Abstract
Bile acids (BA) are actively reabsorbed in the terminal ileum by the apical Na+-dependent bile salt transporter. This review addresses the epidemiology, pathophysiology, diagnosis and treatment of BA diarrhea (BAD). BAD is typically caused by ileal resection or disease; 25–33% of patients with chronic functional diarrhea or irritable bowel syndrome-diarrhea (IBS-D) have BAD, possibly from deficiency in the ileal hormone, FGF-19, which normally provides feedback inhibition of BA synthesis. Diagnosis of BAD is typically based on reduced BA retention of radiolabeled BA (75SeHCAT), increased BA synthesis (serum C4) or increased fecal BA loss. In clinical practice, diagnosis is often based on response to BA sequestrants (e.g., cholestyramine or colesevelam). Diagnostic tests for BA malabsorption (BAM) need to be used more extensively in clinical practice. In the future, farnesoid X receptor agonists that stimulate ileal production of FGF-19 may be alternative treatments of BAD.
Keywords: ASBT, CDCA, colesevelam, DCA, FGF-19, FXR, IBAT, obeticholic acid, secretion, sequestrants
Introduction: bile acids & enterohepatic circulation
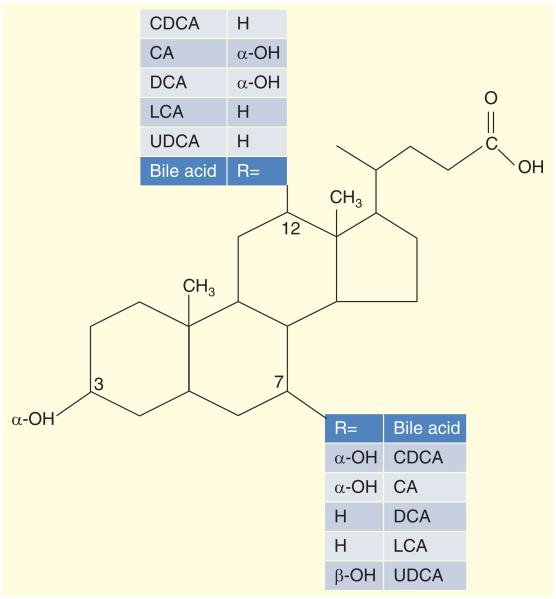
Bile acids (BAs) are detergent molecules [1], excreted from the liver and are responsible for solubilization of the lipolysis products of triglycerides that are fatty acids and monoglycerides, aiding digestion and lipid absorption in the small intestine. BA species are differentiated by their hydroxylation and conjugation status. Chenodeoxycholic acid (CDCA) and cholic acid (CA) are primary BAs (Figure 1) synthesized in the liver from cholesterol [2] and conjugated with taurine and glycine; in the colon, bacteria deconjugate and dehydroxylate the BAs.
Figure 1. Bile acid chemistry: chenodeoxycholic acid, cholic acid, deoxycholic acid, lithocholic acid and ursodeoxycholic acid.
CA: Cholic acid; CDCA: Chenodeoxycholic acid; DCA: Deoxycholic acid; LCA: Lithocholic acid; UDCA: Ursodeoxycholic acid.
Reproduced with pemission from [83].
Taurine or glycine conjugation of the BAs permits the latter to remain ionized in the duodenum, and ionization increases their solubility and renders BAs impermeable to cell membranes, allowing a high enough concentration of BAs to reach the critical micellar concentration, allowing for spontaneous formation of micelles. In the micelles, the polar BAs surround fatty acids and monoglycerides (which are insoluble in the aqueous phase) and can present the hydrophobic fat molecules to the brush border membrane of the small intestine for digestion and absorption. The colonic bacteria avidly deconjugate and dehydroxylate BAs; therefore, the major proportion of fecal BAs consists of the deconjugated secondary BAs, deoxycholic acid (DCA) and lithocholic acid (LCA), with only minor percentages of CDCA and CA [3].
A functional enterohepatic circulation (Figure 2) reabsorbs approximately 95% of BAs in the terminal ileum [4] and transports the BAs back to the liver. The apical Na+-dependent bile salt transporter (ASBT) (also called ileal BA transporter [IBAT] or SLC10A2 [solute carrier family 10, member two]) is responsible for the active reuptake of BAs in the terminal ileum. The molecular mechanisms involved in the enterohepatic circulation are summarized elsewhere [5] and illustrated in Figures 3 & 4. Intestinal BA uptake has direct and indirect impact on hepatic BA homeostasis. Farnesoid X receptor (FXR) is expressed in ileal enterocytes and hepatocytes. BAs are agonists of the FXR, which modulates gene transcription acting in concert with another nuclear receptor, the retinoid X receptor alpha (RXRα). BAs exit the enterocyte via the OSTα/β transporter. Sensing of the enterocyte BA pool by FXR affects the liver by way of the endocrine factor FGF19. FGF19 is released to the portal circulation and activates FGF receptor 4 in hepatocytes and in a process that involves interaction with klotho β on the hepatocyte membrane, results in downregulation of cholesterol 7α-hydroxylase (CYP7A1) and therefore inhibition of the classical BA synthetic pathway, both by small heterodimer partner (SHP) induction and possibly other pathways. Details of ileal feedback regulation of hepatocyte synthesis [5] and the hepatocellular formation of bile [6] are provided in Figures 2 & 3. In cholangiocytes, as in enterocytes, the ASBT, mediates bile salt uptake across the luminal membrane. Ductular bile is modified by chloride channels such as the cystic fibrosis transmembrane conductance regulator (CFTR) and the chloride/bicarbonate exchanger (anion exchanger 2, AE2) [7]. Further details of the major BA transporters in the enterohepatic circulation [8] are shown in Figure 4.
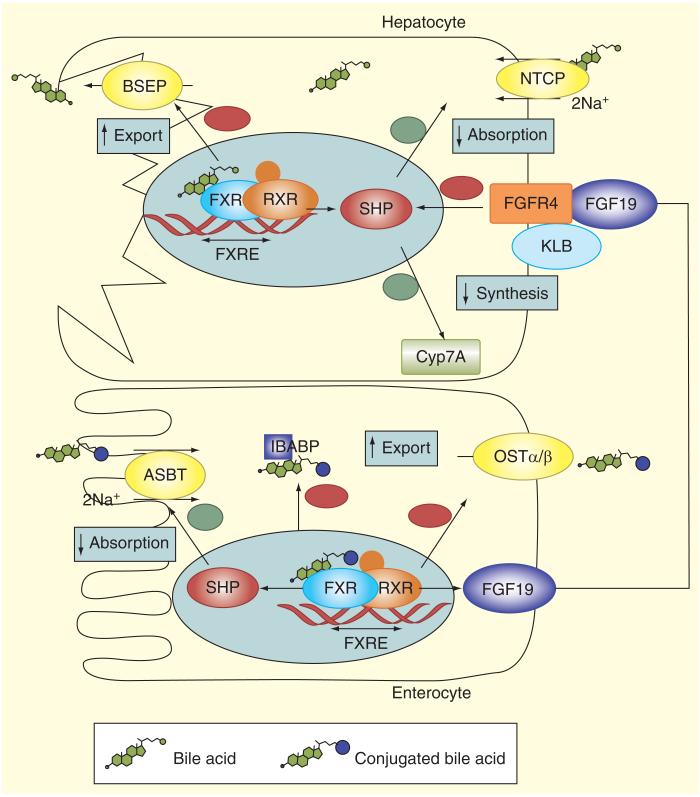
Figure 2. Ileal feedback regulation of bile acid synthesis in hepatocytes.
Intestinal BA uptake has direct and indirect impact on hepatic BA homeostasis. FXR is expressed in ileal enterocytes and hepatocytes. BAs are agonists of the FXR, which modulates gene transcription acting in concert with another nuclear receptor, the retinoid X receptor α (RXRα). BAs exit the enterocyte via the OSTα/β transporter. Sensing of the enterocyte BA pool by FXR affects the liver by way of the endocrine factor FGF19. FGF19 is released to the portal circulation, binds to FGF receptor 4 in hepatocytes, and in a manner that involves biochemical interaction with klotho β, results in downregulation of CYP7A1. This results in inhibition of the classical BA synthetic pathway, both by SHP induction and possibly other pathways. In hepatocytes FXR increases expression of the BSEP and downregulates the expression of NTCP and CYP7A. Since NTCP and BSEP mediate BA uptake from blood and export to bile in hepatocytes and CYP7A catalyzes the limitingrate step in the classical BA biosynthetic pathway, this leads to reduced BA uptake, decreased synthesis and enhanced export to bile.
BA: Bile acid; BSEP: Bile salt export pump; FXR: Farnesoid X receptor; NICP: Na+/taurocholate co-transporting polypeptide transporter; SHP: Small heterodimer partner.
Reproduced with permission [5].
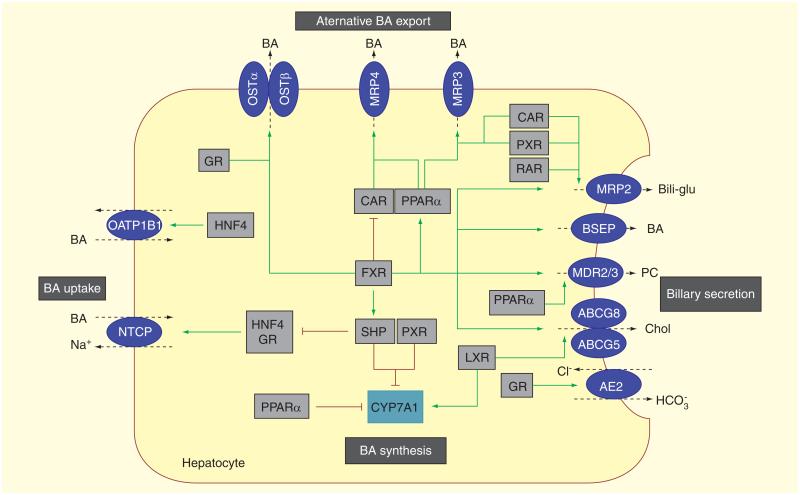
Figure 3. Hepatocellular bile formation: expression of hepatobiliary transporters in hepatocytes determines hepatic bile acid flux and hepatocellular concentrations.
To ensure the balance between synthesis, uptake and excretion, expression of hepatobiliary transporters is tightly regulated by nuclear receptors that provide negative feedback and positive feed-forward mechanisms to control of intracellular concentration of biliary constituents. Several nuclear receptor pathways also influence CYP7A1, the rate limiting enzyme in BA synthesis. (A) Bile acid-activated FXR is a central player that has several effects: first, FXR represses (via interaction with HNF4 in rats and GR in humans) hepatic BA uptake NTCP. FXR also represses the synthesis of BA by CYP7A1; this effect involves SHP. Second, FXR promotes bile secretion via induction of canalicular transporters BSEP, MRP2, cholesterol efflux pump, ATP-binding cassette, subfamily G, member 5/8 [ABCG5/8], MDR3). Third, FXR induces BA elimination via alternative export systems at the hepatic basolateral (sinusoidal) membrane OSTα/β. (B)Other nuclear receptors, CAR and PXR, facilitate adaptation to increased intracellular BA concentrations by upregulation of alternative hepatic export routes (MRP 3 and MRP4) and induction of detoxification enzymes (not shown). Together with RAR, these receptors also regulate the canalicular expression of MRP2. (C) The cholesterol sensor, (LXR, promotes biliary cholesterol excretion via ABCG5/8. Stimulation of the expression of AE2 by GR stimulates biliary bicarbonate secretion thus reducing bile toxicity. Green arrows indicate stimulatory and red lines suppressive effects on target genes. In addition to these transcriptional mechanisms, post-transcriptional processes (e.g., vesicular targeting of transporters to the membrane, phosphorylation of transport proteins) and modification of the bile through cholangiocytes (e.g., bicarbonate secretion) also play an important role in bile formation (not shown). AE2: Anion exchanger 2; BSEP: Bile salt export pump; Bili-glu: Bilirubin glucuronide; BSEP: Bile salt export pump; CAR: Constitutive androstane receptor; CYP7A1: Cholesterol-7a-hydroxylase; FXR: Farnesoid X receptor; GR: Glucocorticoid receptor; HNF4: Hepatocyte nuclear factor 4; LXR: Liver X receptor;MDR3: Multidrug resistance3; MRP: Multidrug resistance-associated protein; NTCP: Na+-dependent taurocholate co-transporting polypeptide; OSTα/β: Organic solute transporter alpha and beta; PC: Phosphatidylcholine; PXR: Pregnane X receptor; RAR: Retinoic acid receptor; SHP: Small heterodimer partner.
Reproduced with permission from [6].
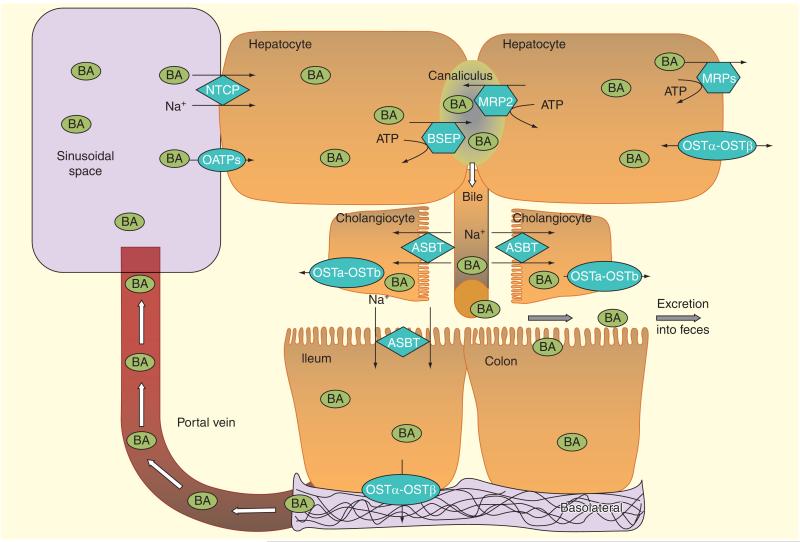
Figure 4. The major bile acid transporters in the enterohepatic circulation.
The transport of BAs across cell membranes in different organs involved in the enterohepatic circulation is complex. Bile acid transport across the basolateral membrane into hepatocytes is mediated mainly by the NTCP and OATPs. BAs efflux across the basolateral membrane of hepatocytes may occur via the organic solute and steroid transporter (OST α-OST β) and/or the multidrug resistance-associated proteins 3 and 4 (MRP3 and MRP4). The secretion of bile acids across the hepatocyte canalicular membrane into bile occurs via two members of the ATP-binding cassette transporters: the BSEP and MRP2. BAs are delivered to the intestinal lumen through bile duct where they aid in emulsifying diet-derived monoglycerides and fatty acids. BAs are actively re-absorbed in the distal ileum (and in cholangiocytes) via Na+-dependent ASBT and are effluxed through OST α-OST β. ASBT: Apical Na+ dependent bile acid transporter; BA: Bile acids; BSEP: Bile acid export pump; NTCP: Na+-dependent taurocholate co-transporting polypeptide; OATPs: Organic anion transporting polypeptides.
Reproduced with permission from [8].
Cholerheic or BA diarrhea is thought to result predominantly from the interruption of the enterohepatic circulation [9].
Pathophysiological mechanisms of bile acid-induced diarrhea
Perfusion of BAs in the human colon results in colonic secretion of water and electrolytes [10] and induction of high amplitude propagated contractions [11]. The presence of two α hydroxyl groups at the 3, 7 (CDCA) or the 3, 12 (DCA) positions in the BA molecules is responsible for their secretory effects [12]. When the colon is exposed to an increased or decreased amount of those BAs, their presence promotes or decreases fluid and electrolyte secretion, which resembles symptoms of chronic diarrhea or constipation [2,13].
BAs induce colonic secretion by activating adenylate cyclase [14], increasing mucosal permeability as a result of their detergent effects [12] and inhibition of apical Cl−/OH− exchange by a process which is dependent on calcium ions and PI3 kinase, but not on IP3 [15]. In addition to the calcium-dependent secretion of chloride, there is recent evidence that CDCA activates CFTR via a cAMP-PKA pathway involving microtubules, implying that this occurs via a basolateral membrane receptor [16]. The effects of bile salt may also be mediated through activation of alternative mechanisms. For example, 4 mm CDC activates the enteric nervous system, at least in part, via release of 5-HT from the enterochromaffin cells [17]. This 5-HT secretory effect is inhibited by the non-selective serotonergic antagonist and methysergide, which inhibited fluid, sodium and mucus secretion in response to 5 mm CDC [18] and by the 5-HT3 antagonist, granisetron [19]. Serotonin also stimulates endotoxin translocation, increasing mucosal permeability via 5-HT3 receptors in the rat ileum [19].
BAs increase colonic contractions. However, there is a wide range of the concentrations that induce propulsive contractions in canine (>20 mm) [20] and human colon (1 mm) [11]. In humans, a relationship was found between fecal BA excretion and colonic motility; however, in those studies, 40% also had significant steatorrhea [21], and the effects on colonic motility cannot be attributed entirely to the malabsorbed BA in such situations. Moreover, the presence of significant steatorrhea is not typical for IBS or functional or osmotic diarrhea where the fecal fat is rarely above 14 g/day [13].
The inter-relationships of altered permeability, secretion, transit and motility that may result from effects of BA malabsorption (BAM) require further elucidation, especially in humans in vivo.
Epidemiology of bile acid diarrhea
Type 1 BA diarrhea is typically caused by (and therefore paradoxically secondary to) ileal disease or resection. The most common conditions associated with ileal disease or resection are, Crohn’s disease and radiation ileitis. The classical papers of Hofmann and Poley [4,22–24] described the association of ileal disease of <100 cm length in association with diarrhea; when the extent of involvement was over 100 cm, there was associated steatorrhea as a result of BA deficiency.
Type 2 BA diarrhea is currently considered diarrhea without morphological abnormalities, and it was originally regarded as idiopathic (and paradoxically ‘primary’), though, as explained below, it is now considered to result from definable abnormalities of function. Several studies have documented BAM in up to 50% of patients with chronic diarrhea or IBS with diarrhea [25–29]. In a systematic review [9], BAM was reported in 32% of patients with IBS-D type symptoms, and there was a dose-response relationship to treatment with BA binders based on severity of BAM. Moreover, up to 35% of patients with microscopic colitis and diarrhea (which presents with a phenotype that overlaps with IBS-D) [30], showed evidence of BAM [28]. However, the prevalence of BAM or BA deficiency in IBS phenotypes and their relation to physiological phenotypes such as transit, colonic motility and permeability are unclear. It has been estimated that 1% of the population of Western countries suffers from BAD [31].
A third, poorly recognized, etiology of BAD is treatment with biguanide drugs, such as metformin, most commonly in patients with Type 2 diabetes mellitus or polycystic ovary syndrome. These treatments are associated with both BAM and vitamin B12 malabsorption and diarrhea [32–34].
Malabsorption of BAs may also play a pathogenic role in patients with AIDS and diarrhea [35], though the precise mechanism is not fully elucidated, and other causes (infectious) or medications (e.g., lopinavir and ritonavir) should be considered first.
Etiopathogenesis of BA diarrhea
The cause of idiopathic BA diarrhea is incompletely understood. However, there is evidence for defective feedback inhibition of BA biosynthesis by FGF19 in BAM. FGF19 is produced in the ileum in response to BA absorption and regulates hepatic BA synthesis [36]. Walters et al. [37] reported lower serum FGF19 in patients with BAM and a significant inverse relationship between FGF19 and serum C4 (a surrogate of the rate of hepatic BA synthesis). We have confirmed inverse correlation between serum C4 and FGF19 in IBS-D (rs = −0.414; p = 0.044) [37] and IBS-C (rs = −0.371; p = 0.028) [2]. C4 levels are also significantly correlated with colonic transit [2].
Alternative mechanisms for BAD in IBS have been proposed:
Accelerated small bowel transit bypassing active BA transport in the ileum; while this is theoretically possible, it seems unlikely given the ASBT’s affinity for BA [40,41]. Thus, Sciarretta et al. measured small bowel transit using lactulose-hydrogen breath test or choledochocecal transit of intravenous 99mTc-HIDA [40]. Some patients had accelerated transit; however, as a group, there was no significant relationship between small bowel transit time and BAM [40]. In a separate study by Sadik et al., there was accelerated small bowel transit in patients with idiopathic BAM that may contribute to the development of BAM; the same report showed accelerated distal colonic transit in both males and females with idiopathic BAM, which may conceivably be the result of the BAM [41]. The potential role of accelerated small bowel transit on BAM is illustrated by the observation that one patient without ileal resection and two with ileal resection of 50 cm showed normalization of BAM on treatment with loperamide [42]. However, there was no improvement in SeHCAT retention with loperamide in those with resections >80 cm, suggesting that, in the latter cases, the accelerated transit was not as important as the lack of the active transport BA mechanism which was lost with the extensive ileal resection [42]. Similarly, diarrhea caused by chronic radiation enteritis is associated with rapid small bowel transit and BA and lactose malabsorption; loperamide slows small intestinal transit, increases BA absorption and is effective in the treatment of the diarrhea [43];
Defective BA uptake into ileal mucosal biopsies was excluded by Bajor et al. [44]. In addition, genetic mutations in ASBT are extremely rare [38]. In the view of the findings of Walters et al. that BAD may result from deficiency of ileal FGF-19 secretion into the portal circulation, studies have been conducted to explore the potential association of genetic variation in one or more of the seven proteins involved in feedback regulation of BA synthesis with the IBS-D phenotype or acceleration of colonic transit (Figure 2) [45], as discussed in the next section;
-
A fourth potential mechanism is that genetic influences that control BA mechanisms may influence gastrointestinal functions that could predispose to BA diarrhea. First, genetic variation in the Klotho B (KLB) gene and six other genes involved in BA synthesis (ASBT, FGFR4, OST-alpha, OST-beta, SHP and CYP7A1 that assessed 15 SNPs and tagSNPs) revealed significant associations of SNP rs17618244 in the KLB gene with colonic transit in IBS-D [46]. In addition, in IBS-C patients, the genotype variants of Klotho B (rs17618244) determined the dose-response effects of administered chenodeoxycholate (CDC) on the emptying rate of the ascending colon [2], suggesting that KLB variation may influence colonic response to BAM.
Second, there is a separate, membrane bound BA receptor, TGR5 or GPBAR1, a member of the G protein-coupled receptor superfamily that functions as a cell surface receptor for BA [47]. The TGR5 receptor is located on colonic epithelial cells [48] and regulates basal and cholinergic-induced secretion in rat colon [49], The TGR5 receptor is also located on cholinergic and nitrergic neurons in the colon and more proximal intestine. TGR5 influences smooth muscle contraction as well as secretion from goblet cells and L cells, which secrete physiologically important peptides, including glucagon-like peptide-1 (GLP-1), glucagon-like peptide-2 (GLP-2), peptide YY (PYY) and oxyntomodulin. Although these peptides do not appear to be involved in BAD, genetic variation in TGR5 rs11554825 [50] has been associated with immunity and inflammation [51], small bowel transit, particularly in IBS-D and with colonic transit [52]. Immune activation and altered colonic transit are recognized pathophysiological mechanisms in IIBS-D [53];
Abnormalities in BA recycling. Whereas the efficiency of ileal extraction in a single pass displayed very little variation (95–97%), it was observed that absorption efficiency per day varied widely (49–86%), implying wide variation in BA enterohepatic recycling frequency [54]. Variations in recycling frequency could explain the wide variations in BA retention observed using 75SeHCAT radioscintigraphy in patients with chronic diarrhea [55]. It could also explain the diarrhea that may follow cholecystectomy [56–58] and vagotomy [59], both of which are associated with reduced BA retention; in such patients increased recycling rate may result from the reported accelerated small bowel transit in patients following vagotomy [60]. However, such an explanation is not likely in patients with post-cholecystectomy diarrhea, since small bowel transit was not accelerated (in contrast to colonic transit) in such patients [61]; the mechanisms of diarrhea post-cholecystectomy are complex and still incompletely understood [62]; at present, the weight of evidence suggests that there is not abnormal recycling in post-cholecystectomy diarrhea.
Impact of diet & drugs on bile acid pool & composition
Both low- and high-fat diets reduce the synthesis and turnover rates of primary bile salts in humans, although probably through different mechanisms [63]. There is evidence that agonists of the FXR stimulate CYP7A1 synthesis of cholesterol and expand the hydrophobic BA pool [64]. In addition, fish oil increases BA synthesis, at least in men with hypertriglyceridemia [65]. Dietary fat can alter the gut microbiota of mice indirectly by changing the animals’ pool of BAs [66]. Milk fat stimulates the growth of B. wadsworthia which thrives in the presence of taurocholic acid [67], and taurocholic acid is thought to be produced more readily during the ingestion of milk fats [68].
About 20–30% of patients who take metformin have gastrointestinal side effects that include diarrhea and nausea [32]; the majority does not have GI symptoms. BAM, due to metformin has been detected by some investigators [69], but not by others [70]. Scarpello et al. reported BAM during metformin treatment, without an effect on orocecal transit time [69]. In that study, dietary macronutrient composition was not characterized. Investigators at Mayo Clinic [Miles, Vella, Camilleri, Unpublished Data] have recorded that metformin treatment produces an increase in 7α-hydroxy-4-cholesten-3-one (C4), a surrogate test for BAM.
Impact of the microbiome on bile acid pool & composition
The colonic microbiome is responsible for the dehydroxylation of primary BAs, cholic and chenodeoxycholic to the secondary BAs, deoxycholic and lithocholic acids. In addition, gut microbiota regulate expression of FGF-15 in the ileum and CYP7A1 in the liver by FXR-dependent mechanisms [71]. The microbiome influences the generation of organic acids and, specifically, BAs in the colon of experimental animals [72]. There are evidences that in humans the BA pool size and composition appear to be major regulators of microbiome structure, which, in turn, appears to be an important regulator of BA pool size and composition [73]. Ongoing research seeks to unravel the contributions of the microbiome and BA composition to diverse conditions including colorectal cancer [74], inflammatory bowel disease [75], IBS[76], cirrhosis [77], NAFLD [78] and obesity [79].
In an exclusive human study that may reflect BAD in patients with IBS-D, fecal counts of total bacteria, Lactobacillus, the bacteroides/prevotella group, coccoides and Faecalibacterium prausnitzii were similar in IBS-D and healthy controls [76]. In contrast, there was a significant increase of Escherichia coli and a significant decrease of Leptum and Bifidobacterium, as well as levels of primary BA in the feces were significantly increased in IBS D patients [76]. It is still unclear whether the effect of the microbiome is mediated partly or wholly through the changes in BAs, or if the changes in microbiome are somehow responsible for changes in function that manifest as BAD.
Differential diagnosis
The main differential diagnoses of non-bloody, chronic diarrhea in adults and the appropriate screening tests are listed in Table 1 [80].
Table 1. Differential diagnoses of bile acid diarrhea.
| Disorder/disease | Diagnosis and disease management |
|---|---|
| Food allergy (e.g., gluten/intolerance) | Dietary exclusion |
| Sugar maldigestion | Sugar-breath H2 test; exclusion diet |
| Celiac disease | IgA tissue transglutaminase serology + duodenal biopsy gluten-free diet |
| Gluten intolerance, not Crohn’s disease | HLA-DQ2/8; trial of gluten-free diet |
| Microscopic/lymphocytic colitis | Fecal calprotectin; colon biopsy; bismuth subsalicylate, budesonide |
| Small bowel bacterial overgrowth | Duodenal aspirate and culture; glucose or lactulose-breath H2 test; find the cause for example, diverticula; treat with antibiotics |
Reproduced with permission from [80].
Diagnosis
At present, the most popular method of diagnosis of BAD is a therapeutic trial of BA binders with symptom improvement; this is the only approach in countries like the USA where non-invasive imaging based on scintigraphic BA retention is unavailable. Unfortunately, this approach may require high doses of the BA sequestrant or binder that patients may not tolerate because of poor palatability and side effects (borborygmi, flatulence and abdominal pain when using certain BA binders [6]. Thus, such a therapeutic trial may be negative, compromising ability to diagnose BAM. In addition, the therapeutic trial is not specific for BAD, since resin formulations such as cholestyramine may also bind and inactivate other etiological agents including Clostridium difficile toxin [81,82]. Hence, the continued need for diagnostic tests for BAD.
There are 4 tools that directly measure BAM: 14C-glycocholate breath and stool test, 75selenium homotaurocholic acid test (SeHCAT), 7 α-hydroxy-4-cholesten-3-one (C4) and fecal BAs. Table 2 summarizes the pros and cons of these diagnostic tests [83].
Table 2. Advantages and disadvantages of bile acid malabsorption diagnostic methods.
| BAM diagnostic methods |
Advantages | Disadvantages |
|---|---|---|
| 14C glycocholate | May identify small bowel bacterial overgrowth | Radiation exposure, β emission, long t1/2 |
| Varying normal values | ||
| Positive breath excretion at 2–4 h does not differentiate BAM from small bowel bacterial overgrowth |
||
| Laborious test method (stool collection) | ||
|
| ||
| 75SeHCAT | γ emission, short t1/2, with decreased radiation to extra-abdominal organs |
Not available in the USA |
| Well-defined normal values; level of isotope retention predicts response to bile acid sequestrant |
Radiation exposure | |
| Simple test method: two patient visits | ||
|
| ||
| Serum C4 | No radiation | Fasting sample, diurnal variation |
| Normal values reported in adults | Requires further validation | |
| Not dependent on age, gender or cholesterol | False-positive in liver disease, treatment with statins and altered circadian rhythm |
|
| Simple blood test: one patient visit | ||
|
| ||
| Fecal BA | No radiation | Variable daily fecal BA excretion, requires at least 48 h sample |
| Measures total and individual BAs | Cumbersome method (stool collection) | |
BA: Bile acid; BAM: Bile acid malabsorption.
Reproduced with permission from [83].
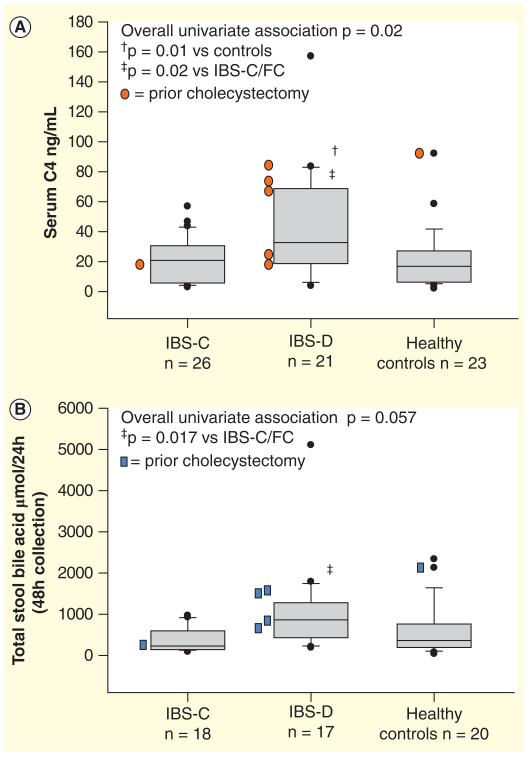
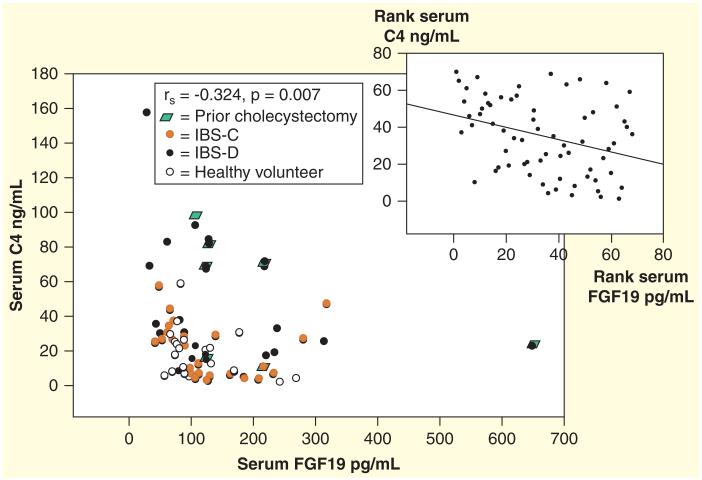
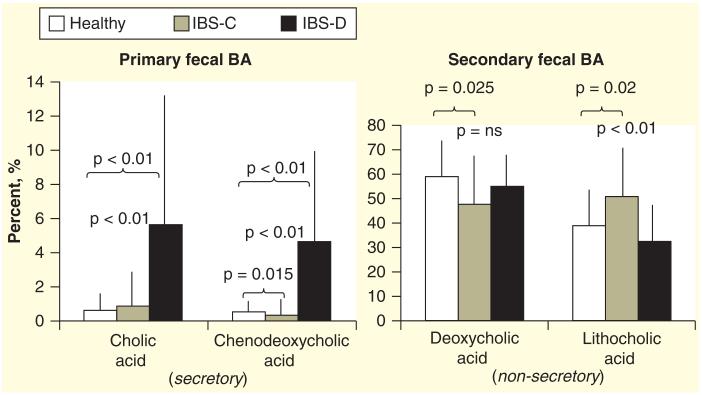
The 14C-glycocholate breath and stool test is laborious and is no longer widely utilized following development of less complex tests without radiation exposure. Serum C4 is a simple, accurate method that is applicable to a majority of patients, but requires further clinical validation. Fecal measurements to quantify total and individual fecal BAs are technically cumbersome and not widely available [84,85]. Recent data show that functional diarrhea or diarrhea-predominant IBS-D are associated with higher serum C4 (Figure 5A), higher total fecal BA (Figure 5B), inverse relationship of serum C4 and FGF-19 (Figure 6) [84], and increased secretory BAs (e.g., CDCA, DCA) (Figure 7). In contrast, constipation-predominant IBS-C is associated with higher fecal LCA levels (Figure 7) [85].
Figure 5. Bile acid measurements in patients with functional bowel disorders.
Measurements of serum C4. (A) BA synthesis marker and (B) fecal total bile acids in IBS-D, IBS-C and healthy controls.
BA: Bile acid; IBS: Irritable bowel syndrome.
Reproduced with permission from [84].
Figure 6. Inverse relationship of serum C4 and FGF-19, with spearman correlation inset.
Reproduced with permission from [84].
Figure 7. Individual predominant fecal bile acids in patients with irritable bowel syndrome-D, irritable bowel syndrome-C and healthy controls.
Note there is an increased percentage of the primary secretory BA, CDCA,in stool in IBS-D. In contrast, there is more of the non-secretory LCA (secondary BA) in IBS-C than in health or IBS-D. BA: Bile acid; CDCA: Chenodeoxycholic acid; IBS: Irritable bowel syndrome; LCA: Lithocholic acid.
Data taken from [85].
An enzymatic assay indirectly measures fecal BA. An NAD+-dependent 3α-steroid dehydrogenase enzyme is used to oxidize deconjugated BAs and produce NADH which is then measured biochemically. This method requires proper stereotactic alignment of enzyme and substrate, and with a variety of conjugations (sulfonation, glucuronidation) of BA while they are in the small intestine; this method would lack precision if it was used to measure concentrations of BA in small bowel fluid or ileostomy effluent. In addition, it does not assess BA with hydroxyl groups in the β configuration, and it tends to underestimate total BAs.
Recently, Pattni et al. [86] have explored the ability of serum FGF-19 to serve as a screen for BAD, given the inverse relationship between C4 and FGF-19 originally described by Walters et al. [32] and confirmed by other groups [33,84]. In the study of Pattni et al. [86] of 258 patients, sensitivity and specificity of FGF-19 at 145pg/ml for detecting a C4 level >28 ng/ml were 58 and 79%, respectively, and for C4 >60 ng/ml (denoting high BA synthesis), the sensitivity and specificity of FGF-19 were 74 and 72%, respectively [87]. Given the ease of the ELISA for FGF-19 rather than the HPLC method required for C4 (or fecal BA) measurement, the test could provide an inexpensive and convenient method to screen for BAD [88], and further validation studies are eagerly awaited.
In summary, cholerrheic diarrhea is most typically confirmed by a therapeutic trial, although it can be diagnosed with the 75SeHCAT retention test or measurement in serum C4 or fecal BAs in a few centers.
Management of BAD
Intraluminal bile acid binders
Cholestyramine is generally considered a first-line treatment in BAD; however, because of poor palatability and low patient compliance [6], alternatives are being used, even though there are no large clinical trials specifically for the indication of BAD. Thus, patients may prefer colesevelam at a dose of up to 1.875 g twice a day (b.i.d.). In a pharmacodynamics study of 24 unselected patients with IBS-D (4 of whom had increased serum C4 levels suggesting increased hepatocyte BA synthesis), emptying of the ascending colon took an average of 4 h longer in patients given colesevelam compared with placebo [89]. Treatment effect was significantly associated with baseline serum C4 levels (p = 0.0025), and colesevelam treatment was associated with greater ease of stool passage (p = 0.048) and somewhat firmer stool consistency (p = 0.12). There is preliminary evidence that pharmacogenetics may influence the response to colesevelam; thus, two genetic variants (FGFR4 rs351855 and KLB rs497501) that impact the rate of hepatocyte BA synthesis rate were associated with differential colesevelam effects on ascending colon emptying and overall colonic transit [90].
Experimental agents inhibiting BAD by cellular mechanisms
FGF-19 stimulation by obeticholic acid [91] provides an opportunity to reverse the deficiency which is considered one of the factors leading to excessive hepatocyte BA synthesis. This treatment was associated with improved stool frequency and consistency in a preliminary study of patients with BAD [92]. Given the observation that BAs chronically downregulate colonic secretory function in colonic epithelial cells [93], an effect that may serve to facilitate normal colonic absorptive function, it is intriguing to note that an FXR agonist, GW4064, induced nuclear translocation of the receptor in T84 cells, attenuated Cl− secretory responses to both Ca2+ and cAMP-dependent agonists, and reduced ovalbumin-induced diarrhea and cholera toxin-induced intestinal fluid accumulation secretion in mice in vivo [94]. These observations led to the proposal that FXR agonists may be efficacious in the treatment of BAD through restoration of FGF-19 production and exertion of antisecretory actions on the colonic epithelium [94].
Expert commentary
BAD secondary to ileal resection and active inflammation is well understood and treated according to the length of ileal disease, as classically described by Hofmann [4,23] and recently reviewed [95]. BAD is increasingly appreciated as a cause of chronic functional diarrhea or IBS-D. If, indeed, one in four cases of IBS-D is caused by BAD [6] and IBS has a global prevalence of 11.2% (95% CI: 9.8–12.8%) [96], with 31.0% (95% CI: 22.0–41.0%) of women with IBS and 50.0% (95% CI: 44.0–56.0%) of men with IBS having IBS-D [97], it is estimated that 1% of all people have BAD.
Therefore, from an epidemiological perspective and, because it can be specifically treated, patients presenting with IBS-D or chronic diarrhea should be screened for BAM; in several countries, this can be achieved with serum C4 or 75SeHCAT retention tests. In countries where 75SeHCAT is unavailable, screening with serum C4 followed by confirmation with either a therapeutic trial with a BA binder or measurement of fecal BA excretion should be done.
The widespread availability of these tests will usher in a more specific treatment for functional diarrhea, avoiding the non-specific treatment of chronic diarrhea which may be associated with adverse effects, as observed with opioids, such as constipation, abdominal pain, diarrhea, headache and nausea [98]. Definitive diagnosis of BAD has the added advantage that it will help physicians convince patients to stay compliant with the BA binder therapy, which is currently a problem with the inexpensive cholestyramine. It will also facilitate the rational use and formulary approval of more expensive agents, like colesevelam. The latter has the added advantages that it reduces serum cholesterol and improves diabetes control.
Finally, it is anticipated that with formal studies, metformin will be proven to cause BAD and diabetologists will be convinced to use alternative treatments for the diabetes, such as DPP-IV inhibitors, which do not appear to be associated with BAD or cause symptomatic gastric emptying delay, a frequent occurrence with GLP-1 agonists.
Five-year view
The mechanism of BAD will require further investigation. While the current prevailing hypothesis is that FGF-19 feedback regulates hepatocyte BA synthesis, the cause of the FGF-19 deficiency is still unclear. In addition, the KLB and FGFR4 genetic polymorphisms described in association with IBS-D or rapid colonic transit require further evaluation to determine if they are associated with increased fecal excretion or hepatocyte excretion of BAs. Given the greater understanding of the hepatocellular pathways in BA synthesis and excretion, the potential role of genetic or acquired variations in hepatocellular synthesis in the causation of BAD requires further study.
The diagnosis of the cause of chronic diarrhea or the non-specific phenotype of IBS-D will be anchored on the identification of specific causes including disaccharidase deficiency, food intolerance and BAD (such as screening with C4 or FGF-19). In the USA, the field of cognitive gastroenterology is making a comeback, in part because the procedural practice is coming under increasing scrutiny for the high costs of care and the unnecessarily frequent surveillance programs for colorectal cancer and Barrett’s adenocarcinoma. Definitive diagnosis with fecal BAs will be widely available in clinical practice. In other countries, 75SeHCAT will remain an important diagnostic test. Thus, BAD will be routinely excluded in patients presenting with chronic functional diarrhea or IBS-D.
From a therapeutic perspective, the efficacy of FXR agonists in the treatment of BAD requires formal study, but the dual actions of stimulation of FGF-19 synthesis (and reduced hepatocyte BA synthesis) as well as their recently described anti-secretory actions appear promising to develop alternatives to BA binders for BAD.
Key issues.
Bile acid diarrhea (BAD) is an important clinical entity that may account for up to a third of patients with chronic diarrhea or diarrhea-predominant irritable bowel syndrome.
At present, the strongest evidence suggests that BAD results from failure of feedback regulation of hepatic synthesis by the ileal hormone FGF-19.
There is a wide array of molecules involved in feedback regulation of BA synthesis, including nuclear receptors and membrane transporters in the ileal enterocyte and hepatocyte that may lead to disturbances in the absorption, synthesis and secretion of BA.
The current diagnosis of BAD is based on either a therapeutic trial with BA binders or measurement of BA retention using 75SeHCAT; simpler serological screening tests are measurement of C4 and possibly FGF-19, farnesoid X receptor (FXR) agonists have the potential to become alternative treatments for BAD in addition to BA binders such as cholestyramine and colesevelam.
Acknowledgements
The author thanks Cindy Stanislav for secretarial support.
Financial & competing interests disclosure
The work has been supported by grant NIH R01-DK92179. M Camilleri had filed a provisional patent on treatment of constipation with delayed release preparation of bile acids. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Hofmann AF, Small DM. Detergent properties of bile salts: correlation with physiological function. Annu. Rev. Med. 1967;18:333–376. doi: 10.1146/annurev.me.18.020167.002001. [DOI] [PubMed] [Google Scholar]
- 2.Rao AS, Wong B, Camilleri M, et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010;139:1549–1558. doi: 10.1053/j.gastro.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann AF. The syndrome of ileal disease and the broken enterohepatic circulation: cholerhetic enteropathy. Gastroenterology. 1967;52:752–757. [PubMed] [Google Scholar]
- 5.Martinez-Augustin O, Sanchez de Medina F. Intestinal bile acid physiology and pathophysiology. World J. Gastroenterol. 2008;14:5630–5640. doi: 10.3748/wjg.14.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halilbasic E, Claudel T, Trauner M. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J. Hepatol. 2013;58:155–168. doi: 10.1016/j.jhep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322–342. doi: 10.1053/j.gastro.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Ballatori N, Li N, Fang F, Boyer JL, Christian WV, Hammond CL. OST alpha-OST beta: a key membrane transporter of bile acids and conjugated steroids. Front Biosci. (Landmark Ed) 2009;14:2829–2844. doi: 10.2741/3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Wedlake L, A’Hern R, Russell D, et al. Systematic Review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2009;30:707–717. doi: 10.1111/j.1365-2036.2009.04081.x. Excellent summary of the literature illustrating the prevalence of BA diraarrhea (BAD) in 25–40% of patients with unexplained, chronic functional diarrhea, as well as the response to bile acid (BA) sequestrants.
- 10.Mekhjian HS, Phillips SF, Hofmann AF. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J. Clin. Invest. 1971;50:1569–1577. doi: 10.1172/JCI106644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bampton PA, Dinning PG, Kennedy ML, et al. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am. J. Physiol. 2002;282:G443–G449. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 12•.Chadwick VS, Gaginella TS, Carlson GL, et al. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J. Lab. Clin. Med. 1979;94:661–674. Classical paper in this field, illustrating the structure-activity relationships of bile acids in mammalian colon and emphasizing the mechanism of action in the induction of colonic secretion; the relevance of 2 α-hydroxyl groups at the 3, 7, or 12 positions is demonstrated.
- 13.Hammer HF, Santa Ana CA, Schiller LR, Fordtran JS. Studies of osmotic diarrhea induced in normal subjects by ingestion of polyethylene glycol and lactulose. J. Clin. Invest. 1989;84:1056–1062. doi: 10.1172/JCI114267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conley DR, Coyne MJ, Bonorris GG, Chung A, Schoenfield LJ. Bile acid stimulation of colonic adenylate cyclase and secretion in the rabbit. Am. J. Dig. Dis. 1976;21:453–458. doi: 10.1007/BF01072128. [DOI] [PubMed] [Google Scholar]
- 15.Alrefai WA, Saksena S, Tyagi S, Gill RK, Ramaswamy K, Dudeja PK. Taurodeoxycholate modulates apical Cl−/OH− exchange activity in Caco2 cells. Dig. Dis. Sci. 2007;52:1270–1278. doi: 10.1007/s10620-006-9090-8. [DOI] [PubMed] [Google Scholar]
- 16.Ao M, Sarathy J, Domingue J, Alrefai WA, Rao MC. Chenodeoxycholic acid stimulates Cl− secretion via cAMP signaling and increases cystic fibrosis transmembrane conductance regulator phosphorylation in T84 cells. Am. J. Physiol. 2013;305:C447–C456. doi: 10.1152/ajpcell.00416.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peregrin AT, Ahlman H, Jodal M, Lundgren O. Involvement of serotonin and calcium channels in the intestinal fluid secretion evoked by bile salt and cholera toxin. Br. J. Pharmacol. 1999;127:887–894. doi: 10.1038/sj.bjp.0702615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camilleri M, Murphy R, Chadwick VS. Pharmacological inhibition of chenodeoxycholic acid induced secretion of fluid and mucus in the rabbit colon. Dig. Dis. Sci. 1982;27:865–869. doi: 10.1007/BF01316567. [DOI] [PubMed] [Google Scholar]
- 19.Yamada T, Inui A, Hayashi N, Fujimura M, Fujimiya M. Serotonin stimulates endotoxin translocation via 5-HT3 receptors in the rat ileum. Am. J. Physiol. 2003;284:G782–G788. doi: 10.1152/ajpgi.00376.2002. [DOI] [PubMed] [Google Scholar]
- 20.Kruis W, Haddad A, Phillips SF. Chenodeoxycholic and ursodeoxycholic acids alter motility and fluid transit in the canine ileum. Digestion. 1986;34:185–195. doi: 10.1159/000199328. [DOI] [PubMed] [Google Scholar]
- 21.Kirwan WO, Smith AN, Mitchell WD, Falconer JD, Eastwood MA. Bile acids and colonic motility in the rabbit and the human. Gut. 1975;16:894–902. doi: 10.1136/gut.16.11.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann AF, Poley JR. Cholestyramine treatment of diarrhea associated with ileal resection. N. Engl. J. Med. 1969;281:397–402. doi: 10.1056/NEJM196908212810801. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann AF, Poley JR. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. I. Response to cholestyramine or replacement of dietary long chain triglyceride by medium chain triglyceride. Gastroenterology. 1972;62:918–934. [PubMed] [Google Scholar]
- 24••.Poley JR, Hofmann AF. Role of fat maldigestion in pathogenesis of steatorrhea in ileal resection. Fat digestion after two sequential test meals with and without cholestyramine. Gastroenterology. 1976;71:38–44. Classical papers illustrating the difference between BA diarrhea in patients with ileal resection or disease of <100 cm length and steatorrhea due to BA deficiency in patients with ileal resection of disease of >100 cm length.
- 25.Williams AJ, Merrick MV, Eastwood MA. Idiopathic bile acid malabsorption–a review of clinical presentation, diagnosis, and response to treatment. Gut. 1991;32:1004–1006. doi: 10.1136/gut.32.9.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merrick MV, Eastwood MA, Ford MJ. Is bile acid malabsorption underdiagnosed? An evaluation of accuracy of diagnosis by measurement of SeHCAT retention. Br. Med. J. 1985;290:665–668. doi: 10.1136/bmj.290.6469.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernández-Bañares F, Esteve M, Salas A, et al. Systematic evaluation of the causes of chronic watery diarrhea with functional characteristics. Am. J. Gastroenterol. 2007;102:2520–2528. doi: 10.1111/j.1572-0241.2007.01438.x. [DOI] [PubMed] [Google Scholar]
- 28.Wildt S, Nørby Rasmussen S, Lysgård Madsen J, Rumessen JJ. Bile acid malabsorption in patients with chronic diarrhoea: clinical value of SeHCAT test. Scand. J. Gastroenterol. 2003;38:826–830. doi: 10.1080/00365520310004461. [DOI] [PubMed] [Google Scholar]
- 29.Gracie DJ, Kane JS, Mumtaz S, Scarsbrook AF, Chowdhury FU, Ford AC. Prevalence of, and predictors of, bile acid malabsorption in outpatients with chronic diarrhea. Neurogastroenterol. Motil. 2012;24:983 e538. doi: 10.1111/j.1365-2982.2012.01953.x. [DOI] [PubMed] [Google Scholar]
- 30.Limsui D, Pardi DS, Camilleri M, et al. Symptomatic overlap between irritable bowel syndrome and microscopic colitis. Inflamm. Bowel Dis. 2007;13:175–181. doi: 10.1002/ibd.20059. [DOI] [PubMed] [Google Scholar]
- 31.Walters JR, Pattni SS. Managing bile acid diarrhoea. Ther. Adv. Gastroenterol. 2010;3:349–357. doi: 10.1177/1756283X10377126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouchoucha M, Uzzan B, Cohen R. Metformin and digestive disorders. Diabetes Metab. 2011;37:90–96. doi: 10.1016/j.diabet.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Caspary WF, Zavada I, Reimold W, Deuticke U, Emrich D, Willms B. Alteration of bile acid metabolism and vitamin-B12-absorption in diabetics on biguanides. Diabetologia. 1977;13:187–193. doi: 10.1007/BF01219698. [DOI] [PubMed] [Google Scholar]
- 34.Scarpello JH, Hodgson E, Howlett HC. Effect of metformin on bile salt circulation and intestinal motility in type 2 diabetes mellitus. Diabet. Med. 1998;15:651–656. doi: 10.1002/(SICI)1096-9136(199808)15:8<651::AID-DIA628>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 35.Bjarnason I, Sharpstone DR, Francis N, et al. Intestinal inflammation, ileal structure and function in HIV. AIDS. 1996;10:1385–1391. doi: 10.1097/00002030-199610000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Lundasen T, Galman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J. Intern. Med. 2006;260:530–536. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- 37••.Walters JR, Tasleem AM, Omer OS, Brydon WG, Dew T, Le Roux CW. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin. Gastroenterol. Hepatol. 2009;7:1189–1194. doi: 10.1016/j.cgh.2009.04.024. Landmark article providing first documentation of a molecular mechanism (deficiency of FGF-19) secretion by ileal enterocytes in patients with BAD.
- 38.Montagnani M, Love MW, Rössel P, Dawson PA, Qvist P. Absence of dysfunctional ileal sodium-bile acid cotransporter gene mutations in patients with adult-onset idiopathic bile acid malabsorption. Scand. J. Gastroenterol. 2001;36:1077–1080. doi: 10.1080/003655201750422693. [DOI] [PubMed] [Google Scholar]
- 39.Montagnani M, Abrahamsson A, Gälman C, et al. Analysis of ileal sodium/bile acid cotransporter and related nuclear receptor genes in a family with multiple cases of idiopathic bile acid malabsorption. World J. Gastroenterol. 2006;12:7710–7714. doi: 10.3748/wjg.v12.i47.7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sciarretta G, Fagioli G, Furno A, et al. 75Se HCAT test in the detection of bile acid malabsorption in functional diarrhoea and its correlation with small bowel transit. Gut. 1987;28:970–975. doi: 10.1136/gut.28.8.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadik R, Abrahamsson H, Ung KA, Stotzer PO. Accelerated regional bowel transit and overweight shown in idiopathic bile acid malabsorption. Am. J. Gastroenterol. 2004;99:711–718. doi: 10.1111/j.1572-0241.2004.04139.x. [DOI] [PubMed] [Google Scholar]
- 42.Valdés Olmos R, den Hartog Jager F, Hoefnagel C, Taal B. Effect of loperamide and delay of bowel motility on bile acid malabsorption caused by late radiation damage and ileal resection. Eur J Nucl. Med. 1991;18:346–350. doi: 10.1007/BF02285463. [DOI] [PubMed] [Google Scholar]
- 43.Yeoh EK, Horowitz M, Russo A, Muecke T, Robb T, Chatterton BE. Gastrointestinal function in chronic radiation enteritis–effects of loperamide-N-oxide. Gut. 1993;34:476–482. doi: 10.1136/gut.34.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bajor A, Kilander A, Fae A, et al. Normal or increased bile acid uptake in isolated mucosa from patients with bile acid malabsorption. Eur. J. Gastroenterol. Hepatol. 2006;8:397–403. doi: 10.1097/00042737-200604000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Bajor A, Gillberg PG, Abrahamsson H. Bile acids: short and long term effects in the intestine. Scand. J. Gastroenterol. 2010;45:645–664. doi: 10.3109/00365521003702734. [DOI] [PubMed] [Google Scholar]
- 46••.Wong BS, Camilleri M, Carlson PJ, et al. A klothoβ variant mediates protein stability and associates with colon transit in irritable bowel syndrome with diarrhea. Gastroenterology. 2011;140:1934–1942. doi: 10.1053/j.gastro.2011.02.063. First documentation of a genetic molecular mechanism that alters feedback regulation (by FGF-19) of hepatocyte BA synthesis, and results in acceleration of colonic transit in patients with IBS-diarrhea.
- 47.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 48.Ward JB, Mroz MS, Keely SJ. The bile acid receptor, TGR5, regulates basal and cholinergic-induced secretory responses in rat colon. Neurogastroenterol. Motil. 2013;25:708–711. doi: 10.1111/nmo.12148. [DOI] [PubMed] [Google Scholar]
- 49.Poole DP, Godfrey C, Cattaruzza F, et al. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol. Motil. 2010;22:814–25. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hov JR, Keitel V, Laerdahl JK, et al. Mutational characterization of the bile acid receptor TGR5 in primary sclerosing cholangitis. PLoS ONE. 2010;5:e12403. doi: 10.1371/journal.pone.0012403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fiorucci S, Cipriani S, Mencarelli A, Renga B, Distrutti E, Baldelli F. Counter-regulatory role of bile acid activated receptors in immunity and inflammation. Curr. Mol. Med. 2010;10:579–595. doi: 10.2174/1566524011009060579. [DOI] [PubMed] [Google Scholar]
- 52.Camilleri M, Vazquez-Roque MI, Carlson P, Burton D, Wong BS, Zinsmeister AR. Association of bile acid receptor TGR5 variation and transit in health and lower functional gastrointestinal disorders. Neurogastroenterol. Motil. 2011;23:995–999. doi: 10.1111/j.1365-2982.2011.01772.x. 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N. Engl. J. Med. 2012;367:1626–1635. doi: 10.1056/NEJMra1207068. [DOI] [PubMed] [Google Scholar]
- 54.Galatola G, Jazrawi RP, Bridges C, Joseph AE, Northfield TC. Direct measurement of first-pass ileal clearance of a bile acid in humans. Gastroenterology. 1991;100:1100–1105. doi: 10.1016/0016-5085(91)90288-v. [DOI] [PubMed] [Google Scholar]
- 55.van Tilburg AJ, de Rooij FW, van den Berg JW, Kooij PP, van Blankenstein M. The selenium-75-homocholic acid taurine test re-evaluated: combined measurement of fecal selenium-75 activity and 3 alpha-hydroxy bile acids in 211 patients. J. Nucl. Med. 1991;32:1219–1224. [PubMed] [Google Scholar]
- 56.Sciarretta G, Furno A, Mazzoni M, Malaguti P. Post-cholecystectomy diarrhea: evidence of bile acid malabsorption assessed by SeHCAT test. Am. J. Gastroenterol. 1992;87:1852–1854. [PubMed] [Google Scholar]
- 57.Suhr O, Danielsson A, Nyhlin H, Truedsson H. Bile acid malabsorption demonstrated by SeHCAT in chronic diarrhoea, with special reference to the impact of cholecystectomy. Scand. J. Gastroenterol. 1988;23:1187–1194. doi: 10.3109/00365528809090189. [DOI] [PubMed] [Google Scholar]
- 58.Kurien M, Evans KE, Leeds JS, Hopper AD, Harris A, Sanders DS. Bile acid malabsorption: an under-investigated differential diagnosis in patients presenting with diarrhea predominant irritable bowel syndrome type symptoms. Scand. J. Gastroenterol. 2011;46:818–822. doi: 10.3109/00365521.2011.574728. [DOI] [PubMed] [Google Scholar]
- 59.Al-Hadrani A, Lavelle-Jones M, Kennedy N, Neill G, Sutton D, Cuschieri A. Bile acid malabsorption in patients with postvagotomy diarrhoea. Ann. Chir. Gynaecol. 1992;81:351–353. [PubMed] [Google Scholar]
- 60.Ladas SD, Isaacs PE, Quereshi Y, Sladen G. Role of the small intestine in postvagotomy diarrhea. Gastroenterology. 1983;85:1088–1093. [PubMed] [Google Scholar]
- 61.Fort JM, Azpiroz F, Casellas F, Andreu J, Malagelada JR. Bowel habit after cholecystectomy: physiological changes and clinical implications. Gastroenterology. 1996;111:617–622. doi: 10.1053/gast.1996.v111.pm8780565. [DOI] [PubMed] [Google Scholar]
- 62.Phillips SF. Diarrhea after cholecystectomy: if so, why? Gastroenterology. 1996;111:816–818. doi: 10.1053/gast.1996.v111.agast961110816. [DOI] [PubMed] [Google Scholar]
- 63.Bisschop PH, Bandsma RH, Stellaard F, et al. Low-fat, high-carbohydrate and high-fat, low-carbohydrate diets decrease primary bile acid synthesis in humans. Am. J. Clin. Nutr. 2004;79:570–576. doi: 10.1093/ajcn/79.4.570. [DOI] [PubMed] [Google Scholar]
- 64.Li T, Matozel M, Boehme S, et al. Overexpression of cholesterol 7α-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology. 2001;53:996–1006. doi: 10.1002/hep.24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jonkers IJ, Smelt AH, Princen HM, et al. Fish oil increases bile acid synthesis in male patients with hypertriglyceridemia. J. Nutr. 2006;136:987–991. doi: 10.1093/jn/136.4.987. [DOI] [PubMed] [Google Scholar]
- 66.Devkota S, Wang Y, Musch MW, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laue H, Denger K, Cook AM. Taurine reduction in anaerobic respiration of Bilophila wadsworthia RZATAU. Appl. Environ. Microbiol. 1997;63:2016–2021. doi: 10.1128/aem.63.5.2016-2021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lindstedt S, Avigan J, Goodman DS, Sjövall J, Steinberg DJ. The effect of dietary fat on the turnover of cholic acid and on the composition of the biliary bile acids in man. Clin. Invest. 1965;44:1754–1765. doi: 10.1172/JCI105283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scarpello JH, Hodgson E, Howlett HC. Effect of metformin on bile salt circulation and intestinal motility in type 2 diabetes mellitus. Diabet. Med. 1998;15:651–656. doi: 10.1002/(SICI)1096-9136(199808)15:8<651::AID-DIA628>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 70.Caspary WF, Zavada I, Reimold W, Deuticke U, Emrich D, Willms B. Alteration of bile acid metabolism and vitamin-B12-absorption in diabetics on biguanides. Diabetologia. 1977;13:187–193. doi: 10.1007/BF01219698. [DOI] [PubMed] [Google Scholar]
- 71.Sayin SI, Wahlström A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 72.Zhao Y, Wu J, Li JV, Zhou NY, Tang H, Wang Y. Gut microbiota composition modifies fecal metabolic profiles in mice. J. Proteome Res. 2013;12:2987–2999. doi: 10.1021/pr400263n. [DOI] [PubMed] [Google Scholar]
- 73.Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids, and gut microbiota: Unraveling a complex relationship. Gut Microbes. 2013;4(5) doi: 10.4161/gmic.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ou J, Carbonero F, Zoetendal EG, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am. J. Clin. Nutr. 2013;98:111–120. doi: 10.3945/ajcn.112.056689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duboc H, Rajca S, Rainteau D, et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62:531–539. doi: 10.1136/gutjnl-2012-302578. [DOI] [PubMed] [Google Scholar]
- 76.Duboc H, Rainteau D, Rajca S, et al. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 2012;24:513–520. doi: 10.1111/j.1365-2982.2012.01893.x. [DOI] [PubMed] [Google Scholar]
- 77.Kakiyama G, Pandak WM, Gillevet PM, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J. Hepatol. 2013;58:949–955. doi: 10.1016/j.jhep.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aron-Wisnewsky J, Gaborit B, Dutour A, Clement K. Gut microbiota and non-alcoholic fatty liver disease: new insights. Clin. Microbiol. Infect. 2013;19:338–348. doi: 10.1111/1469-0691.12140. [DOI] [PubMed] [Google Scholar]
- 79.Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Camilleri M. Do the symptom-based, Rome criteria of irritable bowel syndrome lead to better diagnosis and treatment outcomes? The con argument. Clin. Gastroenterol. Hepatol. 2010;8:129–132. doi: 10.1016/j.cgh.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Surowiec D, Kuyumjian AG, Wynd MA, et al. Past, present, and future therapies for Clostridium difficile-associated disease. Ann. Pharmacother. 2006;40:2155–2163. doi: 10.1345/aph.1H332. [DOI] [PubMed] [Google Scholar]
- 82.Weiss K. Toxin-binding treatment for Clostridium difficile: a review including reports of studies with tolevamer. Int. J. Antimicrob. Agents. 2009;33:4–7. doi: 10.1016/j.ijantimicag.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 83•.Vijayvargiya P, Camilleri M, Shin A, Saenger A. Methods for diagnosis of bile acid malabsorption in clinical practice. Clin. Gastroenterol. Hepatol. 2013;11(10):1232–1239. doi: 10.1016/j.cgh.2013.04.029. Review of methods, applicable in clinical practice, to measure BA malabsorption or BA synthesis.
- 84••.Wong BS, Camilleri M, Carlson P, et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin. Gastroenterol. Hepatol. 2012;10:1009–1015. doi: 10.1016/j.cgh.2012.05.006. Increased total fecal BA excretion is associated with increased hepatocyte BA synthesis in patients with IBS-D; converse is shown in IBS-C.
- 85•.Shin A, Camilleri M, Vijayvargiya P, et al. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2013;11(10):1270–1275 e1. doi: 10.1016/j.cgh.2013.04.020. Increased fecal excretion of secretory BA such as CDC and DCA in patients with IBS-D.
- 86.Pattni SS, Brydon WG, Dew T, Walters JR. Fibroblast growth factor 19 and 7α-hydroxy-4-cholesten-3-one in the diagnosis of patients with possible bile acid diarrhea. Clin. Transl. Gastroenterol. 2012;3:e18. doi: 10.1038/ctg.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pattni SS, Brydon WG, Dew T, et al. Fibroblast growth factor 19 in patients with bile acid diarrhoea: a prospective comparison of FGF19 serum assay and SeHCAT retention. Aliment. Pharmacol. Ther. 2013;38(8):967–976. doi: 10.1111/apt.12466. [DOI] [PubMed] [Google Scholar]
- 88.Camilleri M, Acosta A. Invited commentary: fibroblast growth factor 19 in patients with bile acid diarrhea: a prospective comparison of FGF19 serum assay and SeHCAT retention by Pattni et al. Aliment. Pharmacol. Ther. doi: 10.1111/apt.12466. In press. [DOI] [PubMed] [Google Scholar]
- 89.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin. Gastroenterol. Hepatol. 2010;8:159–165. doi: 10.1016/j.cgh.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wong BS, Camilleri M, Carlson PJ, et al. Pharmacogenetics of the effects of colesevelam on colonic transit in irritable bowel syndrome with diarrhea. Dig. Dis. Sci. 2012;57:1222–1226. doi: 10.1007/s10620-012-2035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang JH, Nolan JD, Kennie SL, et al. Potent stimulation of fibroblast growth factor 19 expression in the human ileum by bile acids. Am. J. Physiol. 2013;304:G940–G948. doi: 10.1152/ajpgi.00398.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92•.Johnston IM, Nolan JD, Dew T, Shapiro D, Walters JRF. A new therapy for chronic diarrhea? A proof of concept study of the FXR agonist obeticholic acid in patients with primary bile acid diarrhea. Gastroenterology. 2013;144(Suppl. 144):S60. Interesting preliminary report demonstrating the efficacy of an FXR agonist that stimulates production of FGF-19, and therefore should reduce BA synthesis by hepatocytes and reduce BAD.
- 93.Keating N, Mroz MS, Scharl MM, et al. Physiological concentrations of bile acids down-regulate agonist induced secretion in colonic epithelial cells. J. Cell Mol. Med. 2009;13:2293–2303. doi: 10.1111/j.1582-4934.2009.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mroz MS, Keating N, Ward J, et al. Farnesoid X receptor agonists attenuate colonic epithelial secretory function and prevent experimental diarrhoea in vivo. Gut. 2013 doi: 10.1136/gutjnl-2013-305088. doi:10.1136/gutjnl-2013-305088. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 95.Nolan JD, Johnston IM, Walters JR. Altered enterohepatic circulation of bile acids in Crohn’s disease and their clinical significance: a new perspective. Expert Rev. Gastroenterol. Hepatol. 2013;7:49–56. doi: 10.1586/egh.12.66. [DOI] [PubMed] [Google Scholar]
- 96.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin. Gastroenterol. Hepatol. 2012;10:712–721. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 97.Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am. J. Gastroenterol. 2012;107:991–1000. doi: 10.1038/ajg.2012.131. [DOI] [PubMed] [Google Scholar]
- 98.Omar MI, Alexander CE. Drug treatment for faecal incontinence in adults. Cochrane Database Syst. Rev. 2013;(6):CD002116. doi: 10.1002/14651858.CD002116.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]