Abstract
PURPOSE
These days, mesenchymal stem cells (MSCs) have received worldwide attention because of their potentiality in tissue engineering for implant dentistry. The purpose of this study was to evaluate various growth inducing factors in media for improvement of acquisition of bone marrow mesenchymal stem cells (BMMSCs) and colony forming unit-fibroblast (CFU-F).
MATERIALS AND METHODS
The mouse BMMSCs were freshly obtained from female C3H mouse femur and tibia. The cells seeded at the density of 106/dish in media supplemented with different density of fetal bovine serum (FBS), 1α, 25-dihydroxyvitamin (VD3) and recombinant human epidermal growth factor (rhEGF). After 14 days, CFU-F assay was conducted to analyze the cell attachment and proliferation, and moreover for VD3, the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay was additionally conducted.
RESULTS
The cell proliferation was increased with the increase of FBS concentration (P<.05). The cell proliferation was highest at the density of 20 ng/mL rhEGF compared with 0 ng/mL and 200 ng/mL rhEGF (P<.05). For VD3, although the colony number was increased with the increase of its concentration, the difference was not statistically significant (P>.05).
CONCLUSION
FBS played the main role in cell attachment and growth, and the growth factor like rhEGF played the additional effect. However, VD3 did not have much efficacy compare with the other two factors. Improvement of the conditions could be adopted to acquire more functional MSCs to apply into bony defect around implants easily.
Keywords: Bone marrow mesenchymal stem cells, Cell proliferation, Fetal bovine serum, 1α, 25-dihydroxyvitamin, Recombinant human epidermal growth factor
INTRODUCTION
Recently, stem cell is the much-anticipated research area especially in cell-based therapeutic strategies,1 and we can obtain the stem cell from several tissues like bone marrow, umbilical cord, adipose and periodontal ligament.2,3 As already known, bone marrow derived mesenchymal stem cells (BMMSCs) have self-renewal capacity and pluripotency so that they can differentiate into other types of cells such as bone cells, cartilage cells and adipose cells.1,4,5
However, the main issue of BMMSCs is how we can get more cells effectively. The isolation and proliferation of MSCs from mouse, human and other species has been more difficult owing to the cultures contaminated by the unexpected non-MSCs for several passages.6,7 A number of techniques have been developed to isolate MSCs and improve the purity.6 Based on the technique, a novel way was introduced that change the media composition.8
Numerous factors can contribute to the cell attachment and growth. Fetal bovine serum (FBS) which is a ubiquitously used essential component for animal cell culture contains hormones, growth factors and adhesion-promoting molecules like fibronectin and vitronectin.9,10 Another interesting factor, 1α, 25-dihydroxyvitamin (VD3), regulates cell proliferation and differentiation,11 and its effects may vary with different types of cells.12 Moreover, the growth factor, like recombinant human epidermal grow factor (rhEGF) is also thought as the enhancer of the growth rate.13
However, till now, no research has completely analyzed the most suitable density of the above three factors for mBMMSC culture. The present study was therefore conducted to focus on the mBMMSCs' response to different concentration of FBS, VD3 and rhEGF in order to make the conclusion regarding which density is the best condition for the cell growth.
MATERIALS AND METHODS
Six to eight weeks specific pathogen free (SPF) grade C3H female mouse weighting 20-25 g were used in this study and fed on a standard diet. The animal husbandry and all procedures performed by the institutional animal care and use committee of the Seoul National University School of Dentistry and conducted according to National Institute of Health guideline.
As the protocol14,15 described, BMMSCs were obtained from C3H mouse femur and tibia. All study procedures were approved by the Institutional Review Board at Seoul National University School of Dentistry (IRB No. SNU-130312-1).
To isolate bone marrow, general anesthesia was done by Ketamine (100-200 mg/kg). Then, the animal skeleton was rinsed with 70% ethanol, followed by making an incision close to the hind limbs where they attach to the trunk and striping the skin by pulling toward the foot, which is cut at the anklebone. This eliminates further contact of the hind limb with the animal's fur, which is a source of contamination. Then, the hind limbs from the trunk of the body were dissected by cutting along the spinal cord with care not to damage the femur. Dissected limbs were stored on ice pack in phosphate-buffered saline (PBS, Gibco by Life Technologies, Grand Island, NY, USA) containing dish and further dissection of the hind limb was performed under the hood. Each hind limb was bisected by cutting through the knee joint. The muscle and connective tissue were removed from the tibia and the femur with forceps.14
Harvesting of the bone marrow was done in a hood using appropriate sterile technique as follows: Firstly, wipe the bone with napkin which is moist by 70% ethanol. Then, cut the ends of the tibia and femur just below the end of the marrow cavity using scissors. Insert a 27-gauge needle attached to a 10-mL syringe containing serum free media (SFM)-(without FBS) into the cut end of the bone. The reason for using SFM is to except the interference of FBS for we test the effects of different density of FBS. Flush the marrow plug out of the cut end of the bone with SFM and collect into a 15-mL tube on ice. Filter the cell suspension through a 70 µm nylon filter mesh (BD Falcon, BD Biosciences-Discovery Labware, Frankin Lakes, NJ, USA) to remove any bone trivial debris or muscle and cell clumps.14 The cells were treated with ACK lysing buffer (Lonza, Wakersville, MD, USA) for 5 min and centrifuged at 1500 rpm (4℃) for 5 min so that the interference of other cells especially hematopoietic cell can be avoided. The yield and viability of cells were determined by Trypan blue (Gibco by Life Technologies, Grand Island, NY, USA) and the cell number was counted using hemocytometer. The mBMMSCs were primary seeded at a density of 106 in 60 mm2 dish and cultured in different conditional media: (1) alpha Minimum Essential Medium (α-MEM)(Gibco by Life Technologies, Grand Island, NY, USA) supplemented with different density (0%, 1%, 5%, 10%, 20%) of fetal bovine serum (FBS)(Equitech-Bio, Inc., Kerrville, Texas, USA), 1% Antibiotic-Antimycotic (Gibco by Life Technologies, Grand Island, NY, USA), 1% L-glutaMAX (Gibco by Life Technologies, Grand Island, NY, USA), 0.1% 2-mercaptoethanol (Gibco by Life Technologies, Grand Island, NY, USA); (2) alpha Minimum Essential Medium (α-MEM) supplemented with 10% FBS media combined with different density (0 nM, 1 nM, 10 nM, 100 nM) of 1α, 25-dihydroxyvitamin (VD3)(Cayman Chemical, Ann Arbor, MI, USA), 1% Antibiotic-Antimycotic, 1% L-glutaMAX, 0.1% 2-mercaptoethanol; (3) alpha Minimum Essential Medium (α-MEM) supplemented with 10% FBS media combined with different density (0 ng/mL, 20 ng/mL, 200 ng/mL) of recombinant human epidermal growth factor (rhEGF) (Prospec, East Brunswick, NJ, USA), 1% Antibiotic-Antimycotic, 1% L-glutaMAX, 0.1% 2-mercaptoethanol. The cells were incubated at 37℃ in 95% humidified air and 5% CO2.
After 72 hours, non-adherent cells were removed by washing with phosphate-buffered saline (PBS) twice and fresh media was added.
The cells cultured in different media condition were tested for colony forming potential. 106 freshly isolated BMMSCs were seeded in each plate. After 14 days, the cultures were washed with PBS twice and stained with 1% toluidine blue solution in 2% paraformaldehyde (PFA, Wako Pure Chemical industries, Ltd., Osaka, Japan), then incubated at room temperature (RT) on rocker for overnight. A cell cluster that had more than 50 cells was counted as a colony under microscopy. All experiments were repeated in triplicate.
The proliferation rate of mBMMSCs cultured in the presence of 1α, 25-dihydroxyvitamin (VD3) was measured by 3-(4,5-dimehylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Briefly, freshly isolated mBMMSCs (104 cells each) were seeded in 96-well plastic culture plates and incubated in 200 µL α-MEM supplemented with different density (0 nM, 1 nM, 10 nM, 100 nM) of VD3 for 1 day, 4 days, 7 days, 10 days, 14 days. Cell proliferation was determined using CellTiter 96 Aqueous One Solution Reagent (Promega, Madison, WI, USA). At every point, after removal of the media, wash with PBS (twice) and add 100 µL SFM with 20 µL MTS solution and incubated for 1-4 hours at 37℃. Formazan absorbance was read at 490 nm using a plate reader and software Accent/MTS. All experiments were repeated in triplicate.
All data were expressed as mean ± SD. Differences between groups were analyzed by using the one-way ANOVA (SPSS version 21.0 for windows 7) with a post hoc Turkey test and were considered statistically significant at P-values of less than .05.
RESULTS
mBMMSCs were harvested from C3H mouse and after 72-hour culture, primary cells attached and non-attached cells were eliminated by PBS washing with media changing. The stem cells formed spindle-like shape and gradually proliferated (Fig. 1).
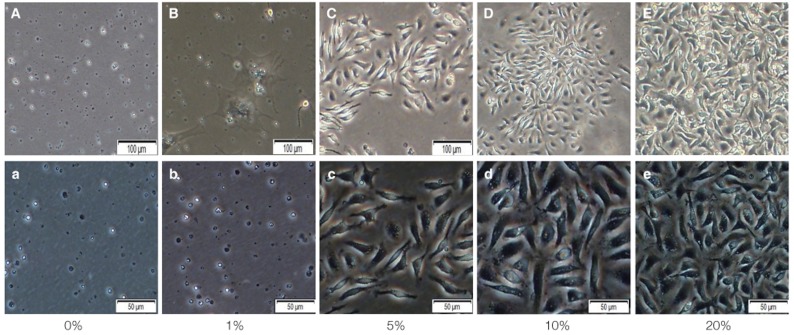
Fig. 1.
Primary isolated mBMMSCs cultured in 0%, 1%, 5%, 10%, 20% FBS medium for 14 days to do colony forming unit (CFU) assay. (A-E) show the mBMMSCs cultured in 0%, 1%, 5%, 10%, 20% FBS medium after 14 days by microscope. Magnification ×100. And (a-e) is magnification ×200.
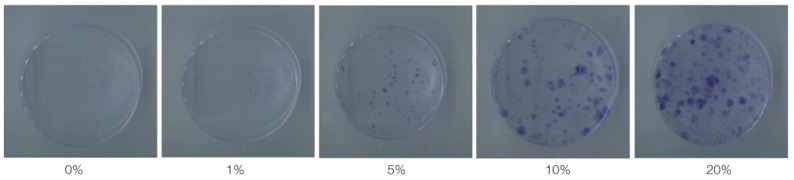
After the mBMMSCs cultured in different density of FBS media, VD3 media and EGF media for 14 days, they represented different growth rate respectively. Light microscopy (Fig. 1) showed that cells cultured in 0%, and 1% FBS gradually died and did not form the colony. With the FBS concentration increasing, the proliferation rate also improved simultaneously and in 5% FBS, the colony began to form. In 10% and 20% FBS, the cell proliferation was significantly increased and the 20% dishes had the higher proliferation than 10% dishes. The toluidine blue staining (Fig. 2) also proved the aforementioned results.
Fig. 2.
The images show the colonies stained by toluidine blue after 14 days of primary cultured in 0%, 1%, 5%, 10%, 20% FBS medium.
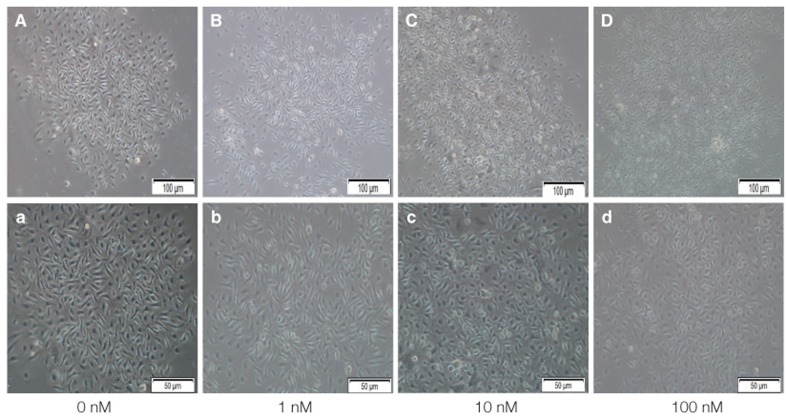
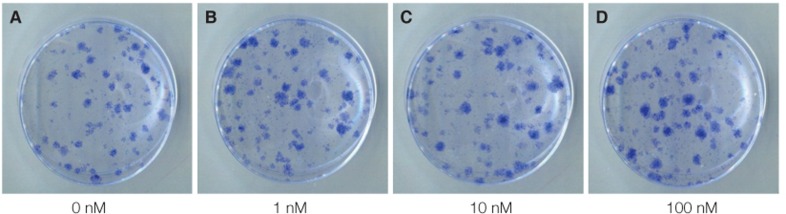
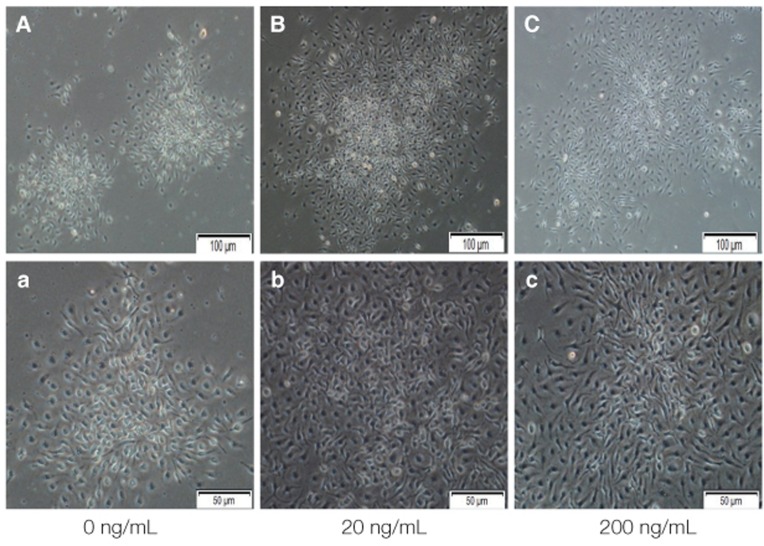
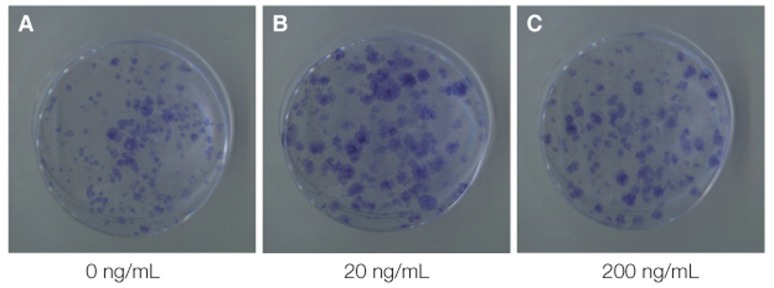
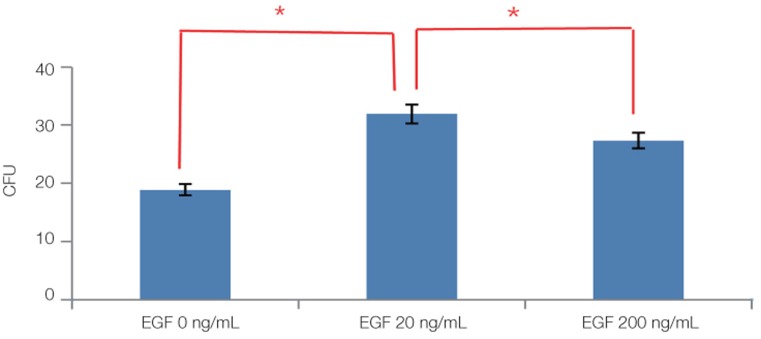
Light microscopy (Fig. 3) showed that the proliferation of cells cultured in different density of VD3. The cell number increased with increasing of VD3 concentration from 0 nM to 10 nM and the cell number did not change any more in 100 nM, but the difference was not statistically significant. The toluidine blue staining (Fig. 4) showed the same trend. Light microscopy (Fig. 5) showed that the media containing with EGF accelerates the cell growth, and the media supplemented with 20 ng/mL EGF had the highest cell proliferation compared with 0 ng/mL and 200 ng/mL EGF. The toluidine blue staining (Fig. 6) also indicated that the 20 ng/mL EGF dish had the most colony numbers compared with the other two dishes.
Fig. 3.
Primary isolated mBMMSCs cultured in 0 nM, 1 nM, 10 nM, 100 nM VD3 medium for 14 days to do colony forming unit (CFU) assay. (A-D) show the mBMMSCs cultured in 0 nM, 1 nM, 10 nM, 100 nM VD3 medium after 14 days by microscope. Magnification ×100. And (a-d) is magnification ×200.
Fig. 4.
The images show the colonies stained by toluidine blue after 14 days of primary cultured in 0 nM, 1 nM, 10 nM, 100 nM VD3 medium.
Fig. 5.
Primary isolated mBMMSCs cultured in 0 ng/mL, 20 ng/mL, 200 ng/mL EGF medium for 14 days to do colony forming unit (CFU) assay. (A-C) show the mBMMSCs cultured in 0 ng/mL, 20 ng/mL, 200 ng/mL EGF medium after 14 days by microscope. Magnification ×100. And (a-c) is magnification ×200.
Fig. 6.
The images show the colonies stained by toluidine blue after 14 days of primary cultured in 0 ng/mL, 20 ng/mL, 200 ng/mL EGF medium.
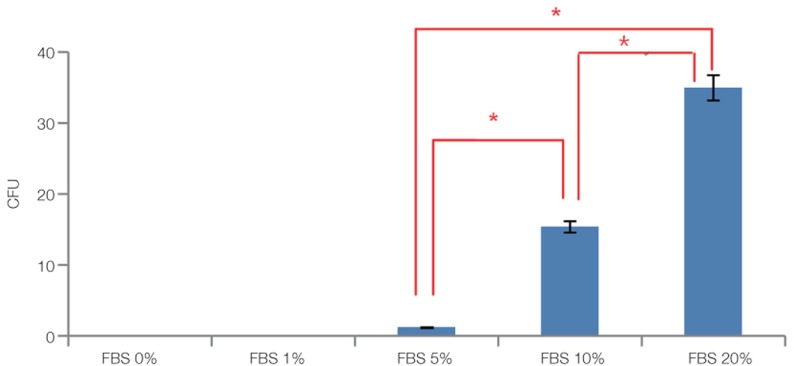
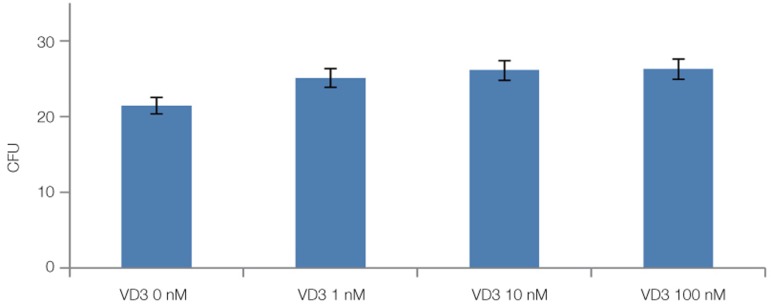
Fig. 7, Fig. 8 and Fig. 9 showed the statistical results of colony number after staining. Fig. 7 showed that the cell proliferation of 20% FBS was significantly higher than that of 10% FBS and 5% FBS (P<.05). Fig. 8 showed that the difference between the growth in 0 nM, 1 nM, 10 nM and 100 nM VD3 was not statistically significant (P>.05). Fig. 9 showed that compared with remaining two groups, the cells cultured in 20 ng/mL EGF exhibited significant increase in cell viability (P<.05).
Fig. 7.
The results of colony forming unit assay for 0%, 1%, 5%, 10%, 20% FBS after 14 days of primary cultured. The data are expressed as the mean ± SD of triplicate determinations (*P<.05).
Fig. 8.
The results of colony forming unit assay for 0 nM, 1 nM, 10 nM, 100 nM VD3 after 14 days of primary cultured. The data are expressed as the mean ± SD of triplicate determinations (P>.05).
Fig. 9.
The results of colony forming unit assay for in 0 ng/mL, 20 ng/mL, 200 ng/mL EGF after 14 days of primary cultured. The data are expressed as the mean ± SD of triplicate determinations (*P<.05).
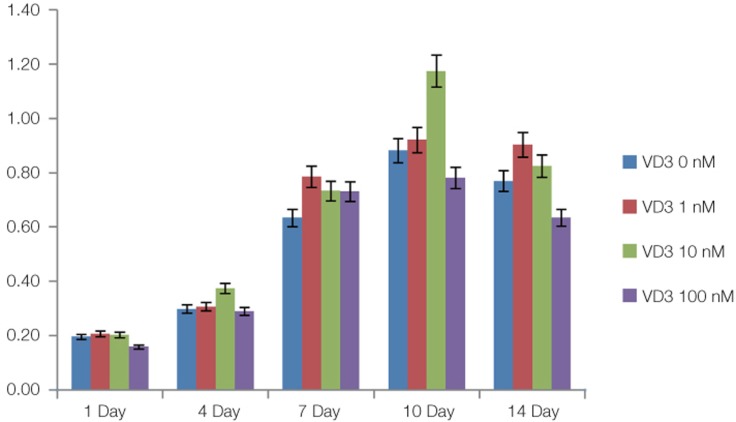
The additional proliferation assay was conducted to further analyze the function of VD3 on mBMMSC. MTS result (Fig. 10) showed that the cell increased over time. At every point, the VD3 did not play the significant effect (P>.05). At day 10, the 10 nM VD3 effect was apparently higher than any other group. But, at day 14, the cell growth rate was all decreased. None specific consequent achieved from this study.
Fig. 10.
Primary isolated mBMMSCs cultured in 0 nM, 1 nM, 10 nM, 100 nM VD3 medium for 14 days. The results of MTS assay for VD3 after 1 day, 4 days, 7 days, 10 days and 14 days of primary cultured. The data are expressed as the mean ± SD of triplicate determinations (P>.05).
DISCUSSION
In this study, we described cellular response to the culture media containing different density of FBS, VD3 and rhEGF. There are several benefits of doing this experiment. First, since the mouse bone marrow mesenchymal stem cell is still difficult to many researchers,7 in this study, we introduced a novel way of supplementing different components into the culture media so that the primary isolated cell number and cell growth rate can be increased.
Previous studies only paid attention to modify the isolation protocol such as employing a centrifugation step,16 the method of plastic adherence,17 immunodepletion way,18 frequent media changing, and changing the plating density.19
Additionally, we choose the FBS, VD3, rhEGF which are very common and can be easily obtained. Conventionally, the MSCs were cultured in FBS containing media which was considered as the basal growth medium in animal cell culture.20 Tropel et al.20 indicated that when the mouse bone marrow stem cells were cultured without serum, they showed senescence signs and died in few days. Li et al.21 published the article about rat bone marrow mesenchymal stem cells cultured in different density of FBS. In the research, they used 10%, 11% and 15% FBS culture medium and finally they found that a serum concentration of 11% is preferential for bMSC propagation. Our experiment also proved some of this point. We designed 0%, 1%, 5%, 10%, 20% five different density of FBS culture medium. In our experiment, the cells cultured in 0% and 1% FBS media were almost impossible to grow, and along with adding more FBS, the cells were gradually grown more quickly and amplified. In 10% FBS media, the cell proliferation was visibly increased and 20% FBS media had stronger effects on cell growth than 10% FBS media. These results indicate that the mBMMSCs could not grow well without FBS and FBS play a pivotal role in cell culture.
1α, 25-dihydroxyvitamin D3 (VD3), the hormonally active form of vitamin D, is a member of a lipophilic family of ligands and is essential for human metabolism.22 Except for its role in calcium and skeletal homeostasis, the evidence increased about its potential for osteoblast differentiation23 and anti-proliferation.24,25 In previous study, Artaza et al.26 cultured mouse C3H 10T1/2 multipotent mesenchymal cells (MMCs) in 10 nM, 25 nM, 50 nM, 100 nM and 500 nM or without VD3 culture medium for 4 days and determined cell proliferation. Their results indicated that starting at 25 nM, VD3 induced a statistically significant reduction in cell number reaching a plateau at 100 nM. Okuno et al.12 also did the research about culturing a murine myogenic cell line C2C12 in 0 nM, 1 nM, 10 nM, 100 nM VD3 containing medium and the results showed that VD3 inhibited the proliferation of C2C12 myoblasts in a dose-dependent manner over 72 hours. So in our study, we used the VD3 to investigate its effect on mouse bone marrow stem cells. We also used 0 nM, 1 nM, 10 nM, 100 nM four different VD3 containing medium. In our study, the cell number slightly increased with increasing of VD3 density, but it no longer increased after exceeding the density of 10 nM. For a more in-depth analysis, except for colony forming unit assay, we performed another MTS assay. But the data did not reveal statistical significance which means that VD3 had little effects on mBMMSCs.
In previous research, several growth factors have been introduced in stem cell culture like VEGF, FGF-2, PDGF and EGF.20,27,28,29 In our study, we choose the EGF for it is inexpensive and easy to manipulate.28 Very recently, You et al.30 did the research about whether epidermal growth factor gene-transfected mesenchymal stem cells (EGF-MSC) would accelerate fibroblast migration and proliferation. The study suggested that EGF increased expression of cell adhesion molecules and had a positive influence on cell migration and proliferation. In another study, Bressan et al.31 used 20 ng.mL-1 EGF supplemented with 40 ng. mL-1 FGF2 to culture mouse epidermal neural crest stem cells (EPI-NCSCs) for 7 days. The results suggest that the combination of EGF-FGF2 stimulates the proliferation and improves the neuronal potential of EPI-NCSCs. In order to explore if EGF alone can has the same stimulating effect to cell growth, we planned this study and the results of our study were in consistent with previous studies. In our study, we used 0 ng/mL, 20 ng/mL, 200 ng/mL EGF, then the EGF represented the promoting role indeed and revealed 20 ng/mL was the best condition for cells.
All these experiments possessed to aim at getting more cells at the initial stage. After treated by three different media, the cells showed different reactions. Therefore, this study can be utilized as the foundation for the future experiments.
CONCLUSION
Collectively, the results of this study indicate that FBS play the main role in primary cell acquisition and proliferation. Growth factors like rhEGF just have the additional effects and VD3 has little effect on cell proliferation. Based on this study, people can do extensive research about changing other media components to increase primary isolated cell number and cell proliferation.
Footnotes
This study was supported by a grant (2011-434) from the Asan Institute for Life Sciences, Seoul, Korea and National Research Foundation of Korea grant funded by the Korea government (No. 2011-0028067), and by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A120304).
References
- 1.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez-Lozano FJ, Insausti CL, Iniesta F, Blanquer M, Ramírez MD, Meseguer L, Meseguer-Henarejos AB, Marín N, Martínez S, Moraleda JM. Mesenchymal dental stem cells in regenerative dentistry. Med Oral Patol Oral Cir Bucal. 2012;17:e1062–e1067. doi: 10.4317/medoral.17925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 6.Nadri S, Soleimani M, Hosseni RH, Massumi M, Atashi A, Izadpanah R. An efficient method for isolation of murine bone marrow mesenchymal stem cells. Int J Dev Biol. 2007;51:723–729. doi: 10.1387/ijdb.072352ns. [DOI] [PubMed] [Google Scholar]
- 7.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 8.Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- 9.Hayman EG, Pierschbacher MD, Suzuki S, Ruoslahti E. Vitronectin--a major cell attachment-promoting protein in fetal bovine serum. Exp Cell Res. 1985;160:245–258. doi: 10.1016/0014-4827(85)90173-9. [DOI] [PubMed] [Google Scholar]
- 10.Jochems CE, van der Valk JB, Stafleu FR, Baumans V. The use of fetal bovine serum: ethical or scientific problem? Altern Lab Anim. 2002;30:219–227. doi: 10.1177/026119290203000208. [DOI] [PubMed] [Google Scholar]
- 11.Miyaura C, Abe E, Kuribayashi T, Tanaka H, Konno K, Nishii Y, Suda T. 1 alpha,25-Dihydroxyvitamin D3 induces differentiation of human myeloid leukemia cells. Biochem Biophys Res Commun. 1981;102:937–943. doi: 10.1016/0006-291x(81)91628-4. [DOI] [PubMed] [Google Scholar]
- 12.Okuno H, Kishimoto KN, Hatori M, Itoi E. 1α,25-dihydroxyvitamin D3 enhances fast-myosin heavy chain expression in differentiated C2C12 myoblasts. Cell Biol Int. 2012;36:441–447. doi: 10.1042/CBI20100782. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda N, Morita N, Matsuda K, Watanabe M. Proliferation and differentiation of human osteoblastic cells associated with differential activation of MAP kinases in response to epidermal growth factor, hypoxia, and mechanical stress in vitro. Biochem Biophys Res Commun. 1998;249:350–354. doi: 10.1006/bbrc.1998.9151. [DOI] [PubMed] [Google Scholar]
- 14.Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4:102–106. doi: 10.1038/nprot.2008.221. [DOI] [PubMed] [Google Scholar]
- 15.Zhu H, Guo ZK, Jiang XX, Li H, Wang XY, Yao HY, Zhang Y, Mao N. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 2010;5:550–560. doi: 10.1038/nprot.2009.238. [DOI] [PubMed] [Google Scholar]
- 16.Dobson KR, Reading L, Haberey M, Marine X, Scutt A. Centrifugal isolation of bone marrow from bone: an improved method for the recovery and quantitation of bone marrow osteoprogenitor cells from rat tibiae and femurae. Calcif Tissue Int. 1999;65:411–413. doi: 10.1007/s002239900723. [DOI] [PubMed] [Google Scholar]
- 17.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–5188. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- 18.Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003;89:1235–1249. doi: 10.1002/jcb.10594. [DOI] [PubMed] [Google Scholar]
- 19.Cholewa D, Stiehl T, Schellenberg A, Bokermann G, Joussen S, Koch C, Walenda T, Pallua N, Marciniak-Czochra A, Suschek CV, Wagner W. Expansion of adipose mesenchymal stromal cells is affected by human platelet lysate and plating density. Cell Transplant. 2011;20:1409–1422. doi: 10.3727/096368910X557218. [DOI] [PubMed] [Google Scholar]
- 20.Tropel P, Noël D, Platet N, Legrand P, Benabid AL, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004;295:395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Zhang Y, Qi G. Evaluation of isolation methods and culture conditions for rat bone marrow mesenchymal stem cells. Cytotechnology. 2013;65:323–334. doi: 10.1007/s10616-012-9497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue ML, Zhu H, Thakur A, Willcox M. 1 alpha,25-Dihydroxyvitamin D3 inhibits pro-inflammatory cytokine and chemokine expression in human corneal epithelial cells colonized with Pseudomonas aeruginosa. Immunol Cell Biol. 2002;80:340–345. doi: 10.1046/j.1440-1711.80.4august.1.x. [DOI] [PubMed] [Google Scholar]
- 23.Finch JL, Dusso AS, Pavlopoulos T, Slatopolsky EA. Relative potencies of 1,25-(OH)(2)D(3) and 19-Nor-1,25-(OH)(2) D(2) on inducing differentiation and markers of bone formation in MG-63 cells. J Am Soc Nephrol. 2001;12:1468–1474. doi: 10.1681/ASN.V1271468. [DOI] [PubMed] [Google Scholar]
- 24.Banerjee P, Chatterjee M. Antiproliferative role of vitamin D and its analogs-a brief overview. Mol Cell Biochem. 2003;253:247–254. doi: 10.1023/a:1026072118217. [DOI] [PubMed] [Google Scholar]
- 25.Kommagani R, Whitlatch A, Leonard MK, Kadakia MP. p73 is essential for vitamin D-mediated osteoblastic differentiation. Cell Death Differ. 2010;17:398–407. doi: 10.1038/cdd.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Artaza JN, Sirad F, Ferrini MG, Norris KC. 1,25(OH)2vitamin D3 inhibits cell proliferation by promoting cell cycle arrest without inducing apoptosis and modifies cell morphology of mesenchymal multipotent cells. J Steroid Biochem Mol Biol. 2010;119:73–83. doi: 10.1016/j.jsbmb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, Um S, Jang JH, Seo BM. Effects of VEGF and FGF-2 on proliferation and differentiation of human periodontal ligament stem cells. Cell Tissue Res. 2012;348:475–484. doi: 10.1007/s00441-012-1392-x. [DOI] [PubMed] [Google Scholar]
- 28.Tamama K, Fan VH, Griffith LG, Blair HC, Wells A. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:686–695. doi: 10.1634/stemcells.2005-0176. [DOI] [PubMed] [Google Scholar]
- 29.Lucarelli E, Beccheroni A, Donati D, Sangiorgi L, Cenacchi A, Del Vento AM, Meotti C, Bertoja AZ, Giardino R, Fornasari PM, Mercuri M, Picci P. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials. 2003;24:3095–3100. doi: 10.1016/s0142-9612(03)00114-5. [DOI] [PubMed] [Google Scholar]
- 30.You DH, Nam MJ. Effects of human epidermal growth factor gene-transfected mesenchymal stem cells on fibroblast migration and proliferation. Cell Prolif. 2013;46:408–415. doi: 10.1111/cpr.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bressan RB, Melo FR, Almeida PA, Bittencourt DA, Visoni S, Jeremias TS, Costa AP, Leal RB, Trentin AG. EGF-FGF2 stimulates the proliferation and improves the neuronal commitment of mouse epidermal neural crest stem cells (EPINCSCs) Exp Cell Res. 2014;327:37–47. doi: 10.1016/j.yexcr.2014.05.020. [DOI] [PubMed] [Google Scholar]