Abstract
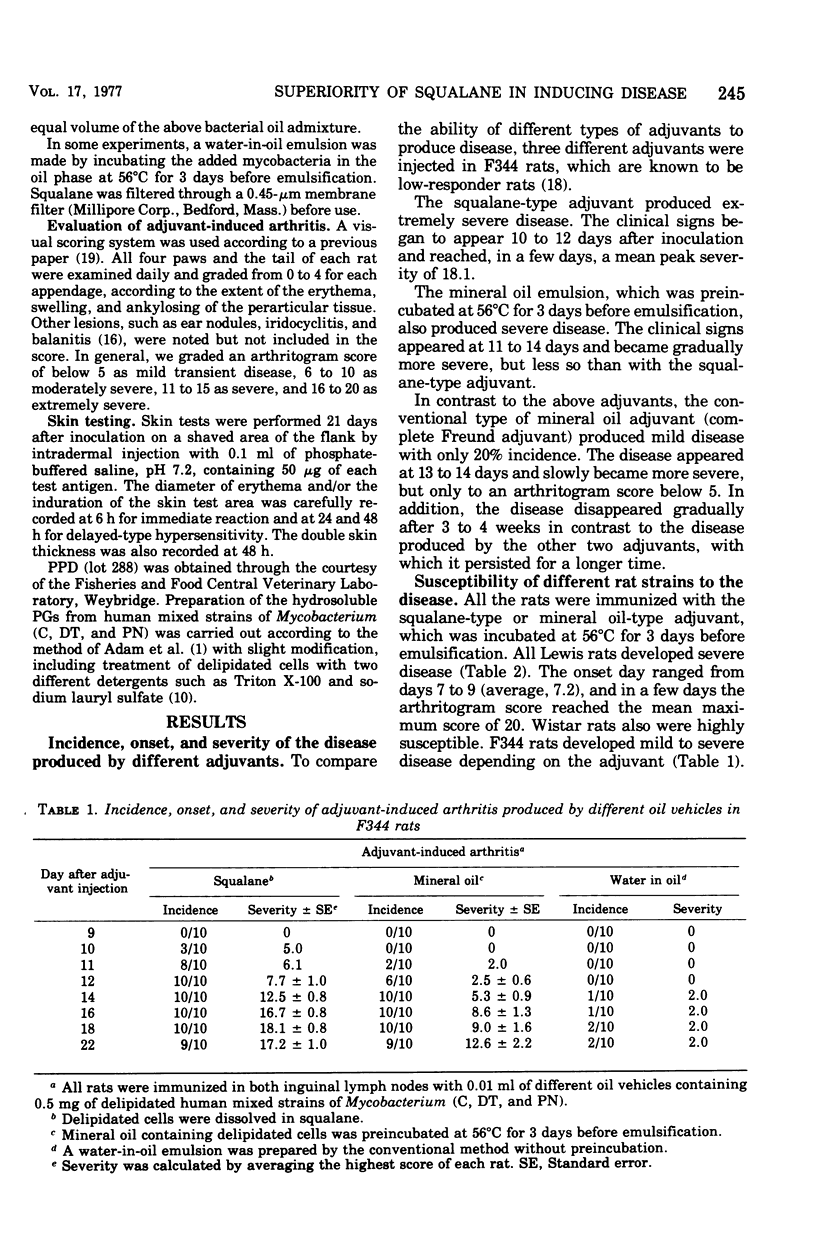
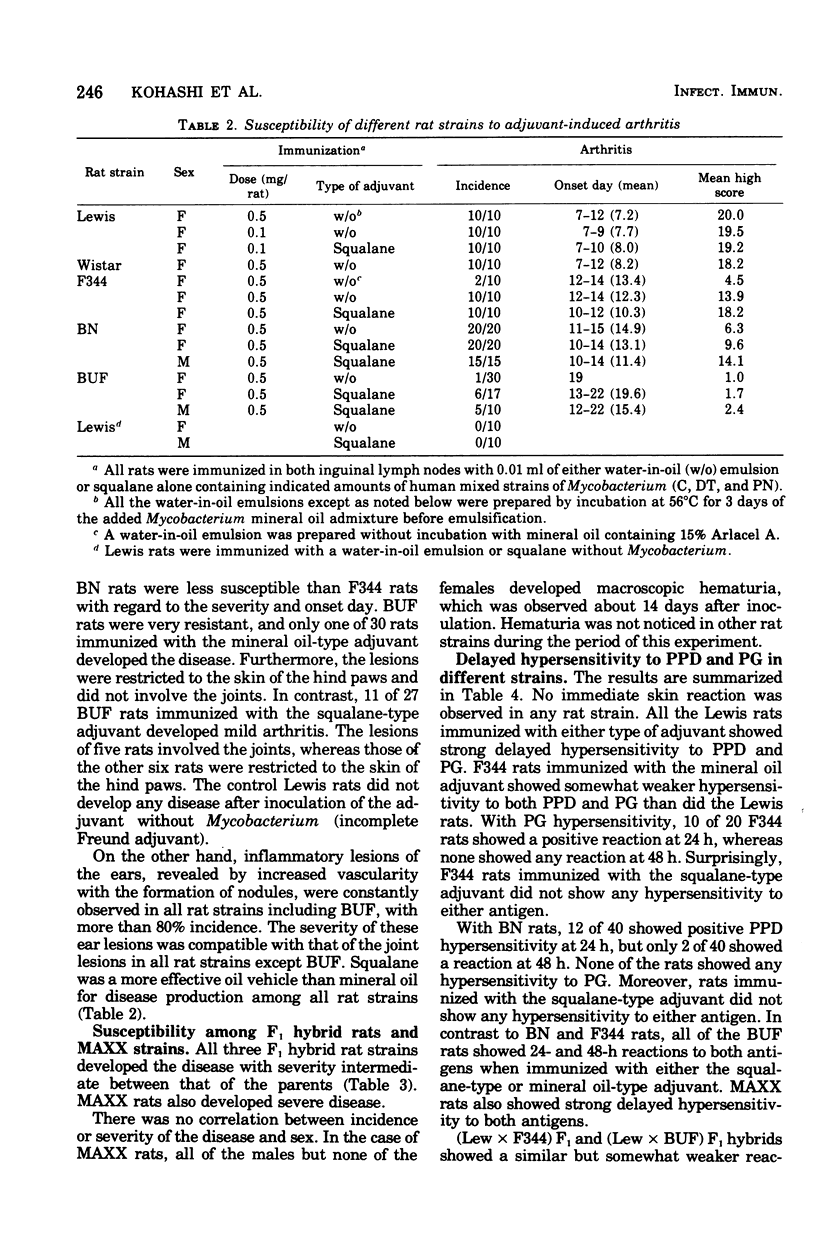
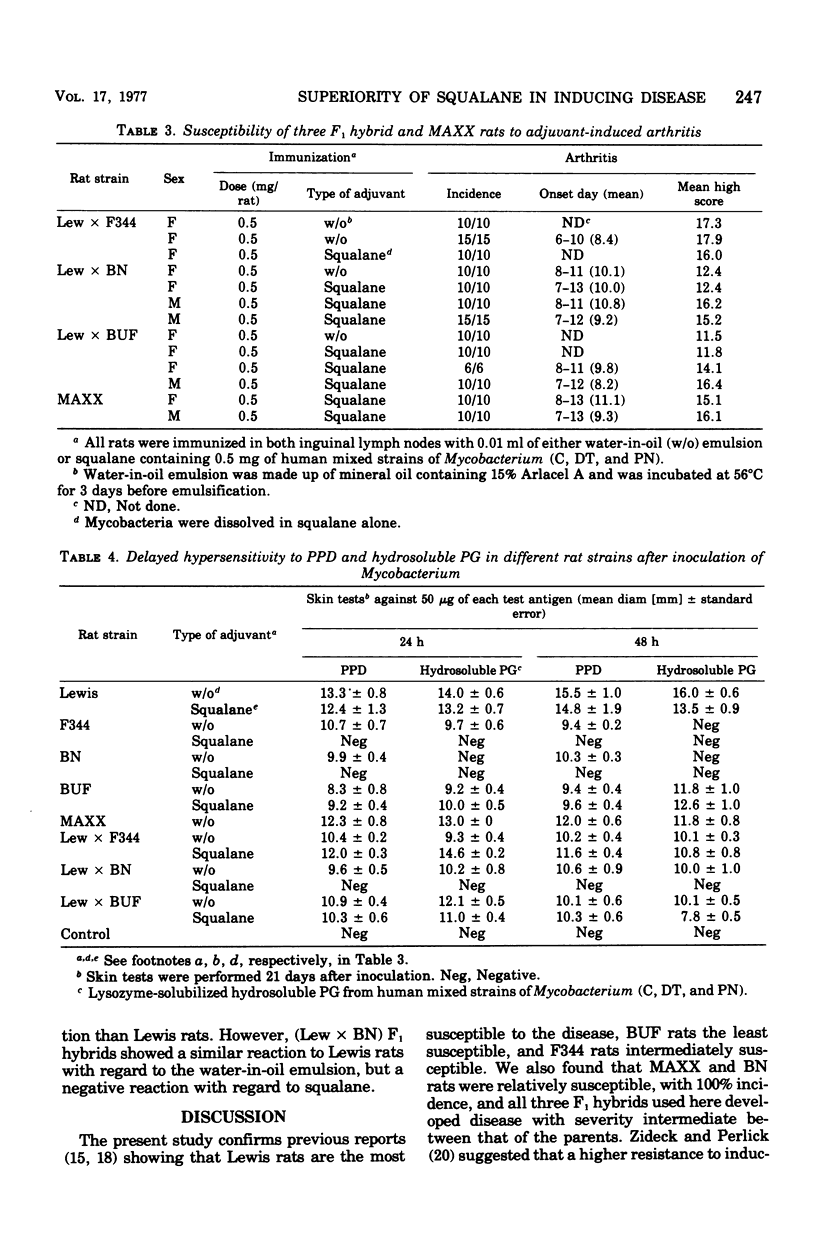
We confirmed that, when immunized with a conventional complete Freund adjuvant (water in oil), Lewis rats were highly susceptible to adjuvant arthritis, Fisher rats were less susceptible, and Buffalo rats were much less susceptible. However, mycobacterial delipidated cells in squalane (squalane-type adjuvant) produced severe arthritis with almost 100% incidence even in the less susceptible rat strains except for Buffalo rats. With regard to an immune response, Freund complete adjuvant induced strong delayed hypersensitivity to purified protein derivative (PPD) and peptidoglycan (PG) in all rat strains used, Whereas the squalane-type adjuvant induced these hypersensitivities only in Lewis and Buffalo rats, but not in Fisher and Brown Norway rats. No correlation was found between development of arthritis and delayed hypersensitivity to either PPD or PG, or both. It seems that PPD hypersensitivity may be inherited differently from PG hypersensitivity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Ciorbaru R., Petit J. F., Lederer E., Chedid L., Lamensans A., Parant F., Parant M., Rosselet J. P., Berger F. M. Preparation and biological properties of water-soluble adjuvant fractions from delipidated cells of Mycobacterium smegmatis and Nocardia opaca. Infect Immun. 1973 Jun;7(6):855–861. doi: 10.1128/iai.7.6.855-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry H., Willoughby D. A., Giroud J. P. Evidence for an endogenous antigen in the adjuvant arthritic rat. J Pathol. 1973 Dec;111(4):229–238. doi: 10.1002/path.1711110403. [DOI] [PubMed] [Google Scholar]
- Ginsberg M. H., Genant H. K., Yü T. F., McCarty D. J. Rheumatoid nodulosis: an unusual variant of rheumatoid disease. Arthritis Rheum. 1975 Jan-Feb;18(1):49–58. doi: 10.1002/art.1780180111. [DOI] [PubMed] [Google Scholar]
- Glenn E. M., Gray J. Adjuvant-induced polyarthritis in rats: biologic and histologic background. Am J Vet Res. 1965 Sep;26(114):1180–1194. [PubMed] [Google Scholar]
- Koga T., Kotani S., Narita T., Pearson C. M. Induction of adjuvant arthritis in the rat by various bacterial cell walls and their water-soluble components. Int Arch Allergy Appl Immunol. 1976;51(2):206–213. doi: 10.1159/000231593. [DOI] [PubMed] [Google Scholar]
- Koga T., Pearson C. M., Narita T., Kotani S. Polyarthritis induced in the rat with cell walls from several bacteria and two Streptomyces species. Proc Soc Exp Biol Med. 1973 Jul;143(3):824–827. doi: 10.3181/00379727-143-37421. [DOI] [PubMed] [Google Scholar]
- Kohashi O., Pearson C. M. Arthritogenicity of Mycobacterium smegmatis subfractions, related to different oil vehicle and different composition. Int Arch Allergy Appl Immunol. 1976;51(4):462–470. doi: 10.1159/000231620. [DOI] [PubMed] [Google Scholar]
- Kohashi O., Pearson C. M., Shimono T., Kotani S. Preparation of various fractions from Mycobacterium smegmatis, their arthritogenicity and their preventive effect on adjuvant disease. Int Arch Allergy Appl Immunol. 1976;51(4):451–461. doi: 10.1159/000231619. [DOI] [PubMed] [Google Scholar]
- Kohashi O., Pearson C. M., Watanabe Y., Kotani S., Koga T. Structural requirements for arthritogenicity of peptidoglycans from Staphylococcus aureus and Lactobacillus plant arum and analogous synthetic compounds. J Immunol. 1976 Jun;116(6):1635–1639. [PubMed] [Google Scholar]
- PEARSON C. M. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956 Jan;91(1):95–101. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- Steffen C., Wick G. Delayed hypersensitivity reactions to collagen in rats with adjuvant-induced arthritis. Z Immunitatsforsch Allerg Klin Immunol. 1971;141(2):169–180. [PubMed] [Google Scholar]
- Swingle K. F., Jaques L. W., Kvam D. C. Differences in the severity of adjuvant arthritis in four strains of rats. Proc Soc Exp Biol Med. 1969 Nov;132(2):608–612. doi: 10.3181/00379727-132-34270. [DOI] [PubMed] [Google Scholar]
- WAKSMAN B. H., PEARSON C. M., SHARP J. T. Studies of arthritis and other lesions induced in rats by injection of mycobacterial adjuvant. II. Evidence that the disease is a disseminated immunologic response to exogenous antigen. J Immunol. 1960 Oct;85:403–417. [PubMed] [Google Scholar]
- Whitehouse M. W., Orr K. J., Beck F. W., Pearson C. M. Freund's adjuvants: relationship of arthritogenicity and adjuvanticity in rats to vehicle composition. Immunology. 1974 Aug;27(2):311–330. [PMC free article] [PubMed] [Google Scholar]
- Wood F. D., Pearson C. M., Tanaka A. Capacity of mycobacterial wax D and its subfractions to induce adjuvant arthritis in rats. Int Arch Allergy Appl Immunol. 1969;35(5):456–467. doi: 10.1159/000230198. [DOI] [PubMed] [Google Scholar]
- Zídek Z., Perlík F. Genetic control of adjuvant-induced arthritis in rats. J Pharm Pharmacol. 1971 May;23(5):389–390. doi: 10.1111/j.2042-7158.1971.tb09938.x. [DOI] [PubMed] [Google Scholar]



