Abstract
Most cell free protein synthesis systems are based on cell extracts, which often contain undesirable activities. The reconstituted systems, by contrast, are composed of a defined number of purified and recombinant components with minimal nuclease and protease activities. This unit describes the use of a particular commercial reconstituted system, "PURExpress®" This system allows in vitro synthesis of proteins from mRNA and circular and linear DNA templates, as well as co-translational labeling of proteins. Unique to this system, all recombinant protein components of the system are His-tagged, allowing purification of the synthesized untagged protein by removing the rest of the system’s components. Newly synthesized proteins can often be visible on a SDS-PAGE gel and directly assayed for their functions without labeling and purification. Certain components of the system, such as ribosomes or release factors, can be omitted for specific applications. Such "delta" versions of the system are well suited for studies of bacterial translation, assays of ribosome functions, incorporation of unnatural amino acids and ribosome display of protein libraries.
Keywords: Reconstituted cell-free protein synthesis, coupled transcription and translation, PURExpress, ribosome display, unnatural amino acid incorporation, isotope labeling
INTRODUCTION
The ability to easily generate a near-infinite variety of DNA sequences has increased the demand for improved protein expression methods. Cell-free protein synthesis systems have allowed researchers to step beyond the limits of traditional in vivo expression by decoupling protein expression from host cell culture and viability (Carlson et al., 2012; Katzen et al., 2005; Rosenblum and Cooperman, 2014; Whittaker, 2013). Therefore, cell-free protein synthesis is well suited for rapidly generating analytical amounts of proteins for high-throughput structural and functional characterization, expressing toxic proteins, and selecting proteins in in vitro directed evolution experiments.
There are two main cell-free protein synthesis technologies, traditional extract-based systems and more recently developed reconstituted systems (Rosenblum and Cooperman, 2014; Whittaker, 2013). One system, "PUREexpress" made by New England Biolabs, is a reconstituted coupled transcription-translation system based on the PURE system (Shimizu et al., 2001).
In this system, and distinct from extract-based cell-free systems, all necessary macromolecule components needed for in vitro transcription and translation are purified from E. coli and supplied as two solution mixes (A and B). Solution A contains tRNAs and small molecules including amino acids and rNTPs, whereas Solution B contains ribosomes and protein components such as T7 RNA polymerase, translation factors, aminoacyl-tRNA synthetases and energy regeneration enzymes. Both solution mixes are tested by standard quality control protocols, showing minimal protease and nuclease contamination. The T7 RNA polymerase in the system allows transcription of a gene under a T7 promoter into mRNA, which is subsequently translated into a protein in the same reaction. Therefore, the system has the flexibility of using plasmid DNA, linear DNA (for example from PCR), or mRNA as the template for in vitro protein synthesis. The average final protein synthesis yield of the system is around 100 ng/µl. All protein components of the system are His tagged, except ribosomes. This feature allows the synthesized protein to be rapidly purified by simply removing ribosomes from the synthesis mix by ultrafiltration and removing His-tagged proteins by treatments using nickel resin, leaving only the pure newly synthesized protein. Using the system, a protein of interest can be synthesized and purified within about 5 hrs.
This unit describes how to use the standard "PURExpress" system kit as well as other related commercial kits. Basic protocols include the assembly of the standard in vitro protein synthesis reaction using DNA or mRNA as a template, expression of disulfide bonded proteins, purification of the synthesized protein, and use of the "PURExpress delta ribosome kit" (∆ribosome) for assaying ribosome activity. Support protocols include DNA and mRNA template design and preparation, synthesis and analysis of fluorescent and 35S-methionine labeled proteins, preparation of bacterial ribosomes, use of a separate PURExpress delta amino acids, tRNA (∆aa, tRNA) kit, and use of a separate PURExpress delta release factors (∆RF123) kit.
BASIC PROTOCOL 1
STANDARD REACTION ASSEMBLY FOR THE IN VITRO TRANSCRIPTION AND TRANSLATION OF PROTEINS USING THE PUREXPRESS KIT
This basic protocol describes the standard procedure for microgram level synthesis in a 25 microliter reaction. For the DNA template, the T7 promoter is required for transcription of the gene of interest by the T7 RNA polymerase included in the system. If the linear DNA template is used, a T7 terminator is also required. When the DNA template is used, both transcription and translation occur in the same in vitro reaction. mRNA can also be used as the template, in this case, only translation occur in the in vitro reaction. Please see Support Protocols 1, 2 and 3 for template design and preparation. The typical yield from the standard reaction assembly is 100 ng/µl for a protein of interest. Other protocols are based on the standard procedure with some modifications. When the protocols specify other commercial reagents, we indicate commercial and noncommercial alternatives.
Materials
A PURExpress® In vitro Protein Synthesis kit (New England Biolabs, http//www.neb.com) containing:
Solution A (yellow tube)
Solution B (red tube)
DHFR control template
Nuclease free microcentrifuge tubes or microtiter plate
Nuclease free H2O
Template DNA (See Support Protocol 1 and 2) or mRNA (See Support Protocol 3)
Murine RNase inhibitor (40 U/µl, New England Biolabs) or RNasin Ribonuclease inhibitor (20–40 U/µl, Promega)
Microcentrifuge
Air incubator set at 37°C
3x SDS-PAGE loading buffer (New England Biolabs)
SDS-PAGE gel (4–20 % Tris-glycine, Life Technologies)
Protocol steps
-
Thaw Solutions A and B on ice.
Do not thaw Solutions A and B at room or higher temperatures as this would result in a loss in activity. Gently mix both Solutions after they are thawed by pipetting a few times, but avoid generating air bubbles. Solution A may appear cloudy, gently mix well before adding to the reaction. Avoid vortexing both Solution A and B. Solutions A and B can be freeze-thawed at least 5 times without a loss of activity if handled as described above and stored separately at −80°C.
-
Assemble the reaction on ice with the reagents in the following order for a total of 25 µl:
- 10 µl Solution A
- 7.5 µl Solution B
- 0.5 µl Murine RNase Inhibitor
- 5 µl Nuclease free H2O
- 2 µl of DNA template (250 ng) or mRNA template (3 µg)
Optimal DNA or RNA template amount for maximum yield will vary for each template used. The range for DNA is 25 ng to 1000 ng per 25 µl reaction. A good starting point is 250 ng DNA per 25 µl reaction. The range for mRNA is 1 µg to 5 µg per 25 µl reaction. A good starting point is 3 µg mRNA per 25 µl reaction. A titration of DNA or mRNA template input may help determine the optimal amount to add to a reaction. See Support Protocol 1, 2 and 3 on template preparation. To ensure the reaction is assembled properly, use the DHFR control template, which directs the synthesis of E. coli DHFR (dihydrofolate reductase, 18 kDa).
After adding all the reagents, gently mix the reaction mixture by pipetting up and down in a pipetman a few times, but avoid generating air bubbles. Adding RNase Inhibitor is optional but recommended especially if the template plasmid DNA is isolated with a commercial plasmid preparation kit (such as QIAprep spin miniprep kit (Qiagen)) as RNase A is often used during the preparation.
Solutions A and B should be mixed in the indicated proportions so that they result in the most efficient transcription and translation reactions. Whatever the scale of the reaction, the researcher should maintain the ratios of Solutions A and B as specified in the accompanying kit instruction manual.
-
Incubate at 37°C for 2 to 4 hrs in an air incubator.
The optimal temperature for the synthesis reaction is 37°C . At that temperature, the reaction normally plateaus after 2 hrs and stops after 4 hrs due to the depletion of small molecules such as NTPs and amino acids as well as the accumulation of molecules such as inorganic phosphate (PPi).
-
Stop the reaction by placing on ice.
The reaction mixture can also be stored in a −20°C freezer for analysis later. Some materials in the reaction mixture may precipitate during storage at −20°C. Be sure to resuspend completely after thawing.
-
Analyze by SDS-PAGE, take 2.5 µl of the reaction mixture, add 7.5 µl of H2O, mix with 5 µl of 3xSDS-PAGE loading buffer, heat at 95°C for 1 min and load the sample (15 µl) and run on a SDS-PAGE gel followed by Coomassie blue staining (XFEF CPMB Chapter 10)
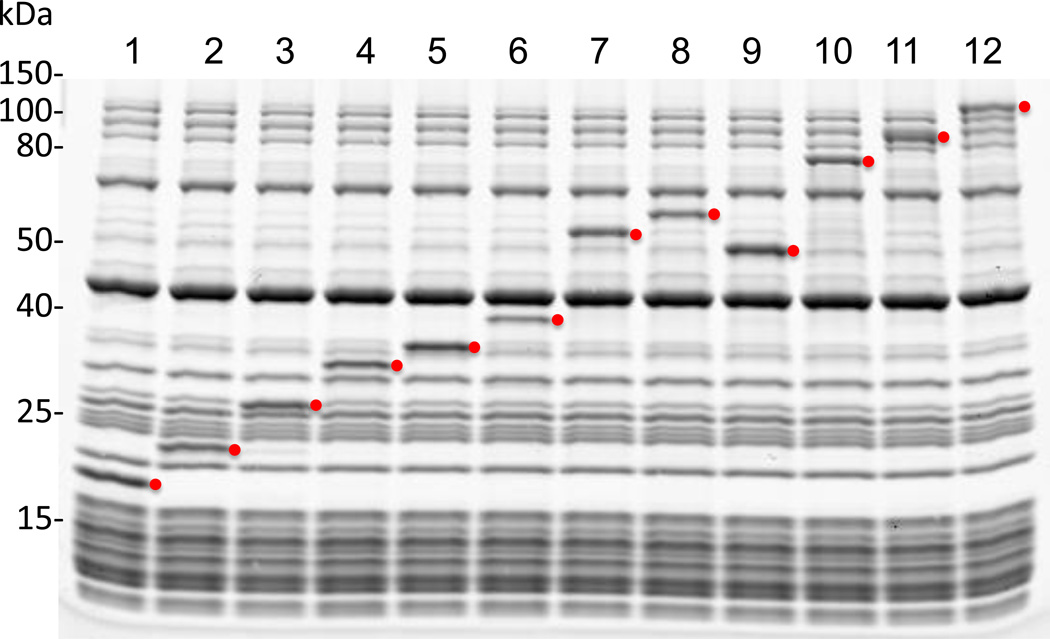
The molecular weight of the synthesized protein can be analyzed by running a SDS-PAGE gel. Because this reconstituted system contains a defined number of different components with distinct molecular weights, , a synthesized protein is often visible on a SDS-PAGE gel. See Figure 1 for results of reactions synthesizing a panel of proteins with a range of molecular weights from 17 to 123 kDa to y, run on a 4–20 % Tris-glycine SDS-PAGE gel (XREF CPMB), and visualized after Coomassie blue staining (XREF CPMB).
Figure 1.
Coomassie blue-stained SDS-PAGE gel of a panel of proteins synthesized in the PUREpxress reactions. Molecular weight standards (kDa) are shown on the left. Red dots indicate the expected positions (bands) of synthesized proteins. Lane 1, dihydrofolate reductase (control protein from DHFR control template); lane 2, CRP transcriptional dual regulator; lane 3, green fluorescent protein; lane 4, Renilla luciferase; lane 5, outer membrane protein A; lane 6, Antarctic phosphatase; lane 7, T4 DNA ligase; lane 8, Firefly luciferase; lane 9, 6-phospho-β-glucosidase A; lane 10, Vent DNA polymerase; lane 11, SP6 RNA polymerase; lane 12, E. coli β-galactosidase.
BASIC PROTOCOL 2
SYNTHESIS OF DISULFIDE-BONDED PROTEINS
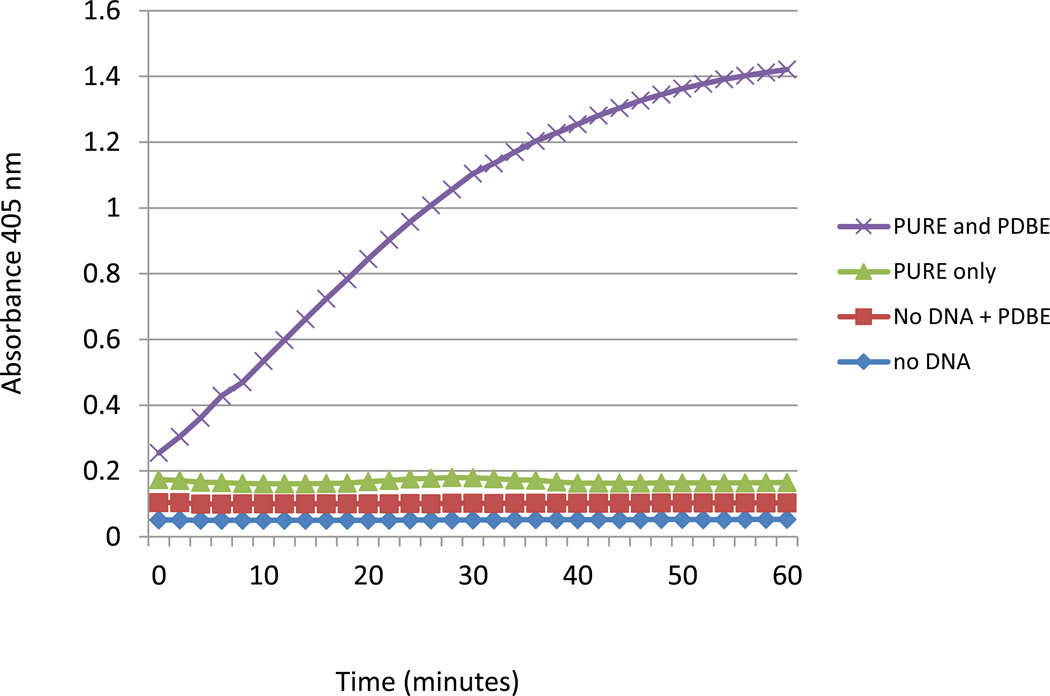
Some proteins, especially those containing multiple disulfide bonds, cannot fold correctly in reducing environments (Gwen: Mehmet Berkman protocol) and have low or no activity after expression in the PURExpress reactions (Figure 2). This class of protein includes many secreted proteins. This basic protocol describes the use of additional components, supplied as "PURExpress Disulfide Bond Enhancer", to help to correctly fold proteins that are known or expected to form disulfide bonds..
Figure 2.
Chromozyme cleavage assay (Roche) for a truncated version of tissue plasminogen activator protein (vtPA) synthesized in the PURExpress reactions with and without PURExpress Disulfide Bond Enhancer (PDBE). This tissue plasminogen activator contains 9 disulfide bonds, 8 of which are non-consecutive. Reactions with the template DNA yielded an equal amount of the protein whether PDBE was present or not (not shown).
Materials
A PURExpress In vitro Protein Synthesis kit (New England Biolabs, http//www.neb.com) containing:
Solution A (yellow tube)
Solution B (red tube)
DHFR control template
PURExpress Disulfide Bond Enhancer (New England Biolabs, http//www.neb.com) containing:
Disulfide Bond Enhancer Solution 1
Disulfide Bond Enhancer Solution 2
microcentrifuge tubes or microtiter plate
Nuclease-free H2O (Integrated DNA technologies)
Template DNA (See Support Protocol 1 and 2) or mRNA (See Support Protocol 3)
Murine RNase inhibitor (40 U/µl, New England Biolabs) or RNasin Ribonuclease inhibitor (20–40 U/µl, Promega)
Microcentrifuge
Air incubator set at 37°C
3x SDS-PAGE loading buffer (New England Biolabs, CPMB Chapter X)
SDS-PAGE gel (4–20% Tris-glycine, Life Technologies, CPMB Chapter X)
Protocol steps
-
Thaw Solutions A and B and Disulfide Bond Enhancer Solution 1 and 2 on ice.
Do not thaw Disulfide Bond Enhancer Solutions at room or higher temperatures as this would result in a loss of activity. Disulfide Bond Enhancer Solutions should be stored at −20°C after use. Also see Step annotation 1 in Basic Protocol 1 describing a basic reaction step up with PURExpress.
-
Assemble the reaction on ice with the reagents in the following order for a total of 25 µl:
- 10 µl Solution A
- 7.5 µl Solution B
- 1.5 µl Murine RNase Inhibitor
- 1 µl PURExpress Disulfide Bond Enhancer Solution 1
- 1 µl PURExpress Disulfide Bond Enhancer Solution 2
- 3 µl nuclease-free water
- 2 µl of 250 ng of DNA template or 3 µg mRNA template
Improved folding and/or activity can sometimes be achieved by altering the amount of Disulfide Bond Enhancer 1 and 2 to add. By titrating each from 0.25 µl to 2 µl per 25 µl reaction one may find the optimal amounts for the maximal activity. For example one could keep Solution 1 constant at 1 µl while changing the amount of Solution 2 added from 0.25 to 2 ul in increments of 0.25 or .5 ul per 25 ul final reaction volume and then do the same with Solution 1.
Incubate at 37°C for 2 to 4 hrs in an air incubator.
Stop the reaction by placing on ice.
-
Analyze by SDS-PAGE, take 2.5 µl of the reaction mixture, add 7.5 µl of H2O, mix with 5 µl of 3xSDS-PAGE loading buffer, heat at 95°C for 1 min and load the sample (15 µl) and run on a SDS-PAGE gel followed by Coomassie blue staining.
It is often possible to directly take an aliquot of the synthesis reaction for biochemical analysis of the activity of synthesized proteins (Figure 2).
BASIC PROTOCOL 3
PURIFICATION OF IN VITRO SYNTHESIZED PROTEINS
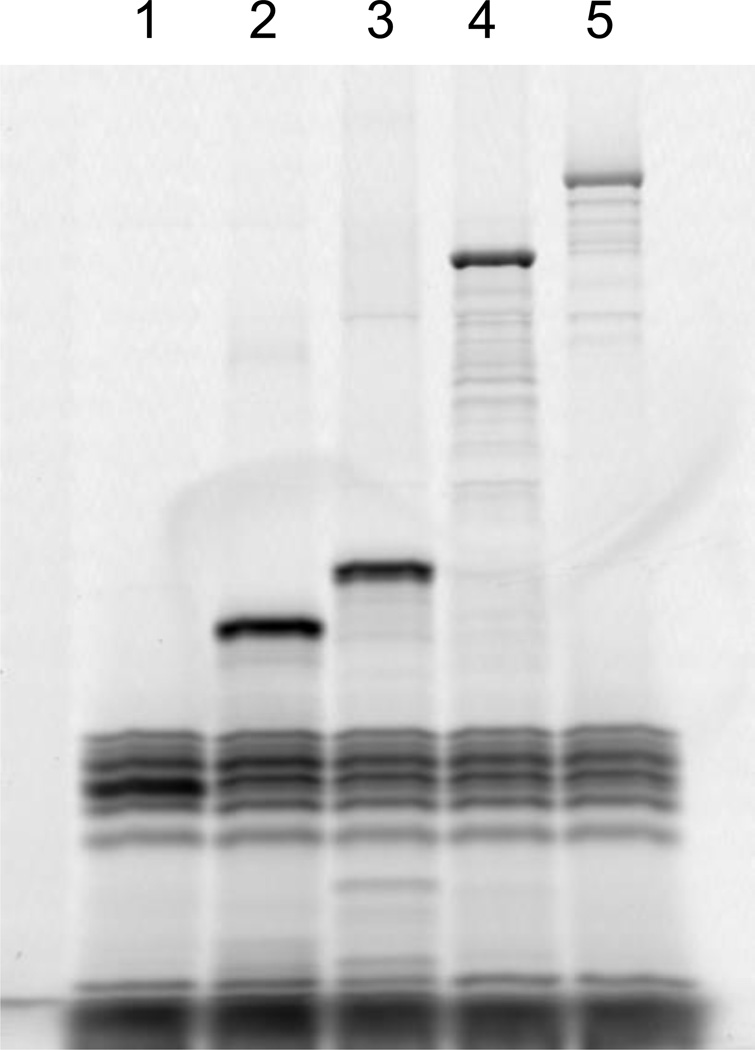
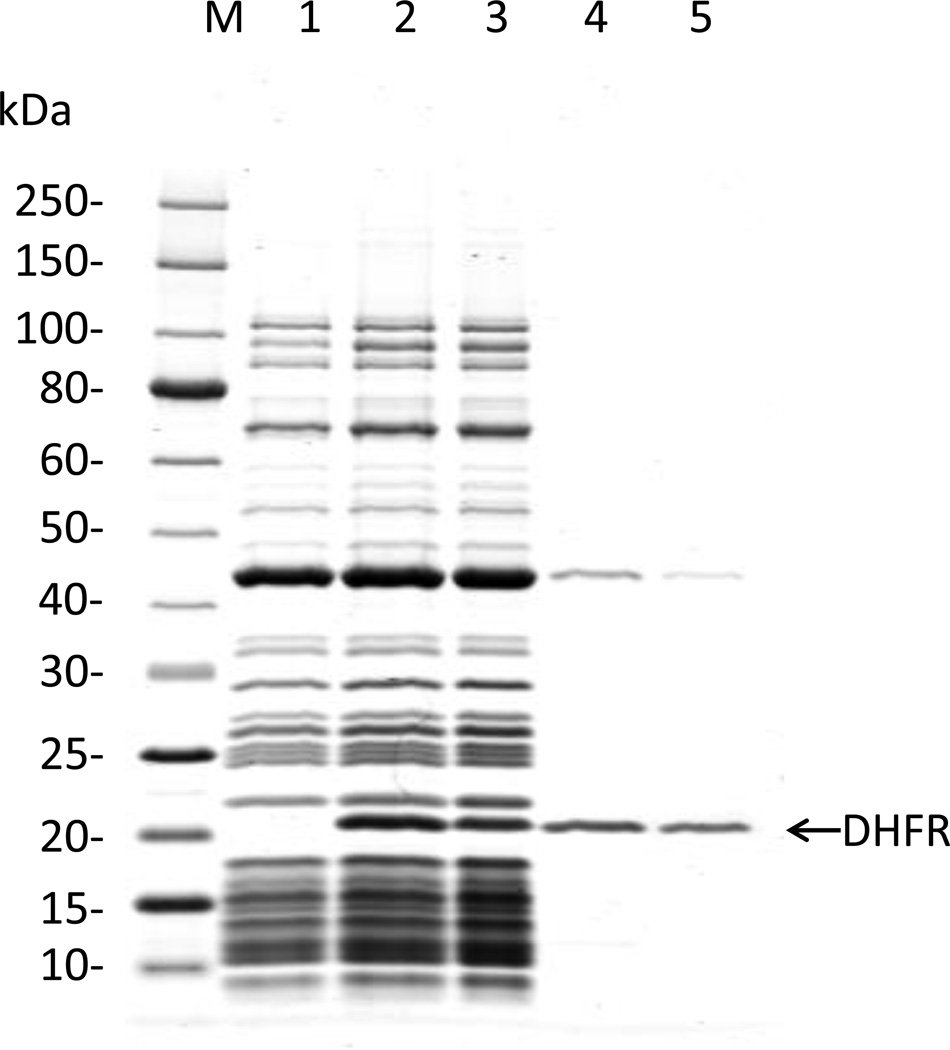
Although the activity of a synthesized protein can often be directly assessed from the synthesis reaction, it may be desirable to purify it. Unlike other extract-based cell-free systems, our system allows purification of the synthesized protein by removing the rest of the system’components. Due to their large molecular weight (>2.5 MD), ribosomes can be removed simply by ultrafiltration. In a second step, all His-tagged protein components of the system can be removed by passage through an NTA Nickel agarose column. These two treatments leave the synthesized protein in the flow through fraction. Figure 3 shows a Coomassie-blue stained SDS-PAGE of fractions from a typical reverse purification of the DHFR control protein synthesized in the PURExpress reaction.
Figure 3.
SDS-PAGE analysis of the reverse purification of the DHFR control protein synthesized in the PURExpress reaction. M: molecular weight standards (kDa); Lane 1: Control PURExpress reaction with no input template, Lane 2: PURExpress reaction with the DHFR template; Lane 3: Retentate from Amicon (0.5 ml, 100 kDa) concentrator ultrafiltration; Lane 4: Permeate (Flow through) from Amicon (0.5 ml, 100 kDa) concentrator ultrafiltration; Lane 5: Eluate (flow-through) from Ni-NTA agarose column.
Materials
A PURExpress In vitro Protein Synthesis kit (New England Biolabs, http//www.neb.com)
10 mM magnesium acetate
Amicon Ultracel −0.5 ml-100 K MW cut off spin concentrator (Millipore)
Microcentrifuge at 4°C
Ni-NTA agarose (Qiagen)
Microcentrifuge tubes
Bio-Rad micro-spin column (Bio-Rad)
Protocol steps
Set up a synthesis reaction with your template encoding a protein of interest (see Basic Protocol 1 steps 1 and 2. Incubate at 37°C for 2 to 4 hrs as described in basic protocol 1 step 3.
-
Add at least an equal volume of diluent containing 10 mM magnesium acetate to the sample.
10 mM magnesium acetate is required to keep the ribosomes intact. This is important for successfully removing the ribosomes in the ultrafiltration step. Up to 400 mM final concentration of NaCl can be included as well but is not required for the protocol. Use of 400 mM NaCl may in general minimize protein aggregation and non-specific binding. However, the salt addition in the diluent may interfere with the downstream assays and/or analysis. In such cases, salt should be avoided. It is recommended to add diluent to a minimal volume of 100 µl to reduce losses in the 2 steps during purification. Scaling up to a larger reaction may also improve recovery in this protocol.
Apply the diluted sample to an Amicon Ultracel −0.5 ml-100 K spin concentrator and centrifuge for 30 to 60 minutes at 14,000 x g at 4°C.
Transfer the permeate (flow through) to a new microcentrifuge tube.
Add 0.25 volumes of Ni-NTA agarose and mix thoroughly for 30 minutes at 4°C. This will allow the his-tagged protein components of the kit to bind the resin.
Transfer the reaction slurry to a Bio-Rad micro-spin column and centrifuge at 1,500 x g for 2 minutes at 4°C.
Collect the eluate (flow through) containing your protein of interest and proceed with analysis and/or assay.
BASIC PROTOCOL 4
ASSAY OF BACTERIAL RIBOSOMES USING PUREXPRESS ΔRIBOSOME KIT
This particular basic protocol describes the use of PURExpress Δribosome kit, in which the ribosomes are removed from the rest of the components of the standard PURExpress kit. Thus, the protein synthesis capacity of PURExpress Δribosome kit can only be restored by addition of the user-supplied ribosomes. This kit can be used to test the activity of mutant ribosomes or ribosomes from other bacteria species and is a non-radioative alternative to the traditional polyPhe assay for ribosomes that uses [14C] Phenylalanine (Kopaskie et al., 2013; Korostelev et al., 2006; Shi et al., 2011; Zhou et al., 2012).
Materials
A PURExpress® ∆Ribosome Kit (New England Biolabs, http//www.neb.com) containing:
Solution A
Factor Mix (essentially Solution B without ribosomes)
Purified E. coli ribosomes (13.3 µM, as positive control)
DHFR control template
User-supplied bacterial ribosomes
microcentrifuge tubes or microtiter plate
Nuclease-free H2O (Integrated DNA technologies)
Murine RNase inhibitor (40 U/µl, New England Biolabs) or RNasin Ribonuclease inhibitor (20–40 U/µl, Promega)
Microcentrifuge
Air incubator set at 37°C
3x SDS-PAGE loading buffer (New England Biolabs, CPMB Chapter X)
SDS-PAGE gel (4–20% Tris-glycine, Life Technologies, CPMB Chapter X)
Protocol steps
-
Thaw Solution A, factor mix and ribosomes on ice.
Gently mix Solution A and factor mix after thawed by pipetting a few times, but avoid generating air bubbles. Solution A may appear cloudy. In this case, mix well before adding to the reaction.
-
Prepare bacterial ribosome to be tested at a concentration around 13 µM. The concentration of ribosome can be estimated by absorbance at 260 nm. In the case of E. coli ribosome, 1 A260 = 60 µg/ml = 0.024 µM.
The kit is supplied with E. coli ribosome as control enough for two reactions at 13.3 µM. A total of 60 pmols of the control ribosome is used in a standard 25 µl reaction. If the ribosome to be tested has concentration higher than 13.3 µM, make up the ribosome volume to 4.5 µl with 10 mM magnesium acetate for every 25 µl reaction. Note that a lower concentration of ribosome may be used but the protein yield may decrease.
- Assemble the reaction on ice with the reagents in the following order for a total of 25 µl:
- 10 µl Solution A
- 3 µl Factor Mix
- 4.5 µl Ribosomes (13.3 µM)
- 0.5 µl Murine RNase Inhibitor
- 5 µl Nuclease-free H2O
- 2 µl DNA template (125 ng/µl)
Incubate at 37°C for 2 to 4 hrs in an air incubator.
Stop the reaction by placing on ice.
-
For SDS-PAGE gel analysis, take 2.5 µl of the reaction mixture, add 7.5 µl of H2O, mix with 5 µl of 3xSDS-PAGE loading buffer, heat at 95°C for 1 min and load the sample (15 µl) and run on a SDS-PAGE gel followed by Coomassie blue staining.
If the control (DHFR) DNA template is used, the activity of the ribosome is correlated to the expression of DHFR as analyzed by the SDS-PAGE gel. Other reporter DNA templates such as those expressing GFP or luciferase can be used. In this case, the activity of the ribosome can be tested by taking aliquots of the reaction and measuring protein activity by fluorescence or luminescence.
SUPPORT PROTOCOL 1
PREPARATION OF PLASMID (CIRCULAR) DNA TEMPLATE
This protocol describes the essential elements of plasmid (circular) DNA template for use with the PURExpress system. Since the system contains T7 RNA polymerase to allow coupled transcription and translation of proteins under a T7 promoter,. The DNA template design and quality and the coding sequence of the protein of interest all affect the efficiency of transcription, translation and therefore protein yield. The yields can vary between less than 10 ng/ml to more than 100 ng/ml.
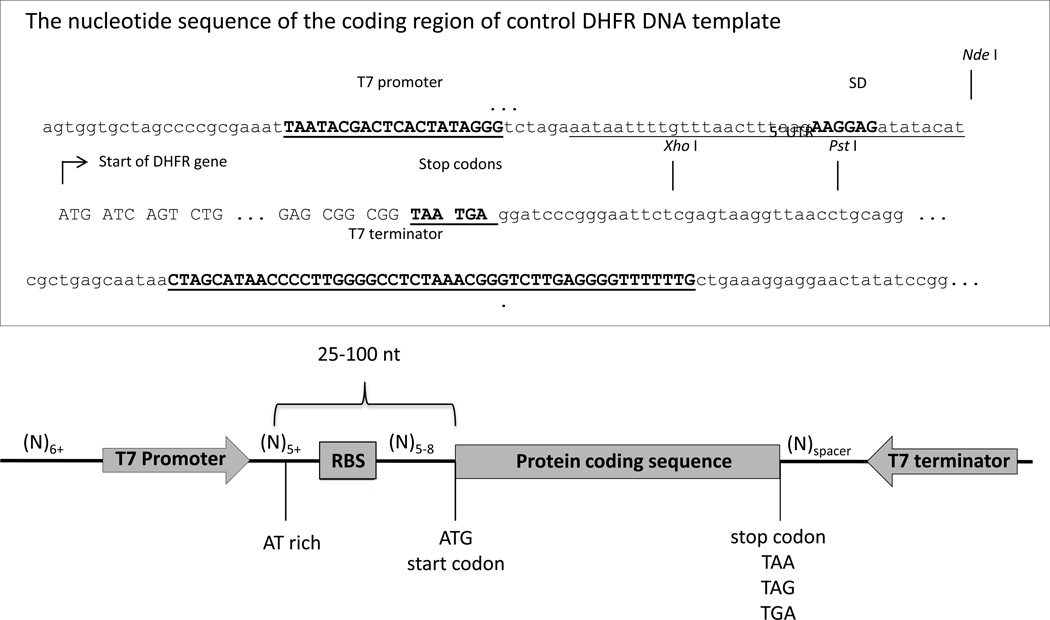
Generally, a plasmid suitable for expression in the system must have a 5’ untranslated region (5’ UTR), containing a T7 promoter followed by a ribosome binding site (Shine-Delgarno sequence (SD)) (xref CPMB) upstream of the ATG start codon of the protein coding sequence, and a 3’ untranslated region (3’UTR), containing a T7 terminator sequence (Figure 5). The region between the T7 promoter and the ribosome-binding site should be AT rich and at least 5 nucleotides long. The ribosome-binding site should be 5 to 8 nucleotides from the start codon. Researchers can start by cloning a gene of interest into a commercial T7 vector such as a pET vectors (Chapter xx, available through Novagen) or the DHFR control template plasmid provided with the system that expresses E. coli dihydrofolate reductase (DHFR) (see the sequence and restriction sites in Figure 5).
Figure 5.
Circular (plasmid) DNA template design.
If a different promoter, such as SP6 promoter or an E. coli sigma 70 promoter, is used in the plasmid, the specific RNA polymerase, such as 2 µl per 25 µl final volume of SP6 RNA polymerase (20 unit/µl, New England Biolabs, CPMB xxxx) or sigma 70-saturated E. coli RNA polymerase holoenzyme (1 unit/µl, New England Biolabs, CPMB chapter X), respectively, must be added to the reaction (Asahara and Chong, 2010).
Template purity is very important for successful in vitro transcription/translation. For best results, template DNA should be free of nucleases (DNases and RNases) and other potential inhibitors of transcription or translation, e.g., NaCl (>50 mM), glycerol (>6%), EDTA (>1 mM), and magnesium or potassium salts (>1 mM). Plasmid DNA prepared from many commercial kits, such as QIAprep Spin Miniprep kit (Qiagen) or GenElute™ Plasmid kit (Sigma-Aldrich), often contain inhibitory amounts of RNase A. To remove RNase A, one can use the methods of phenol/chloroform extraction and ethanol precipitation (CPMB xxx) or include a commercially available RNase A Inhibitor (e.g., Murine RNAse Inhibitor, (New England Biolabs) or RNasin® (Promega)) to the PURExpress reaction.
Finally, the protein synthesis yield of the system is affected not only by the region flanking the protein coding sequence, but also by the protein coding sequence itself. Codon optimization of the gene of interest can sometimes improve the protein expression level.
Materials
QIAprep Spin Miniprep kit (Qiagen) or other commercial kits
NanoDrop (Thermo Scientific) or a similar UV spectrophotometer.
Protocol steps
Follow manufacturer’s instructions to purify plasmid DNA from E. coli culture. Resuspend the plasmid DNA in 10 mM Tris buffer (pH 8.0). Alternatively, the method of alkaline lysis followed by cesium chloride ultracentrifugation can be used.
Quantitate the plasmid concentration using NanoDrop or a UV spectrophotometer. For double-stranded DNA, 1 A260 is equivalent to 50 ng/µl. Approximately 125 ng/µl or greater is preferred for using in the PURExpress kits.
SUPPORT PROTOCOL 2
PREPARATION OF LINEAR DNA TEMPLATE BY POLYMERASE CHAIN REACTION (PCR)
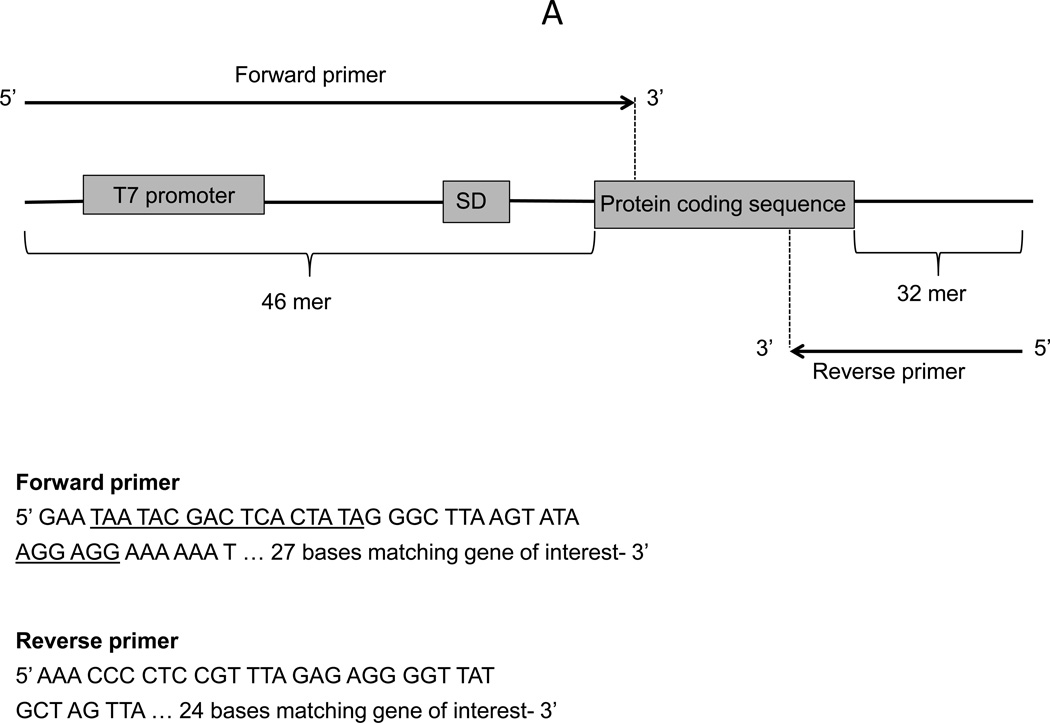
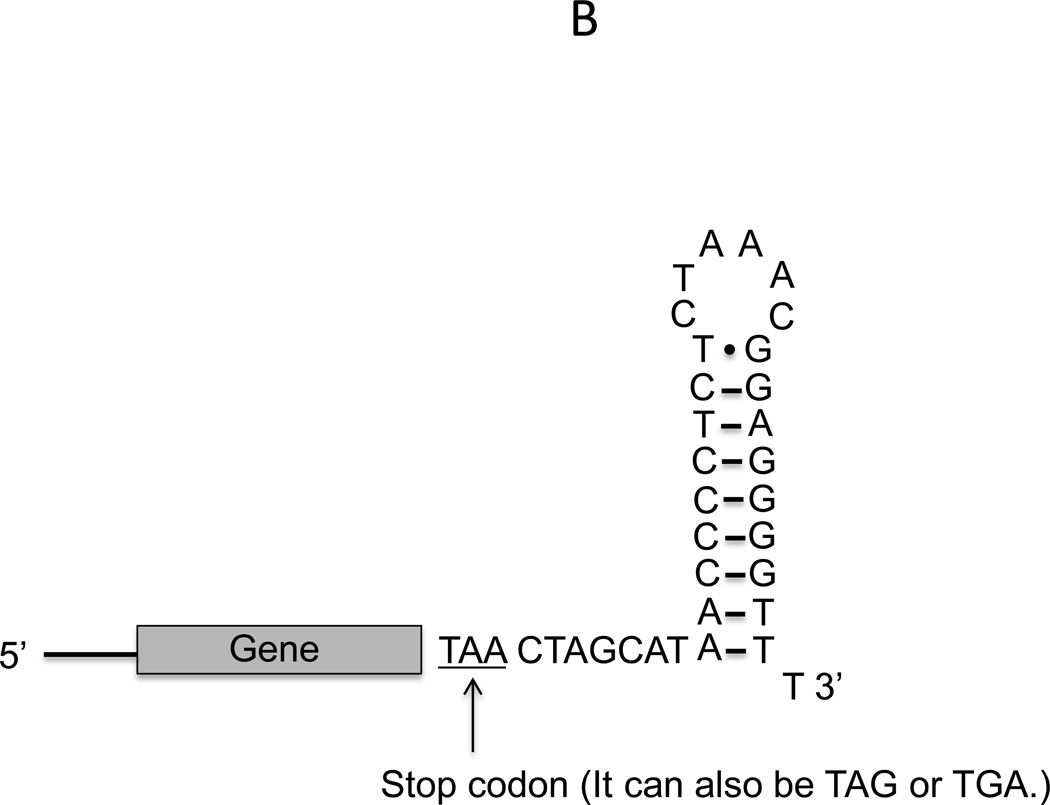
This protocol describes the preparation of the linear template DNA by using PCR to amplify the gene of interest from a plasmid, genomic DNA or cDNA. The gene of interest (the protein coding sequence) often does not have the necessary 5’ UTR and 3’ UTR necessary for expression in our system that is based on T7 promoter and T7 RNA polymerase for transcription. In this case, a T7 promoter, the ribosome binding site and T7 terminator have to be included in the PCR primer design. Researchers should use the exact sequences of the forward and reverse primers as recommended (Figure 6A). Note that the reverse primer contains a truncated shorter version of the T7 terminator to avoid a long primer. This truncated T7 terminator can still form the stem-loop structure of a transcription terminator (Figure 6B) and results in a similar protein synthesis yield as the full-length T7 terminator (Chong lab, unpublished results).
Figure 6.
A. Linear DNA template and primer design; B. The stem-loop structure formed by the 3’ UTR of the reverse primer.
Materials
Primers designed according to Figure 6A
A plasmid, genomic DNA or cDNA containing the protein coding sequence
Q5® High-Fidelity 2x Master Mix (New England Biolabs) or a commercial PCR kit (e.g, AccuPrime Pfx SuperMix, Life Technologies)
QIAquick PCR Purification Kit (Qiagen)
NanoDrop (Thermo Scientific)
Protocol step
-
Amplify protein coding sequences from the plasmid, genomic DNA or cDNA following the protocol of Q5® High-Fidelity 2x Master Mix or other protocol from a commercial PCR kit. Perform two PCR reactions (50 µl each) for each protein coding sequence.
Using the optimal annealing temperature calculated by NEB Tm Calculator (https://www.neb.com/sitecore/content/nebsg/home/tools-and-resources/interactive-tools/tm-calculator) or other calculator is recommended.
Combine the two reactions and purify the PCR products by QIAquick PCR Purification Kit or other commercial PCR purification kit.
-
Elute the linear DNA template in 50 µl Tris buffer (pH 8.0) and measure the DNA concentration by NanoDrop.
A typical concentration of purified DNA is between 100 ng/µl to 200 ng/µl.
-
Take 1 µl and run a 1% agarose gel to check the quality of DNA.
The products of the PCR reaction should be examined on an agarose gel prior to the addition to the protein synthesis reaction to ensure the linear DNA template with the correct size is the dominant band. If many additional bands are observed, PCR conditions should be optimized to eliminate these bands, which otherwise may be translated into truncated products, thereby decreasing the yield of the correct product. Purifying the linear DNA template from the agarose gel is not recommended as we observe that gel purified DNA results in lower yield in our system than kit purified DNA (unpublished observation).
SUPPORT PROTOCOL 3
PREPARATION OF mRNA TEMPLATE BY IN VITRO TRANSCRIPTION
This protocol describes the preparation of mRNA by in vitro transcription as mRNA can also be used as the template for protein synthesis in our system. The sequence elements for mRNA template should contain proper 5’UTR and 3’ UTR that include the ribosome binding site and a stem loop structure at the 3’ region as described in Figure 6. The amount of input mRNA template (transcribed in vitro or purified from cells) can affect the protein synthesis yield and should be determined empirically. Generally, 1–5 µg mRNA template per 25 µl reaction is a good starting point.
Materials
T7 Quick High Yield RNA Synthesis kit (New England Biolabs) containing
NTP buffer mix
T7 RNA polymerase mix
Fluc control template
DNase I (RNase-free)
LiCl solution
Linear DNA template with T7 promoter and 5’ UTR and 3’ UTR as described in Figure 6.
MEGAclear™ Transcription Clean-up kit (Life Technologies) or other commercial kit for mRNA purification.
Nanodrop (Thermo Scientific)
Lonza Flash Gel RNA Cassette 1.2% agarose gel (Lonza)
Novex TBE-Urea gels 5–15% (Life Technologies)
Protocol steps
-
Assemble the transcription reaction mix on ice with the reagents in the following order for a total of 20 µl:
- 3 µl Nuclease-free water
- 10 µl NTP buffer mix
- 5 µl Linear DNA Template (30 ng/ul)
- 2 µl T7 RNAP Polymerase mix
We recommend using 1 µg linear plasmid DNA or 0.1–0.5 µg PCR fragment as template in each 20 µl transcription reaction
-
Mix thoroughly and pulse-spin in a microfuge. Incubate at 37 °C for 2 hrs.
The transcription reaction time will depend on the template amount, quality and the length of RNA transcript. For transcripts longer than 0.3 kb, the maximum yield can be reached at about 2 hour incubation. For short RNA transcript (<0.3 kb), the yield can be improved by incubating at 4 hrs or longer. Additional recommendation for transcription reaction assembly for short transcripts (<0.3 kb) can be found at https://www.neb.com/protocols/2013/04/02/standard-rna-synthesis-e2050. An extended incubation at 16 hrs (overnight) has no detrimental effects on the products of the reaction. For reaction within 1 hour, a water bath or heating block may be used. However, for reaction longer than 1 hour, a dry air incubator or a thermocycler is recommended to minimize evaporation from the liquid at the bottom of the tube and condensation on the walls and lids.
-
Optional: Remove DNA template from the reaction using DNase I. Dilute the reaction mixture by adding 30 µl nuclease-free water to each 20 µl reaction. Add 2 µl of DNase I (RNase-free), mix and incubate for 15 minutes at 37 °C.
Up to 10 mg/ml of RNA can be generated in a standard transcription reaction, resulting a quite viscous reaction mixture. Therefore, it is easier to perform the DNase treatment after the reaction mixture is diluted.
Proceed with purification of synthesized RNA following the instructions provided by MEGAclear™ kit.
Determine the RNA transcript concentration by UV absorption at 260 nm. For single-stranded RNA, 1 A260 is equivalent to an RNA concentration of 40 µg/ml.
Analyze transcription products by gel electrophoresis. Use a 1 % agarose gel for transcripts larger than 0.3 kb, and a denaturing polyacrylamide gel (5–15 %) such as TBE-Urea gels (crossref with CPMB, Urea or other gels) for smaller transcripts (<0.3 kb). The denaturing polyacrylamide gel is run under denaturing conditions to prevent formation of secondary structures of the transcript.
SUPPORT PROTOCOL 4
SYNTHESIS AND ANALYSIS OF FLUORESCENT LABELED PROTEINS
This support protocol describes the use of FluoroTect™GreenLys in vitro Translation Labeling System from Promega to fluorescently label a protein synthesized in the PURExpress system. The FluoroTect™GreenLys system contains a charged epsilon-labeled fluorescent lysine-tRNALys, which can be incorporated into the lysine codons of synthesizing proteins. Labeling the synthesized protein facilitates the downstream analysis on a SDS-PAGE gel, especially when the expression level is low or the synthesized protein runs at the same positions as one of the protein components of the reconstituted synthesis mixture. To detect the fluorescence, the SDS-PAGE gel should be analyzed without Coomassie blue staining by a laser scanner capable of biomolecular imaging of fluorescence (Figure 4).
Figure 4.
Scanned image of a SDS-PAGE gel of proteins synthesized in the PURExpress reactions and labeled with FluoroTect™ GreenLys. Lane 1: DHFR; lane 2: GFP; lane 3: Renilla luciferase; lane 4: Firefly luciferase; lane 5: E. coli β-galactosidase.
Materials
FluoroTect GreenLys in vitro Translation Labeling System (Promega) containing
FluoroTect GreenLys tRNA
PURExpress® In vitro Protein Synthesis kit (New England Biolabs) containing:
Solution A (yellow tube)
Solution B (red tube)
DHFR control template
Nuclease free microcentrifuge tubes or microtiter plate
Nuclease free H2O
Template DNA (See Support Protocol 1 and 2) or mRNA (See Support Protocol 3)
Murine RNase inhibitor (40 U/µl, New England Biolabs))
Microcentrifuge
Air incubator set at 37°C
3x SDS-PAGE loading buffer (New England Biolabs)
SDS-PAGE gel (4–20 % Tris-glycine, Life Technologies)
Typhoon or other fluorescent Imaging System (GE Healthcare Life Sciences) with excitation and emission wavelengths of 532 nm and 526 nm, respectively..
Protocol steps
Thaw Solutions A and B on ice. Thaw a tube containig FluoroTect GreenLys tRNA by quick hand-warming and immediately place on ice
- Assemble the reaction on ice with the reagents in the following order for a total of 25 µl:
- 10 µl Solution A
- 7.5 µl Solution B
- 0.5 µl Murine RNase Inhibitor
- 1 µl of FluoroTect GreenLys tRNA
- 4 µl Nuclease free H2O
- 2 µl of DNA template (250 ng) or mRNA template (3 µg).
Incubate at 37 °C for 2 to 4 hr. Stop the reaction by placing on ice.
Analyze by SDS-PAGE, take 2.5 µl of the reaction mixture, add 3.5 µl of H2O, mix with 3 µl of 3xSDS-PAGE loading buffer, heat at 95°C for 1 min and load the sample (9 µl) and run on a SDS-PAGE gel.
-
After gel electrophoresis, scan the gel using Typhoon Scanner with Ex/Em = 532 nm/526 nm.
We observe that the FluoroTect™ GreenLys tRNA cause some low molecular weight bands on the SDS-PAGE gel that can co-migrate with the synthesized protein (e.g., Figure 4, lane 1).
SUPPORT PROTOCOL 5
SYNTHESIS AND ANALYSIS OF [35S]-METHIONINE LABELED PROTEINS
This support protocol describes the use of [35S]-L-methionine to radiolabel a protein synthesized in our system. Radiolabeling facilitates the analysis of the size and yield of the synthesized protein and the translational efficiency, especially when the expression level is low or the synthesized protein runs at the same positions as one of the protein components of the in vitro synthesis reaction on a SDS-PAGE gel.
Materials
PURExpress In vitro Protein Synthesis kit (New England Biolabs) containing:
Solution A (yellow tube)
Solution B (red tube)
DHFR control template
Nuclease free microcentrifuge tubes or microtiter plates
Nuclease free H2O
Template DNA (See Support Protocol 1 and 2) or mRNA (See Support Protocol 3)
Murine RNase inhibitor (40 U/µl, New England Biolabs)
[35S]-L-Methionine (15 mCi/ml, 1000 Ci/mmol) (PerkinElmer)
Microcentrifuge
Air incubator set at 37°C
3x SDS-PAGE loading buffer (New England Biolabs)
SDS-PAGE gel (4–20 % Tris-glycine, Life Technologies)
Filter paper (Whatman)
Vacuum gel dryer
X-ray film or phosphorimager
Protocol steps
Thaw Solutions A and B and [35S]-L-Methionine on ice
- Assemble the reaction on ice with the reagents in the following order for a total of 25 µl:
- 10 µl Solution A
- 7.5 µl Solution B
- 0.5 µl Murine RNase Inhibitor
- 3 µl Nuclease free H2O
- 2 µl of DNA template (250 ng) or mRNA template (3 µg).
- 2 µl [35S]-L-Methionine
Incubate at 37 °C for 2 hrs and stop the reaction by placing on ice.
Take 2.5 µl of the reaction mixture, add 3.5 µl of H2O, mix with 3 µl of 3xSDS-PAGE loading buffer, heat at 95°C for 1 min and load the sample (9 µl) and run on a SDS-PAGE gel.
Dry the gel onto filter paper for 30 min at 80°C and expose to X-ray film or analyze by a phosphorimager.
SUPPORT PROTOCOL 6
PREPARATION OF BACTERIAL RIBOSOMES
This protocol describes rapid purification of ribosomes from E. coli or other bacterial species using ultracentrifugation following a protocol modified from (Ohashi et al., 2007a). If further purification is necessary, a butyl column can be used (Ohashi et al., 2007b). Buffer conditions may vary depending on the source of bacterial ribosomes. Other reported protocols can also be used (Ederth et al., 2009; Expert-Bezancon et al., 1974; Le Goffic et al., 1974; Maguire et al., 2008; Trauner et al., 2011).
Materials
Centrifuge (BECKMAN COULTER)
JS-4.2 rotor (BECKMAN COULTER)
JLA-16.25 rotor (BECKMAN COULTER)
Incubator
Cell Disrupter (CONSTANT CELL DISRUPTION SYSTEMS)
Ultracentrifuge (BECKMAN COULTER)
SW32 rotor (BECKMAN COULTER)
Ultra-Clear Centrifuge Tubes, 1 × 3½ in. (BECKMAN COULTER)
Slide-A-Lyzer, 10,000 MWCO, 3–12 ml capacity (Thermo Scientific)
NanoDrop (Thermo Scientific)
Protocol steps
-
Harvest bacterial cells in mid-log growth phase at OD600 = 0.6 – 0.7.
Harvested cells can be stored at −80°C. All steps are performed at 4°C or on ice.
Resuspend 80 g (wet weight) of cells with 200 ml of Lysis Buffer.
Centrifuge at 4,000 rpm for 15 min using JS-4.2 rotor.
Wash the cells by resuspending with 200 ml of Lysis Buffer.
Centrifuge at 4,000 rpm for 15 min using JS-4.2 rotor.
Resuspend the cells with 200 ml of Lysis Buffer.
-
Lyse the cells by Cell Disrupter at 32 kpsi.
FRENCH® Press (Thermo IEC) can also be used to lyse cells. Sonication is not recommended.
Centrifuge at 30,000 g for 30 min using JLA-16.25 rotor.
Collect the supernatant and repeat step 8.
Layer 25 ml of supernatant onto 13 ml of Sucrose Buffer in 6 centrifuge tubes (1 × 3½ in).
Spin in ultracentrifuge at 28,000 rpm for 22 hrs using SW-28 rotor.
Discard supernatant.
Wash pellets 2 times with 5 ml of Storage Buffer to completely remove a yellow layer on the pellets.
Add 1 ml of Storage Buffer, cover the tubes with parafilm and keep them in a cold chamber overnight to dissolve the pellets. Occasionally stir the solution gently.
Use Slide-A-Lyzer (10,000 MWCO, 3–12 ml capacity) to dialyze the ribosome solution against 1 L of Storage Buffer at 4°C overnight. Centrifuge the dialyzed solution at 30,000 g for 10 min to remove any precipitated material.
-
Measure A260 using NanoDrop and calculate the ribosome concentration.
The concentration of ribosomes can be estimated by absorbance at 260 nm. In case of E. coli ribosome, 1 A260 = 60 µg/ml = 0.024 µM. For accurate determination of ribosome concentration, make serial dilutions of the ribosome solution with H2O, confirm the linear correlation between dilutions and A260 readings, and extrapolate the A260 reading to 1x dilution.
SUPPORT PROTOCOL 7
PROTEIN SYNTHESIS USING the PUREXPRESS ∆(aa, tRNA) kit
This protocol describes assembling a reaction using the PURExpress∆ (aa, tRNA) kit, in which amino acids and tRNA mix are omitted from Solution A and provided separately as controls. This kit can be used for applications such as incorporation of unnatural (i.e, beyond the canonical 20) amino acids into the protein, labeling with amino acids that contain stable isotopes (Tada et al., 2012; Welsh et al., 2011), or any cases requiring protein synthesis in vitro in which the where the research wish to control the concentrations or contents of amino acids and tRNAs (Martinez et al., 2014; Rosenblum et al., 2013).
Materials
A PURExpress∆ (aa, tRNA) kit (New England Biolabs) containing:
Solution A (minus aa, tRNA)
Solution B
Amino acid mix (control)
E. coli tRNA mix (control)
DHFR control template
Amino acids in solution at least 3 mM in concentration if desired
tRNA supplement at 20 mg/ml if desired
Nuclease free microcentrifuge tubes or microtiter plate
Nuclease free water
Template DNA (support protocol 1 and 2) or mRNA (support protocol 3)
Murine RNase inhibitor (40U/µl, New England Biolabs)
Microcentrifuge
Air incubator at 37°C
Protocol steps
-
Thaw all reagents on ice.
Solution A (minus aa, tRNA) has a cloudy appearance after thawed. Mix well and add to the reaction assembly. The cloudiness will disappear once the reaction is fully assembled.
- Assemble the reaction on ice with the reagents in the following order for a total of 25 µl:
- 5 µl Solution A (minus aa, tRNA)
- 2.5 µl 3 mM control amino acid mix or your amino acid mix
- 2.5 µl control E. coli tRNA (20 µg/µl) or your tRNA mix
- 7.5 µl Solution B
- 0.5 µl Murine RNAse Inhibitor
- 5 µl Nuclease free H2O
- 2 µl of DNA template (100 – 150 ng, plasmid or PCR product)
Incubate at 37 °C for 2 to 4 hrs in an air incubator.
Analyze by SDS-PAGE-take 2.5 µl of the reaction mixture, add 7.5 µl of H2O, mix with 5 µl of 3x SDS-PAGE loading buffer, heat at 95 °C for 1 min and load the sample (15 µl) and run on a SDS-PAGE gel followed by Coomassie blue staining.
Step annotations
SUPPORT PROTOCOL 8
PROTEIN SYNTHESIS USING PUREXPRESS ∆RF123 kit
This protocol describes assembling a reaction using a kit, in which the release factors 1, 2 and 3 are omitted from Solution B and provided separately as controls. This kit can be used for applications such as unnatural amino acid incorporation via nonsense suppression, ribosome display and mRNA display, or any application where researchers wish to prevent the release factors from releasing the nascent polypeptide chain from ribosomes and maintain the association of mRNA with the nascent polypeptide chain (Kogure et al., 2013; Ohashi et al., 2007a; Shimizu et al., 2001).
Materials
A PURExpress ∆RF123 kit (New England Biolabs, http//www.neb.com) containing:
Solution A
Solution B (minus RF1, RF2, RF3)
Solutions of purified RF1, RF2 and RF3 (controls)
DHFR control template
Nuclease free microcentrifuge tubes or microtiter plate
Nuclease free water
Template DNA (support protocol 1 and 2) or mRNA (support protocol 3)
Murine RNase inhibitor (40U/µl, New England Biolabs)
Microcentrifuge
Air incubator at 37°C.
Protocol steps
Thaw all reagents on ice.
- Assemble the reaction on ice with the reagents in the following order for a total of 25 µl:
- 10 µl Solution A
- 7.5 µl Solution B (minus RF1, RF2, RF3)
- 0.5 µl each of release factors 1, 2, and/or 3 (optional)
- 0.5 µl Murine RNase Inhibitor
- 5 µl Nuclease free H2O
- 2 µl of DNA template (100 – 150 ng, plasmid or PCR product).
Incubate at 37 °C for 2 to 4 hrs in an air incubator.
Analyze by SDS-PAGE-take 2.5 µl of the reaction mixture, add 7.5 µl of H2O, mix with 5 µl of 3x SDS-PAGE loading buffer, heat at 95 °C for 1 min and load the sample (15 µl) and run on a SDS-PAGE gel followed by Coomassie blue staining
REAGENTS AND SOLUTIONS
Lysis Buffer (20 mM Tris-HCl, pH 7.5, 100 mM NH4Cl, 10.5 mM MgCl2, 0.5 mM EDTA, 2 mM DTT, 6 mM β-melcaptoethanol). It should be prepared fresh.
Sucrose Buffer (20 mM Tris-HCl, pH 7.5, 500 mM NH4Cl, 10.5 mM MgCl2, 0.5 mM EDTA, 2 mM DTT, 6 mM β-melcaptoethanol, 1.1 M Sucrose). It should be prepared fresh.
Storage Buffer (25 mM Hepes-KOH, pH 7.5, 100 mM NH4OAc, 10 mM Mg(OAc)2, 2 mM DTT, 6 mM β-melcaptoethanol). It should be prepared fresh.
COMMENTARY
Background Information
The PURExpress system from New England Biolabs is based on the PURE system originally developed by the Ueda group (Shimizu et al., 2001), and later commercialized as PURESYSTEM® (Shimizu and Ueda, 2010). Our system maintains the original two-tube format (Solution A and Solution B) of the Ueda system and follows essentially the same protocols to purify the ribosomes and each protein component.
Critical Parameters
Solutions A and B in PURExpress® kits are a balanced mixtures of more than 70 individual components. So care should be taken to handle both solutions. Always thaw on ice and store at −80°C after use. Avoid multiple freeze-thaw cycles (>5 times). Aliquot Solutions A and B separately if necessary.
Template quality, design and amount will significantly impact the yield of protein synthesis. It is important to follow the guidelines described in the protocols. When using the PCR-generated DNA fragment as the template, make sure to check the quality of DNA and purify DNA with a commercial spin column. Gel purification is not recommended. When using PURExpress Disulfide Bond Enhancer, the activity of the synthesized disulfide-bonded protein can sometimes be further enhanced by carefully titrating both Disulfide Bond Enhancer 1 and 2 to an optimal ratio. When performing the reverse purification, make sure to dilute the reaction to at least 100 µl in volume. This reduces losses in the ultrafiltration and chromatography steps. Scaling up to a larger reaction is also recommended.
Troubleshooting
Low protein yield or no protein
Solutions A and B inactivated
If no protein product is detected, the DHFR control template should be used to verify that Solutions A and B have not been compromised. Ensure the solutions are stored properly at −80 °C and freeze/thaw cycles are minimized.
Nuclease and other contamination
Ensure that nucleases are not introduced with DNA or RNA templates prepared by a commercial kit or from environmental contamination. Work with RNase-free reagents, wear gloves and use an RNase inhibitor, especially if the commercial DNA prep kit includes RNase A in the reagents. If template contamination is suspected, mix the control DHFR template with the template in question and use the mixture in a PURExpress reaction. If contamination is present, DHFR will not be translated. A phenol:chloroform extraction followed by ethanol precipitation may remove contaminants from your template. Be careful to remove all traces of ethanol. The template should be of a high quality. DNA purified from the agarose gel is not recommended, as inhibitors of translation can be present.
Poor template design
5’ UTR can be less than optimal and form unfavorable secondary structure that reduces translation initiation. The sequence flanking the ATG start codon is especially important (Voges et al., 2004). In general, the nucleotides flanking the ATG start codon should be AT-rich, since GC-rich sequences can form more stable secondary structure, especially with the ribosome-binding site, which can adversely affect translation initiation (Voges et al., 2004).. For a plasmid, a T7 terminator at the 3’ UTR is recommended so that energy in the system is not wasted on transcription beyond the protein-coding region. When using a PCR-generated template, make sure the T7 terminator or suggested truncated T7 terminator is present in the 3’ UTR (Figure 6). This stabilizes the transcribed mRNA to ensure availability for translation. It is important to include all recommended 5’ UTR and 3’ UTR elements in the template (see Figure 5 and 6). Lastly, optimizing the gene of interest to E. coli codon usage may help to increase the yield in our E. coli based system. Many gene synthesis companies offer online tool to optimize the codon usage for a given gene for expression in E. coli (e.g., .https://www.idtdna.com/CodonOpt)
Sub-optimal template concentration
The optimal amount of template for the maximal yield may be template-dependent. For best results, titrate the amount of the template as outlined in Basic protocol 1 step 2 annotation).
Poor resolution on the SDS-PAGE gel
The synthesized protein can run at the same position as one of the components of the PURExpress reaction on the 4–20% SDS-PAGE gel. One can use 4% or 18% non-gradient SDS-PAGE gel to better separate large or small proteins, respectively. If overlapping still occurs, label the synthesized protein with FluoroTect™ GreenLys tRNA (Promega) or [35S]-L-Methionine and repeat the gel analysis by following Support Protocols 4 and 5 (Figure 4).
Aggregation of the synthesized protein
Some proteins may aggregate after the synthesis and precipitate out of the solution. Researchers can assess the possibility of protein aggregation by running SDS-PAGE gels before and after centrifugation (21,600 g for 30 min) of the reaction mixture (Niwa et al., 2012; Niwa et al., 2009). The aggregated protein is absent from the bands on the SDS-PAGE gel after the centrifugation. Performing the PURExpress reaction at lower temperatures, e.g., 30 °C for 16 hrs (overnight), may improve the solubility (but at the cost of a lower yield). Alternatively, adding purified chaperones (such as DnaK or GroE chaperones) can also help to prevent aggregation (Shimizu et al., 2005).
Secondary translation products
Rare codons, internal start codons, internal ribosome binding site and premature termination all can result in the synthesis of amino or carboyxl truncated protein products. Researchers can sometimes diminish premature termination by supplementing the PURExpress reaction with rare tRNAs or by codon optimization of the gene of interest. They can sometimes diminish internal translational starts by changing the gene sequence to produce less favorable downstream ribosome binding sites and mutagenizing the sequence to eliminate downstream ATGs. Other causes of secondary products include the presence of additional DNA fragments in the PCR-generated template. Since gel purification of DNA template is not recommended, one should optimize the PCR reaction to eliminate the other fragments..
Low protein recovery after reverse purification
The reverse purification protocol can result in an essentially pure protein. The recovery yield of the reverse purification can be low due to several reasons. Because liquid can be lost on the two columns, diluting the PURExpress reaction to an input volume of 100 µl or more will reduce recovery. The Amicon concentrator (100 kDa MW cutoff) may not allow high-molecular-weight proteins to pass through even if they are smaller than 100 kDa. In fact, the ultrafiltration step is not recommended for proteins larger than 70 kDa. For the same reason, strong protein-protein and/or protein-ribosome interactions may result in effective molecular weights higher than the cutoff and reduce recovery. Using 400 mM NaCl in addition to 10 mM magnesium acetate may help dissociate such protein complexes without disrupting the integrity of the ribosome. Ribosomal proteins are not his-tagged so will contaminate your protein of interest if the ribosome is disrupted.
Purified ribosomes have low activity
Cell growth critically impacts the activity of bacterial ribosomes purified by ultracentrifugation. The ribosomes isolated from exponentially growing cells (at mid-log phase) often give the highest activity. Freshly harvested cells should be used for purification. Cells should be broken by a cell disruptor or French press (crossreference protein purification chapter). Sonication is not recommended. 10 mM or higher Mg2+ should be maintained throughout the purification process.
Anticipated Results
The average yield is approximately 100 µg/ml, or 2.5 ug per 25ul reaction. Proteins larger than E. coli β-galactosidase (123 kDa) may be translated but with a reduced yield. If the protein synthesis is successful, the synthesized protein can often be visible on a Coomassie blue stained SDS-PAGE gel (Figure 1). If the expression is low or the synthesized protein runs at the same position as one of the components of the PURExpress reaction, the protocols for fluorescent labeling or radiolabeling proteins should be followed. In this case, only the newly synthesized protein is labeled and visualized on a SDS-PAGE gel.
The typical yield of the ribosome purification (Support Protocol 6) is 1–2 mg per 1 g (wet weight) of cells.
Time Considerations
Typical reactions run for 2 to 4 hrs. SDS-PAGE analysis and purification can be performed immediately afterwards or reactions can be stored at −20°C for later analysis. Running SDS-PAGE gel followed by Coomassie blue staining usually take 2–3 hrs. All Basic Protocols can be performed within a day. Synthesis and analysis of [35S]-L-methionine labeled proteins (Support Protocol 5) can take 2 days due to gel drying and exposure to X-ray film. Ribosome preparation (Support Protocol 6) can take 3 days starting from cell lysis.
ACKNOWLEDGEMENT
We would like to thank Drs. Dongxian Yue, Eric Cantor and Chris Noren for comments and suggestions. This work was funded by NIH grant GM086930.
Footnotes
INTERNET RESOURCES (optional)
For more information on kits, updates, and frequently asked questions (FAQs).
LITERATURE CITED
- Asahara H, Chong S. In vitro genetic reconstruction of bacterial transcription initiation by coupled synthesis and detection of RNA polymerase holoenzyme. Nucleic Acids Res. 2010;38:e141. doi: 10.1093/nar/gkq377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ED, Gan R, Hodgman CE, Jewett MC. Cell-free protein synthesis: applications come of age. Biotechnol Adv. 2012;30:1185–1194. doi: 10.1016/j.biotechadv.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ederth J, Mandava CS, Dasgupta S, Sanyal S. A single-step method for purification of active His-tagged ribosomes from a genetically engineered Escherichia coli. Nucleic Acids Res. 2009;37:e15. doi: 10.1093/nar/gkn992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expert-Bezancon A, Guerin MF, Hayes DH, Legault L, Thibault J. Preparation of E. coli ribosomal subunits without loss of biological activity. Biochimie. 1974;56:77–89. doi: 10.1016/s0300-9084(74)80357-3. [DOI] [PubMed] [Google Scholar]
- Katzen F, Chang G, Kudlicki W. The past, present and future of cell-free protein synthesis. Trends Biotechnol. 2005;23:150–156. doi: 10.1016/j.tibtech.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Kogure H, Handa Y, Nagata M, Kanai N, Guntert P, Kubota K, Nameki N. Identification of residues required for stalled-ribosome rescue in the codon-independent release factor YaeJ. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopaskie KS, Ligtenberg KG, Schneewind O. Translational regulation of Yersinia enterocolitica mRNA encoding a type III secretion substrate. J Biol Chem. 2013;288:35478–35488. doi: 10.1074/jbc.M113.504811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korostelev A, Trakhanov S, Laurberg M, Noller HF. Crystal structure of a 70S ribosome-tRNA complex reveals functional interactions and rearrangements. Cell. 2006;126:1065–1077. doi: 10.1016/j.cell.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Le Goffic F, Baca B, Moreau N. A new rapid and powerful technique to obtain purified ribosomes. FEBS Lett. 1974;41:69–72. doi: 10.1016/0014-5793(74)80956-7. [DOI] [PubMed] [Google Scholar]
- Maguire BA, Wondrack LM, Contillo LG, Xu Z. A novel chromatography system to isolate active ribosomes from pathogenic bacteria. Rna. 2008;14:188–195. doi: 10.1261/rna.692408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez AK, Gordon E, Sengupta A, Shirole N, Klepacki D, Martinez-Garriga B, Brown LM, Benedik MJ, Yanofsky C, Mankin AS, Vazquez-Laslop N, Sachs MS, Cruz-Vera LR. Interactions of the TnaC nascent peptide with rRNA in the exit tunnel enable the ribosome to respond to free tryptophan. Nucleic Acids Res. 2014;42:1245–1256. doi: 10.1093/nar/gkt923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa T, Kanamori T, Ueda T, Taguchi H. Global analysis of chaperone effects using a reconstituted cell-free translation system. Proc Natl Acad Sci U S A. 2012;109:8937–8942. doi: 10.1073/pnas.1201380109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa T, Ying BW, Saito K, Jin W, Takada S, Ueda T, Taguchi H. Bimodal protein solubility distribution revealed by an aggregation analysis of the entire ensemble of Escherichia coli proteins. Proc Natl Acad Sci U S A. 2009;106:4201–4206. doi: 10.1073/pnas.0811922106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi H, Shimizu Y, Ying BW, Ueda T. Efficient protein selection based on ribosome display system with purified components. Biochem Biophys Res Commun. 2007a;352:270–276. doi: 10.1016/j.bbrc.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Ohashi H, Shimizu Y, Ying BW, Ueda T. Efficient protein selection based on ribosome display system with purified components. Biochem Biophys Res Commun. 2007b;352:270–276. doi: 10.1016/j.bbrc.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Rosenblum G, Chen C, Kaur J, Cui X, Zhang H, Asahara H, Chong S, Smilansky Z, Goldman YE, Cooperman BS. Quantifying elongation rhythm during full-length protein synthesis. J Am Chem Soc. 2013;135:11322–11329. doi: 10.1021/ja405205c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum G, Cooperman BS. Engine out of the chassis: cell-free protein synthesis and its uses. FEBS Lett. 2014;588:261–268. doi: 10.1016/j.febslet.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Zhang X, Jiang X, Yuan H, Lee JS, Barry CE, 3rd, Wang H, Zhang W, Zhang Y. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science. 2011;333:1630–1632. doi: 10.1126/science.1208813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Inoue A, Tomari Y, Suzuki T, Yokogawa T, Nishikawa K, Ueda T. Cell-free translation reconstituted with purified components. Nat Biotechnol. 2001;19:751–755. doi: 10.1038/90802. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Kanamori T, Ueda T. Protein synthesis by pure translation systems. Methods. 2005;36:299–304. doi: 10.1016/j.ymeth.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Ueda T. PURE technology. Methods Mol Biol. 2010;607:11–21. doi: 10.1007/978-1-60327-331-2_2. [DOI] [PubMed] [Google Scholar]
- Tada S, Andou T, Suzuki T, Dohmae N, Kobatake E, Ito Y. Genetic PEGylation. PLoS One. 2012;7:e49235. doi: 10.1371/journal.pone.0049235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauner A, Bennett MH, Williams HD. Isolation of bacterial ribosomes with monolith chromatography. PLoS One. 2011;6:e16273. doi: 10.1371/journal.pone.0016273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voges D, Watzele M, Nemetz C, Wizemann S, Buchberger B. Analyzing and enhancing mRNA translational efficiency in an Escherichia coli in vitro expression system. Biochem Biophys Res Commun. 2004;318:601–614. doi: 10.1016/j.bbrc.2004.04.064. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Bonomo J, Swartz JR. Localization of BiP to translating ribosomes increases soluble accumulation of secreted eukaryotic proteins in an Escherichia coli cell-free system. Biotechnol Bioeng. 2011;108:1739–1748. doi: 10.1002/bit.23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker JW. Cell-free protein synthesis: the state of the art. Biotechnol Lett. 2013;35:143–152. doi: 10.1007/s10529-012-1075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Asahara H, Gaucher EA, Chong S. Reconstitution of translation from Thermus thermophilus reveals a minimal set of components sufficient for protein synthesis at high temperatures and functional conservation of modern and ancient translation components. Nucleic Acids Res. 2012;40:7932–7945. doi: 10.1093/nar/gks568. [DOI] [PMC free article] [PubMed] [Google Scholar]