-targeted granulocyte-macrophage colony-stimulating factor-secreting whole cell vaccination to augment CD8+ T cell effector function and tumor-free survival in HER-2/neu transgenic mice. J Immunol. 2003;171:2161–9. doi: 10.4049/jimmunol.171.4.2161. [DOI] [PubMed] [Google Scholar]
- 16.Kim PS, Armstrong TD, Song H, Wolpoe ME, Weiss V, Manning EA, et al. Antibody association with HER-2/neu-targeted vaccine enhances CD8 T cell responses in mice through Fc-mediated activation of DCs. J Clin Invest. 2008;188:1700–11. doi: 10.1172/JCI34333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor C, Hershman D, Shah N, Suciu-Foca N, Petrylak DP, Taub R, et al. Augmented HER-2-specific immunity during treatment with Trastuzumab and chemotherapy. Clin Cancer Res. 2007;13:5133–43. doi: 10.1158/1078-0432.CCR-07-0507. [DOI] [PubMed] [Google Scholar]
- 18.Ladoire S, Arnould L, Mignot G, Apetoh L, Rebe C, Martin F, et al. T-bet expression in intratumoral lymphoid structures after neoadjuvant trastuzumab plus docetaxel for HER-2-overexpressing breast carcinoma predicts survival. Br J Cancer. 2011;105:366–71. doi: 10.1038/bjc.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guy CT, Webster MA, Schaller M, Parstons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–82. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reilly RT, Gottlieb MB, Ercolini AM, Machiels JP, Kane CE, Okoye FL, et al. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res. 2000;60:3569–76. [PubMed] [Google Scholar]
- 21.Zhang H, Wang Q, Montone KT, Peavey JE, Drebin JA, Greene MI, et al. Shared antigenic epitopes and pathobiological functions of anti-p185 (HER-2/neu) monoclonal antibodies. Exp Mol Pathol. 1999;67:15–25. doi: 10.1006/exmp.1999.2266. [DOI] [PubMed] [Google Scholar]
- 22.Manning EA, Ullman JGM, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res. 2007;13:3951–9. doi: 10.1158/1078-0432.CCR-07-0374. [DOI] [PubMed] [Google Scholar]
- 23.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 24.Davis-Sproul JM, Harris MP, Davidson NE, Kobrin BJ, Jaffee EM, Emens LA. Cost-effective manufacturing of an allogeneic GM-CSF-secreting breast tumor vaccine in an academic cGMP facility. Cytotherapy. 2005;7:46–56. doi: 10.1080/14653240510018082. [DOI] [PubMed] [Google Scholar]
- 25.Disis ML, Schiffman K, Gooley TA, McNeel DG, Rinn K, Knutson KL. Delayed-type hypersensitivity response is a predictor of peripheral blood T cell immunity after HER-2/neu peptide immunization. Clin Cancer Res. 2000;6:1347–50. [PubMed] [Google Scholar]
- 26.Yao X, Ahmadzdeh M, Liu F, Schrump DS, Steinberg SM, Rosenberg SA, et al. Levels of peripheral CD4+FoxP3+ regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood. 2012;119:5688–96. doi: 10.1182/blood-2011-10-386482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin Y, Gallardo HF, Ku GY, Li H, Manukian G, Rasalan TS, et al. Optimization and validation of a robust human T cell culture method for monitoring phenotypic and polyfunctional antigen-specific CD4+ and CD8+ T cell responses. Cytotherapy. 2009;11:912–22. doi: 10.3109/14653240903136987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dauer M Obermaier B, Herten J, Haerle C, Pohl K, Rothenfusser S, et al. Mature dendritic cells derived from human monocytes within 48 hours: a novel strategy for dendritic cell differentiation from blood precursors. J Immunol. 2003;170:4069–76. doi: 10.4049/jimmunol.170.8.4069. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 32.Brookmeyer R, Crowley J. A K-sample median test for censored data. J Am Stat Assoc. 1982;77:433–40. [Google Scholar]
- 33.Disis ML, Wallace DR, Gooley TA, Dang Y, Slota M, Lu H, et al. Concurrent Trastuzumab and HER-2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27:4685–92. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:45–56. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 35.Hsueh EC, Essner R, Foshag LJ, Ollila DW, Gammon G, O’Day SJ, et al. Prolonged survival after complete resection of disseminated melanoma and active immunotherapy with a therapeutic cancer vaccine. J Clin Oncol. 2002;20:4549–54. doi: 10.1200/JCO.2002.01.151. [DOI] [PubMed] [Google Scholar]
- 36.Lopez MN, Pereda C, Segal G, Munoz L, Aquilera R, Gonzalez FE, et al. Prolonged survival of dendritic cell-vaccinated melanoma patients correlates with tumor-specific delayed type hypersensitivity response and reduction of tumor growth factor beta-expressing T cells. J Clin Oncol. 2009;27:945–52. doi: 10.1200/JCO.2008.18.0794. [DOI] [PubMed] [Google Scholar]
- 37.Barth RJ, Jr, Fisher DA, Wallace PK, Channon JY, Noelle RJ, Gui J, et al. A randomized trial of ex vivo CD40L activation of a dendritic cell vaccine in colorectal cancer patients: tumor-specific immune responses are associated with improved survival. Clin Cancer Res. 2010;16:5548–56. doi: 10.1158/1078-0432.CCR-10-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harari A, Dutoit V, Cellerai C, Bart PA, Du Pasquier RA, Pantaleo G. Functional signatures of protective antiviral T-cell immunity in viral infections. Immunol Rev. 2006;211:236–54. doi: 10.1111/j.0105-2896.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 39.Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205:3119–31. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thakur A, Pedersen LE, Jungersen G. Immune markers and correlates of protection for vaccine-induced immune responses. Vaccine. 2012;30:4907–20. doi: 10.1016/j.vaccine.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 41.Ndhlovu ZM, Proudfoot J Cesa K, Alvino DM, McMullen A, Vine S, et al. Elite controllers with low to absent effector CD8+ T cell responses maintain highly functional, broadly directed central memory responses. J Virol. 2012;86:6959–69. doi: 10.1128/JVI.00531-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reyes-Sandoval A, Berthoud T, Alder N, Siani L, Gilbert SC, Nicosia A, et al. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T cell responses. Infect Immun. 2010;78:145–53. doi: 10.1128/IAI.00740-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kyte JA, Gaudernack G, Dueland S, Trachsel S, Julsrud L, Aamdal S. Telomerase peptide vaccination combined with temezolomide: a clinical trial in stage IV melanoma patients. Clin Cancer Res. 2011;17:4568–80. doi: 10.1158/1078-0432.CCR-11-0184. [DOI] [PubMed] [Google Scholar]
- 44.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–33. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morse MA, Hobeika AC, Osada T, Serra D, Neidzwiecki D, Lyerly HK, et al. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112:610–8. doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanka A, Szczylik C, et al. Multipeptide immune response to cancer vaccine IMA901 after single dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–61. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 47.Sevko A, Sade-Feldman M, Kanterman J, Michels T, Falk CS, Umansky L, et al. Cyclophosphamide promotes chronic inflammation-dependent immunosuppression and prevents antitumor response in melanoma. J Invest Dermatol. 2013;133:1610–9. doi: 10.1038/jid.2012.444. [DOI] [PubMed] [Google Scholar]
- 48.Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother. 2013;62:909–18. doi: 10.1007/s00262-013-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, et al. Frequencies of circulating MDSC correlated with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother. 2014;63:247–57. doi: 10.1007/s00262-013-1508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 51.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER-2 monoclonal antibody in women who have HER-2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 52.Baselga J, Gelmon KA, Verma S, Wardley A, Conte P, Miles D, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–44. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER-2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]