1. Introduction
Aromatic heterocycles are highly important structural units that are found in a vast number of biologically active natural compounds, pharmaceuticals, and materials.1 Aromatic heterocycles are important intermediates in organic synthesis, often providing access to other highly desirable structures.2 A myriad of methodologies and protocols have been developed for their synthesis,1 and, although generally high efficiencies and selectivities can be achieved, a large number of these methodologies are limited to the preparation of heterocycles with particular substitution patterns. Thus, there is a compelling need to develop novel and more general methods for the synthesis of heterocycles.
In recent years, the use of transition-metal-catalyzed transformations truly revolutionized the area of heterocyclic chemistry.3–15 Many research groups have focused on the development of general methods that utilize readily accessible starting materials under mild reaction conditions for the synthesis of densely functionalized heterocyclic cores to achieve better functional group compatibilities and greater levels of molecular complexity. A particularly attractive approach toward this goal involves the incorporation of molecular rearrangement steps into the transition-metal-catalyzed cycloisomerization cascade reactions. In most cases, this approach provides a significant advantage over alternative routes in the convergent preparation of heterocycles with new substitution patterns. This review covers the most important recent advances in the Cu-,11,16–19 Ag-,12 and Au-catalyzed20–27 syntheses of five-membered aromatic heterocycles proceeding with 1,n migrations of various groups during the assembly of the heterocyclic ring. The main organization of this review is based on the type of migrating group, and the discussion of a particular migrating group is structured by the type of its 1,n shift. The concepts underlying a given transformation and the synthetic applicability of the corresponding method are emphasized. A brief discussion of the mechanism is given where needed to shed some light on possible reaction intermediates in the catalytic transformation.
2. Synthesis of Heterocycles via Migratory Cycloisomerizations
2.1. Formal Hydrogen Migration
Among a variety of transition-metal-catalyzed syntheses of aromatic heterocycles containing five-membered rings, the cycloisomerization of single-component acyclic precursors represents the most versatile, atom-economical, and direct approach.15,21,28 Moreover, a major fraction of the corresponding multicomponent syntheses10,29,30 also relies on the fundamental reactivities of these key acyclic precursors. Extensive research on the synthesis of aromatic heterocycles through transition-metal-catalyzed cycloisomerizations has been stimulated by the pioneering work of Heilbron (1947; Hg, 2-en-4-yne-1-ols),31 Castro (1966; Cu, ortho-alkynyl anilines),32 Miller (1969; Hg, alkynyl epoxides),33 Huang (1986 and 1987; Pd, alkynyl ketones34 and propargyl ketones35), and Marshall (1990; Ag, Rh, allenyl ketones).36 The cycloisomerizations of allenyl- or alkynyl-containing substrates, catalyzed by Ag, Cu, or Au complexes,11–13,28 have been extensively utilized in the construction of heterocyclic cores (Figure 1). Mechanistically, these reactions proceed via formal 1,2- or 1,3-hydrogen migrations (prototropic isomerizations, G = H), which limit these methodologies to the preparation of heterocyclic frameworks with at least one unsubstituted position. In recent years, researchers have sought to develop novel strategies that might help overcome this limitation. One of the possible solutions involves the introduction of a migrating group other than hydrogen into the transition-metal-catalyzed cascade cycloisomerizations of allenes and alkynes. Subsequent sections of this review describe advances in this exciting and growing area.
Figure 1.
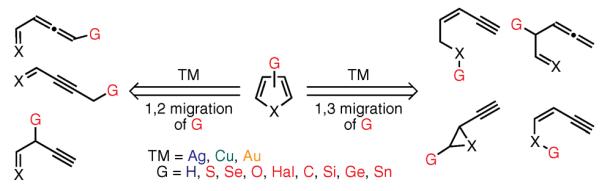
Retrosynthetic Analysis of Traditional Transition-Metal-Catalyzed Cycloisomerizations Leading to Aromatic Heterocycles.(Ref. 11–13, 28)
2.2. Sulfur and Selenium Migrations
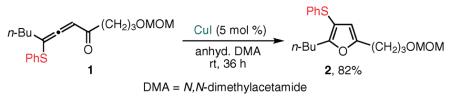
In 2003, our group discovered that the Cu(I)-catalyzed cycloisomerization of 4-thio-substituted allenone 1 proceeded very efficiently with a 1,2 migration of the phenylsulfanyl group,37,38 providing 3-thio-substituted furan392 in high yield.40 This discovery led us to formulate a general concept of transition-metal-catalyzed cascade cycloisomerizations of alkynyl and allenyl systems involving the formal 1,2 migration of different functional groups as the key step in a rapid assembly of densely functionalized heterocyclic cores (vide infra).
 |
eq 1 |
(Ref. 40)
 |
eq 2 |
 |
eq 3 |
(Ref. 48)
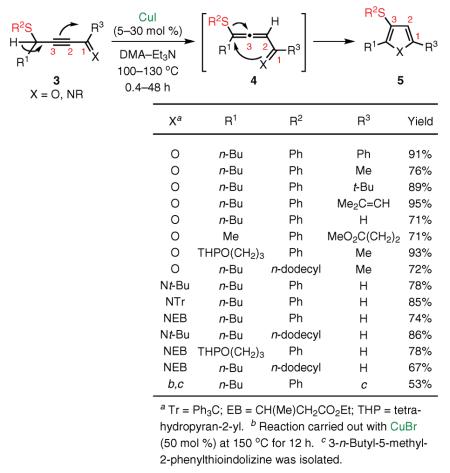
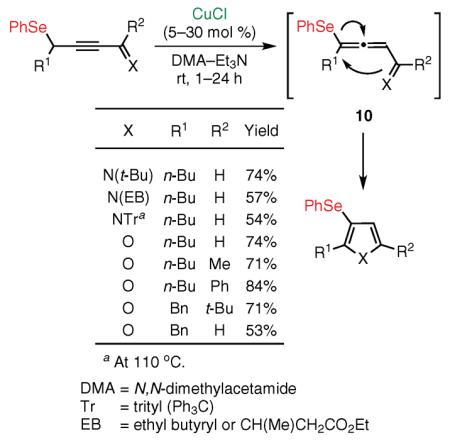
This report had been preceded by our disclosure in 2001 and 2002 that alkynyl imines and ketones (see Figure 1; G = H, X = NR or O) could be transformed highly efficiently into pyrroles41,42 and furans43 via the Cu(I)-catalyzed cycloisomerization. In this way, the alkynyl imines and ketones serve as surrogates of reactive allenyl intermediates generated in situ by the base-assisted propargyl–allenyl isomerization. In light of these findings from 2001–2003, our group next attempted a migratory cycloisomerization of substituted propargyl sulfides 3, undoubtedly superior precursors when compared with their allenyl sulfide analogues from a synthetic point of view. We found that 3-sulfanyl-substituted furans, pyrroles,44 and even indolizines455 are efficiently accessed via the Cu(I)-catalyzed migratory cycloisomerization of the corresponding propargyl sulfides (eq 2).40,46 The alkylsulfanyl group migrates with efficiency comparable to that of its phenylsulfanyl analogue, and a variety of functional groups are perfectly tolerated under these reaction conditions. It is believed that, mechanistically, this transformation proceeds through the Cu(I)-catalyzed cycloisomerization of reactive allenyl sulfide 4, wherein a 1,2 migration of an alkylthio or arylthio group47 occurs via a thiirenium intermediate (vide infra). This transformation represents the first example of 1,2 migration of the sulfanyl group from an olefinic sp2 carbon to an sp center.37
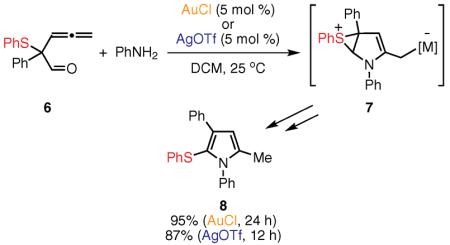
Recently, Wang and co-workers reported another example of a 1,2-sulfur migration that was utilized in the assembly of polysubstituted pyrroles 8 via an acid-catalyzed cascade reaction sequence of skipped allenyl aldehydes 6 and anilines. They also demonstrated that this reaction could be catalyzed by Au(I) or Ag salts, wherein the 1,2 migration of the sulfanyl group occurs intramolecularly via the proposed thiiranium intermediate 7 (eq 3).48
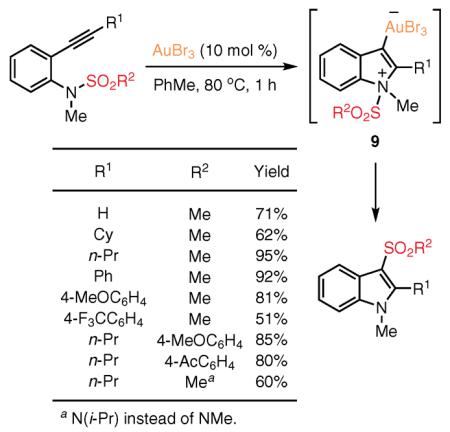
In 2007, Yamamoto's group showed that cycloisomerization of ortho-alkynylsulfonanilides in the presence of a Au(III) catalyst produces 3-sulfonylindoles via a 1,3 migration of a sulfonyl group (eq 4).49,50 This reaction is compatible with a variety of alkyl, aryl, and terminal alkynes and provides the indole products in generally high yields. The proposed mechanism involves Au-catalyzed 5-endo-dig cyclization of ortho-alkynylsulfonamides to the zwitterionic indolyl–gold intermediate 9, in which the 1,3 migration of the sulfonyl group occurs intramolecularly.
Our group recently extended the 1,2-sulfur migration approach (see eq 2) to the related 1,2-selenium migration in the Cu(I)-catalyzed cycloisomerization cascade of propargyl selenides into polysubstituted 3-selenylfurans and pyrroles (eq 5).46 Remarkably,
 |
eq 4 |
(Ref. 50) the 1,2 migration of the seleno group is more facile than that of the thio groups; this fact permits such cycloisomerizations to be carried out under significantly milder reaction conditions. The proposed mechanism for selenium migration is analogous to that suggested for the Cu(I)-catalyzed cycloisomerization of propargyl sulfides and involves formation of the reactive allenyl intermediate 10 via the initial prototropic rearrangement.
2.3. Halogen Migration
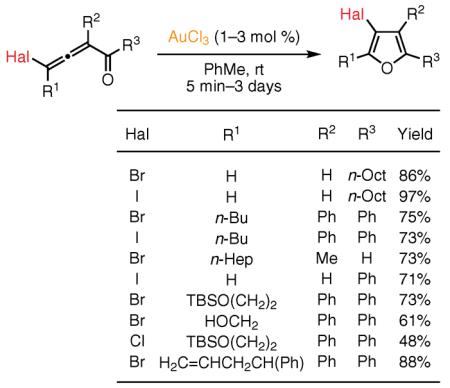
In 2005, our group reported a very efficient and regiodivergent Au-catalyzed haloallenyl ketone cycloisomerization that proceeds with a 1,2 migration of iodine, bromine, or chlorine atoms,51 and leads to 3-halofurans with 1–4 substituents (eq 6).52 Remarkably, in the presence of the Au(III) catalyst, 1,2 migrations of bromine and iodine are more facile than 1,2-alkyl and even 1,2-hydrogen shifts in these allenyl ketones. In contrast, employment of a Au(I) catalyst, Et3PAuCl, for the cycloisomerization of ambident C-4 monohalo-substituted allenones (see eq 6, R1 = H) furnishes 2-halofuran products via exclusive 1,2-hydrogen migration. It was demonstrated that various functionalities, including alkene and free hydroxyl groups, are tolerated under the reaction conditions. Iodo- and bromo-substituted substrates were shown to be more efficient in this cycloisomerization than their chloro-substituted analogues. The 3-halofurans thus obtained can easily be further functionalized at the C-3 position via cross-coupling protocols.53
Thorough mechanistic studies, including high-level Density Functional Theory (DFT) calculations, have indicated that activation of the distal double bond of the allene with either a Au(I) or Au(III) catalyst leads to the formation of the gold–carbene intermediate 11, wherein a kinetically favored 1,2-halogen migration gives 3-halofuran 12 (Scheme 1).54 However, the use of Au(PR3)L (L = Cl, OTf; R = Et, Ph) catalysts in the case of ambident haloallenones triggers the stepwise counterion- or ligand-assisted55 hydrogen shift, leading to 2-halofurans 13. This observation indicates that, in these Au-catalyzed processes, whether hydrogen or bromine migrates is determined by the nature of the ligand on Au.52,54 In addition, switching the reaction solvent from toluene to THF, which is capable of assisting the stepwise 1,2-hydrogen migration, provides the regiodivergent formation of 2-halofurans 13.52
Scheme 1.
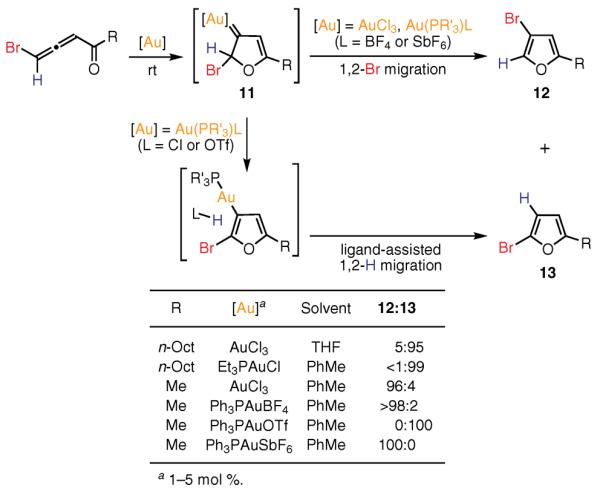
Ligand-Controlled Hydrogen vs Bromine 1,2 Migration.(Ref. 52,54)
2.4. Carbon Migration
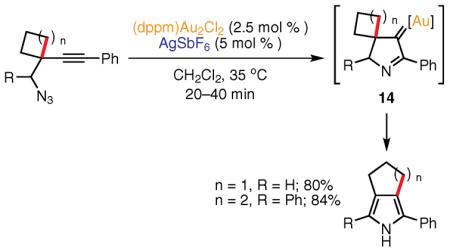
The first example of a 1,2-alkyl shift56,57 in the Au-catalyzed synthesis of heterocycles was reported by Toste and co-workers in 2005.58 In the presence of a cationic Au(I) catalyst, homopropargylic azides possessing cyclobutyl or cyclopentyl substituents undergo an acetylenic Schmidt reaction leading to C-3–C-4-fused pyrroles in good yields (eq 7).58 This protocol allows for the rapid assembly of N-unprotected pyrroles possessing 1–4 substituents. According to the mechanistic hypothesis, the Au(I) catalyst activates the alkyne moiety toward nucleophilic attack by the azide to produce gold–carbene 14 with loss of dinitrogen. A subsequent 1,2 migration of a CH2 group in the cyclobutyl or cyclopentyl ring to the gold–carbene center furnishes the pyrrole product after tautomerization.
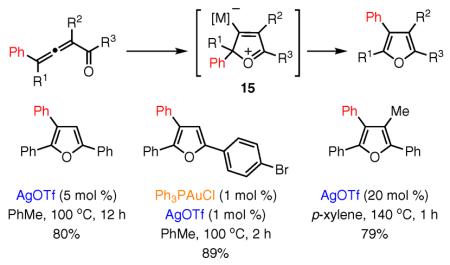
Aiming to incorporate various 1,2-migratory groups into the cycloisomerization cascade, we developed an efficient synthesis of furans with 1–4 substituents. The synthesis proceeds by a 1,2 migration of alkyl or aryl groups in allenyl ketones in the presence of π-philic Au(I), Ag, Cu(I), or Cu(II) catalysts (eq 8).59 Based on studies of the migratory aptitude of various groups, we proposed this cycloisomerization to occur via metal–oxonium ion intermediate 15. The latter can be viewed as a resonance form of a metal–carbene intermediate analogous to 11, but with the carbon atom attached to the metal possessing more cationic than carbene-like character.46,59
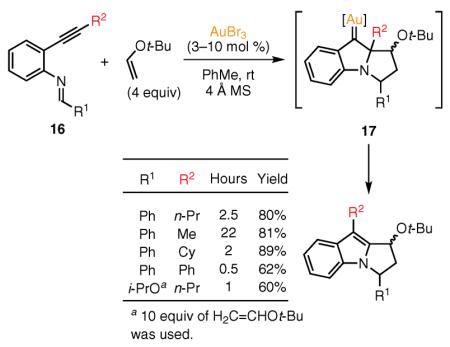
In 2006, Iwasawa and co-workers established an efficient Au(III)-catalyzed protocol for the construction of N-1–C-2-fused polycyclic indole skeletons via a cycloisomerization–
 |
eq 5 |
(Ref. 46)
 |
eq 6 |
(Ref. 52)
 |
eq 7 |
(Ref. 58)
 |
eq 8 |
 |
eq 9 |
(Ref. 60)
 |
eq 10 |
(Ref. 62) cycloaddition reaction sequence of alkene enol ethers with ortho-alkynylphenylanilides 16.60 Iwasawa's group showed that the latter substrates (i.e., ortho-alkynylphenylanilides), upon activation with the Au(III) catalyst, generate reactive azomethine ylides.61 Interception of such ylides with tert-butyl vinyl ether via a [3 + 2] cycloaddition leads to the formation of the key gold–carbene intermediates. 1,2-Alkyl migration to the Au-stabilized carbene center in the latter intermediates affords fused indole products in moderate-to-high yields (eq 9).60
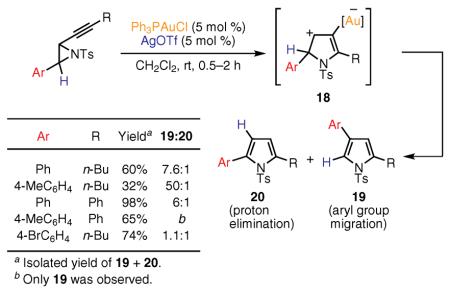
Very recently, another example of the synthesis of heterocycles via a 1,2-aryl group migration to a cationic center was reported by Davies and Martin.62 In this study, alkynyl aziridines were shown to undergo the Au(I)-catalyzed cycloisomerization, providing mixtures of regioisomeric pyrroles 19 and 20 (eq 10). According to the proposed mechanism, ring-opening of the aziridine and subsequent nucleophilic attack of the nitrogen atom at the distal position of the alkyne, activated by the Au(I) catalyst, affords the metal-substituted intermediate 18. Interestingly, 1,2 migration of the phenyl group is preferred in this intermediate over the generally facile proton elimination, leading to pyrrole 19 as the major product. Introduction of electron-rich aryl groups provides 19 exclusively, whereas the isomerization of substrates with electron-deficient aryl substituents (e.g., 4-bromo) exhibits poor selectivity. The authors also demonstrated that the use of a more basic tosylate-containing, instead of triflate-containing, Au(I) catalyst55 favors the proton elimination pathway, furnishing pyrroles 20 as the sole regioisomers in excellent yields.
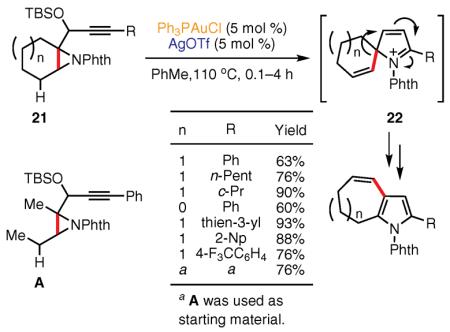
The utility of aziridines in the synthesis of pyrroles was further demonstrated by Tu and co-workers, who reported that cycloisomerization of skipped alkynyl aziridines 21 in the presence of a Au(I) catalyst affords polysubstituted 3-vinylpyrroles via a formal 1,2-alkenyl shift (eq 11).63 Various alkyl-, aryl-, and heteroaryl-substituted alkynes were easily transformed into pyrrole products in good yields. A mechanistic hypothesis for this transformation cascade features the 1,2-alkenyl migration in the spirocyclic iminium intermediate 22, while formation of the alkenyl unit in the latter arises from the prior proton elimination step. The authors showed that this reaction could equally efficiently proceed with a ring expansion of the five- and six-membered rings fused to the aziridine moiety, as well as with a 1,2-propenyl shift in the case of acyclic substrates.
Besides migratory cycloisomerizations proceeding by 1,2 shifts, several groups have recently reported examples of Au- and Ag-catalyzed counterparts taking place by 1,3 migrations of alkyl,
 |
eq 11 |
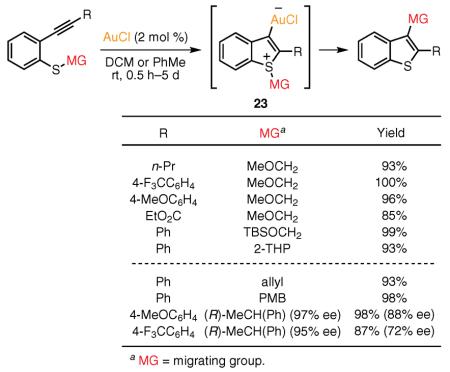
(Ref. 63) alkenyl, and carbonyl groups. Similarly to the Pt(II)-catalyzed migratory cycloisomerizations leading to benzofuran64–68 and indole64,68,69 cores, Yamamoto's group disclosed the Au(I)-catalyzed cycloisomerization of ortho-alkynylthiophenol alkyl ethers into 2,3-disubstituted benzothiophenes in excellent yields. This transformation is believed to occur by an intramolecular 1,3 migration of the alkyl group attached to sulfur in intermediate 23 (eq 12).70 Remarkably, a variety of 1,3-migrating groups, including Si-containing and cyclic tetrahydropyranyl ones, were easily incorporated into this transformation cascade. Other sulfur substituents capable of stabilizing the incipient positive charge, such as allyl and para-methoxybenzyl,71,72 were also efficiently employed, leading to 3-allyl- or 3-benzylbenzothiophenes, respectively. According to the proposed mechanism, this reaction cascade begins with the Au-catalyzed 5-endo-dig cyclization of the ortho-alkynylthiophenol alkyl ether to give the gold-substituted benzothiophenium intermediate 23, which subsequently undergoes intramolecular 1,3-alkyl migration to give the rearranged final product.
Following this report, the same group later disclosed that the Au(I)-catalyzed carbothiolation reaction of optically active ortho-alkynylphenyl 1-phenylethyl sulfides proceeded with predominant retention of the configuration in the 1-phenylethyl migrating group (see eq 12, last two entries).73 This observation indicates that, at least in the case of 1-arylethyl groups, 1,3-alkyl migration proceeds through formation of a contact ion pair during the migration process.
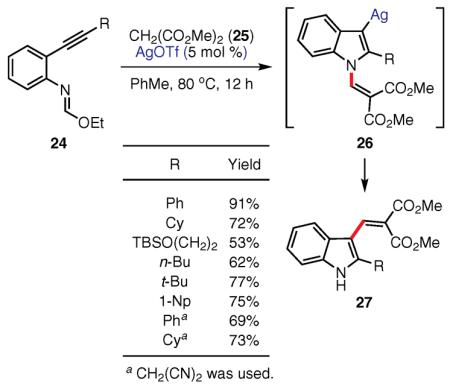
Oh and co-workers have very recently demonstrated that a variety of 3-vinylindoles, possessing different alkyl and aryl substituents at C-2, can be accessed efficiently through a 1,3-alkenyl shift in a Ag-catalyzed cascade reaction (eq 13).74 The authors have proposed that the initial condensation of N-(alkynylphenyl)formimidate 24 with malonate derivative 25, and subsequent cyclization in the presence of the silver catalyst, provide N-alkenyl intermediate 26. The 1,3 shift of the alkenyl group in this intermediate from N to C-3 gives indole derivative 27. However, the exact mechanism and nature of this interesting 1,3 migration remain unknown.
In 2007, Istrate and Gagosz reported that N,N-disubstituted (Z)-(2-en-4-ynyl)amines, with a second allyl group attached to the nitrogen atom, undergo a Au(I)-catalyzed cycloisomerization with a 1,3-allyl shift to afford tri- and tetrasubstituted pyrroles (eq 14, conditions A).75 This transformation allows for the synthesis of homoallyl-substituted pyrroles bearing various functional groups in good-to-excellent yields. The proposed mechanism involves, in the first step, activation of the alkyne moiety toward 5-exo-dig cyclization to give the cyclic vinyl–gold intermediate 28. A subsequent 1,3-allyl shift64,76 occurs via a Au(I)-catalyzed aza-Claisen-type rearrangement, furnishing the corresponding pyrrole. More recently, Heugebaert and Stevens applied the same concept to the synthesis of isoindoles from N-allyl-benzylamine derivatives (see eq 14, conditions B).77
Zhang and co-workers recently synthesized a variety of tri- and tetrasubstituted N–C-2-fused pyrroles by the Au(I)-catalyzed cycloisomerization of (Z)-(2-en-4-ynyl)lactams 29 (eq 15).78 This interesting synthesis proceeds by a formal ring expansion of the β-lactam moiety via a 1,3-carbonyl migration.79 Similarly to Gagosz's rationale, the Au(I)-catalyzed 5-exo-dig cyclization is followed by lactam ring opening, which leads to the formation of nucleophilic vinyl–Au species 30. A subsequent 1,2 addition of the latter functional group to the activated carbonyl function produces the fused pyrrole product.
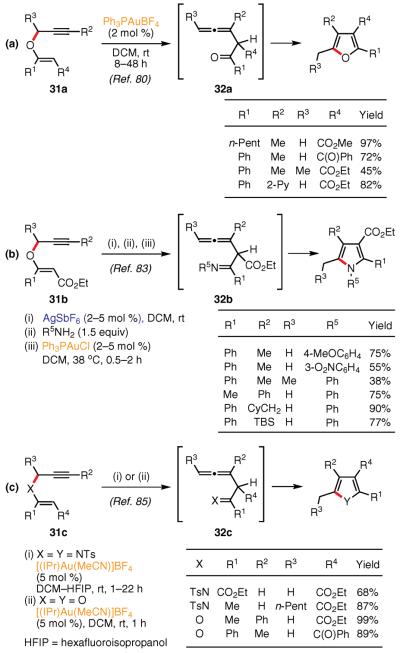
Finally, several migratory cycloisomerizations involving a Claisen-type rearrangement of the carbon skeleton of the substrate prior to the heterocyclization step have been reported. Kirsch and co-workers first reported that vinyl propargyl ethers 31 could be converted into densely substituted furans via a Au(I)-catalyzed cycloisomerization reaction (Scheme 2, Part (a))80,81 Thus, a variety of tetrasubstituted alkyl, aryl, and heteroarylfurans possessing a carbonyl group at C-3 were obtained under very mild reaction conditions. It is believed that this cascade process begins
 |
eq 12 |
 |
eq 13 |
(Ref. 74)
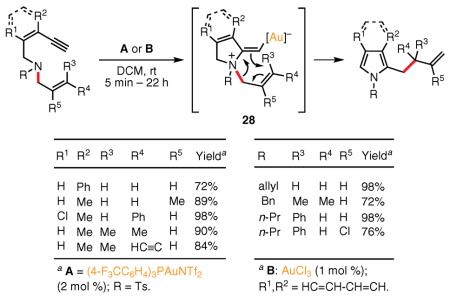 |
eq 14 |
(Ref. 75,77) with a Au(I)-catalyzed Claisen-type rearrangement82 and leads to the formation of the reactive skipped allenyl ketone intermediate 32, which undergoes a Au(I)-catalyzed 5-exo-dig cyclization to produce the furan ring.
Scheme 2.
Migratory Cycloisomerizations Involving a Claisen-Type Rearrangement Prior to Heterocyclization.
The same group later disclosed that vinyl propargyl ethers 31 could also be employed in a very efficient synthesis of densely substituted pyrroles via the Ag–Au(I)-catalyzed condensation- cycloisomerization reaction sequence (see Scheme 2, Part (b))83 Thus, skipped allenyl ketones 32, formed by the Ag-catalyzed
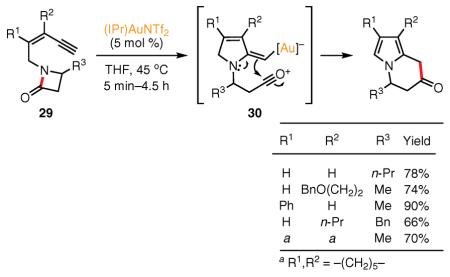 |
eq 15 |
(Ref. 78) Claisen rearrangement, were intercepted by the amination reaction with various anilines and the resulting imines underwent the Au(I)-catalyzed 5-exo-dig cyclization to furnish the corresponding pyrroles. The authors demonstrated that a variety of pyrroles possessing different labile groups could be rapidly obtained in moderate-to-high yields under mild reaction conditions. In addition, the reaction was quite general with respect to the aromatic amine component, whereas aliphatic amines (R5 = Me, i-Pr, Bn) did not undergo this transformation at all.
More recently, Saito et al. applied the above methodology to the direct synthesis of pyrroles from vinyl propargyl amines 31 (X = Y = NTs) in the presence of an (NHC)Au(I) catalyst84 (see Scheme 2, Part (c)).85 Furthermore, this new catalytic system was shown to be highly efficient for the cycloisomerization of ether analogues as well (X = Y = O, see Scheme 2, Part (c), conditions (ii)).
2.5. Silicon, Germanium, and Tin Migrations
Aiming at the efficient synthesis of not-so-easily accessible C-2-substituted indolizines, our group developed a highly efficient, Au(III)-catalyzed cascade cycloisomerization of skipped propargylpyridines 33 into indolizines 35 (eq 16).86,87 This cascade is proposed to occur with a facile 1,2 migration of a silyl, stannyl, and even germyl group via an alkyne–vinylidene isomerization88 of propargyl substrate 33 to give the reactive organogold species 34. A subsequent cyclization of the intermediate, 34, followed by a series of 1,2-hydride shifts, furnishes the corresponding indolizine. We have further demonstrated the synthetic utility of this methodology by carrying out the facile synthesis of various N-fused heterocycles, including pyrrolo[1,2-a]quinoxaline, pyrrolo[1,2-a]pyrazine, and pyrrolo[2,1-b]thiazole.
Yamamoto's group has developed the Au(I)-catalyzed, high-yield synthesis of 3-silyl-substituted benzothiophenes through a 1,3-silyl group migration during the cycloisomerization cascade of ortho-alkynylthiophenol silyl ethers (eq 17).89 However, lower yields were obtained for substrates bearing very bulky or strong electron-withdrawing substituents, or containing less nucleophilic silyl migrating groups. In contrast to the intramolecular nature of the 1,3-alkyl-group migration in the analogous system (see eq 12), the observed crossover of two different silyl groups during the cycloisomerization of two different substrates indicated that the 1,3 migration of the silyl group proceeds intermolecularly. The observed crossover was rationalized by the suggested longer
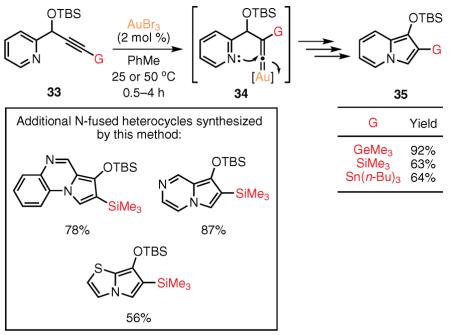 |
eq 16 |
(Ref. 86) lifetime of the gold–silylsulfonium ion intermediate 36 due to the lower migratory ability of a silyl group relative to that of an alkyl group.
2.6. Acyloxy, Phosphatyloxy, and Sulfonyloxy Migrations
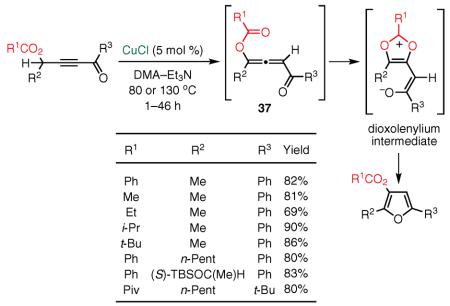
In 2004, our group envisioned that highly reactive allenyl substrates could be accessed via a formal 1,3 migration in propargyl acetates,90–92 phosphates, or sulfonates.93 This early concept later evolved into a series of highly efficient, practical, and general methodologies for the assembly of polysubstituted furans and indolizines. Accordingly, we demonstrated that an array of densely functionalized 3-acyloxyfurans could be synthesized via a Cu(I)-catalyzed cycloisomerization cascade of conjugated alkynyl ketone acetates proceeding through a formal 1,2-acyloxy-group migration (eq 18).93 The nature of the base required for the selective formation of the 3-acyloxy regioisomer supported possible involvement of allene intermediate 37, which is generated upon initial prototropic rearrangement of the substrate. Based on further mechanistic studies involving 17O-labeled substrates, we proposed that this formal 1,2-acyloxy-group migration94,95 likely occurs through the involvement of a dioxolenylium intermediate.96
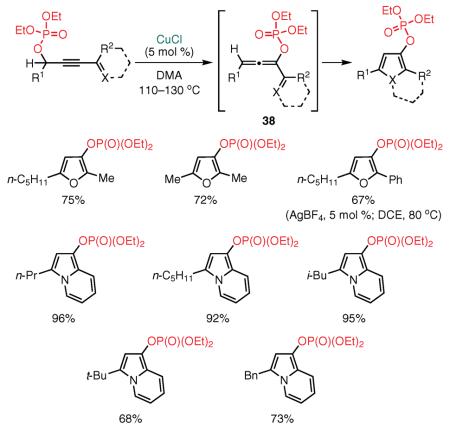
Our group also investigated the Cu(I)-catalyzed cycloisomerization of conjugated keto or pyridino propargyl phosphates in the absence of a base (eq 19).96 This transformation allowed for a highly efficient synthesis of 3-phosphatyloxy furans and indolizines97 via a formal 1,3-phosphatyloxy group migration.96 Thorough mechanistic studies of this transformation with the aid of 17O-labeled substrates revealed that the cycloisomerization proceeds via an initial formal [3,3]-sigmatropic rearrangement of propargyl phosphates into the reactive allenyl phosphates 38. It should be noted that the phosphatyloxy-containing furans and indolizines represent versatile synthons, as the phosphatyloxy group can efficiently be substituted with various alkyl and aryl groups by the Kumada cross-coupling reaction.96
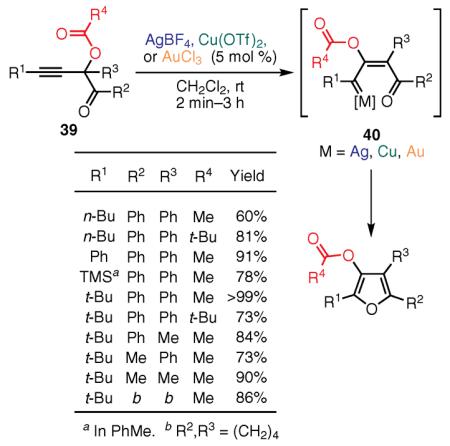
Next, we developed an alternative route to tetrasubstituted and even to fused furans via a transition-metal-catalyzed migratory cycloisomerization of skipped propargylic substrates. Thus, alkynyl acetates 39 underwent, in the presence of a silver catalyst at room temperature, a formal 1,2-acyloxy-group migration furnishing fully substituted 3-acyloxyfurans in high yields (eq 20).93,96 The
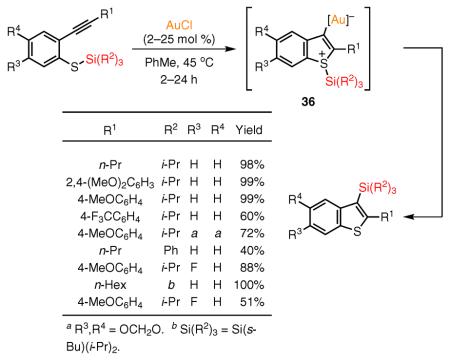 |
eq 17 |
(Ref. 89)
 |
eq 18 |
 |
eq 19 |
(Ref. 96)
 |
eq 20 |
(Ref. 93,96) mechanism for the cycloisomerization of skipped alkynyl ketones 39 is suggested to follow a Rautenstrauch-type 1,2 migration,98 resulting in intermediate 40, and subsequent cycloisomerization to the furan ring.96 Several other transition-metal complexes, including Cu(II) and Au(III), also catalyzed this transformation. Furthermore, phosphatyloxy and tosyloxy groups underwent an analogous 1,2 migration from propargyl (41) and allenyl (42) substrates, providing 3-phosphatyloxy- and 3-tosyloxyfurans, respectively (Scheme 3). In the case of skipped phosphatyloxy alkynyl ketones, 41, cycloisomerization proceeds via two consecutive 1,2 migrations leading to the formation of allene intermediate 42 and a formal 1,3 shift. Subsequent 1,2 migration gives rise to the final product, 3-phosphatyloxy- or 3-tosyloxyfuran.
Scheme 3.
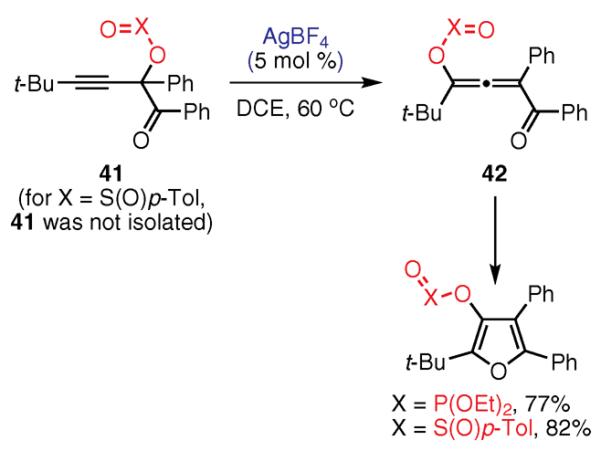
Tetrasubstituted Furans by the Migratory Cycloisomerization of Skipped Propargylic Substrates. (Ref. 93,96)
3. Conclusions and Outlook
This review highlighted a growing interest in the development of novel cascade transformations that incorporate various molecular rearrangements and functional-group migrations. Recent reports featuring this approach established new, general, highly efficient, and atom-economical transformations that lead to complex and densely functionalized aromatic heterocycles with diverse substitution patterns. These heterocycles are not easily available via alternative routes. A variety of functional groups—including S-, Se-, Hal-, C-, Si-, Ge-, Sn-, and O-containing functionalities—undergo various types of 1,n migrations during these heterocycle syntheses. In recent years, in addition to the continuing interest in the traditional Ag and Cu catalysts, the focus of many research groups has shifted to the remarkably efficient and mild gold catalysis. Although further development of novel, more general, and efficient migratory methodologies is certainly highly warranted, the progress achieved so far in this area bodes well for broad application in organic synthesis.
Acknowledgements
We thank the National Institutes of Health (GM-64444) and National Science Foundation (CHE-0710749) for financial support of this work. A. S. D. is grateful to the University of Illinois at Chicago Graduate College for a Dean's Scholar Award. N. C. thanks the Department of Chemistry of the University of Illinois at Chicago for the Moriarty Graduate Fellowship.
Biographies

Alexander S. Dudnik was born in Krasnodar, Russia. He received his B.S. degree in 2005 from the M. V. Lomonosov Moscow State University. Between 2003 and 2005, he worked as a visiting researcher at the Zelinsky Institute of Organic Chemistry of the Russian Academy of Sciences. He is currently a fifth-year graduate student in Professor Gevorgyan's group at the University of Illinois at Chicago. A major part of his research in Prof. Gevorgyan's group is devoted to the development of new Lewis acid and gold-, copper-, and silver-catalyzed methodologies involving C–C or C–heteroatom bond-forming reactions and molecular rearrangements leading to carbo- and heterocycles. Alexander has recently received a University of Illinois Graduate College Dean's Scholar Award. After completion of his Ph.D. requirements, he will join the laboratory of Professor Gregory C. Fu at the Massachusetts Institute of Technology as a postdoctoral associate.

Natalia Chernyak was born in Riga, Latvia. She received her B.S. degree in 2002 and M.S. degree in 2005 from Riga Technical University. Between 2000 and 2005, she worked as a researcher at the Latvian Institute of Organic Synthesis. In 2005, she joined the laboratory of Professor Gevorgyan at the University of Illinois at Chicago, where she is currently a fifth-year graduate student. Her dissertation research has focused on the development of novel Pd-catalyzed direct/directed CH-functionalization processes, C–C-bond-forming reactions proceeding via CH activation, and the Cu-catalyzed multicomponent synthesis of heterocycles. Recently, her research achievements were recognized with a Moriarty Graduate Fellowship. Upon graduation, she will join the laboratory of Professor Stephen L. Buchwald at the Massachusetts Institute of Technology, as a postdoctoral associate.

Vladimir Gevorgyan was born in Krasnodar, Russia. He received his B.S. degree in 1978 from Kuban State University and his Ph.D. degree in 1984 from the Latvian Institute of Organic Synthesis, where he was promoted to Group Leader in 1986. He spent two years (1992–1994) in Tohoku University in Sendai, Japan, the first as a Japan Society for the Promotion of Science (JSPS) Postdoctoral Fellow and the second as a Ciba-Geigy International Postdoctoral Fellow. The following year (1995), he was a Visiting Professor at Consiglio Nazionale delle Ricerche (CNR) in Bologna, Italy. He returned to Tohoku University in 1996 as an Assistant Professor and was promoted to Associate Professor in 1997. In 1999, he moved to the University of Illinois at Chicago as an Associate Professor, and was promoted to the rank of Professor in 2003. Prof. Gevorgyan's current research interests cover four main areas: (i) highly selective Pd-catalyzed benzannulations, (ii) novel transition-metal-catalyzed reactions for the synthesis of heterocyclic and naturally occurring compounds, (iii) selective Lewis acid catalyzed bond formation and cleavage reactions, and (iv) the chemistry of strained-ring systems. In 2008, his contributions to the field of organic chemistry were recognized with the University of Illinois at Chicago Researcher of the Year Award.
5. References and Notes
- (1).Katritzky AR, Ramsden CA, Scriven EFV, Taylor RJK, editors. Comprehensive Heterocyclic Chemistry III. Vols. 3 and 11. Elsevier; Oxford, U.K.: 2008. [Google Scholar]
- (2).For reviews, see: Gribble GW, Saulnier MG, Pelkey ET, Kishbaugh TLS, Yanbing L, Jiang J, Trujillo HA, Keavy DJ, Davis DA, Conway SC, Switzer FL, Roy S, Silva RA, Obaza-Nutaitis JA, Sibi MP, Moskalev NV, Barden TC, Chang L, Habeski WM, Pelcman B, Sponholtz WR, III, Chau RW, Allison BD, Garaas SD, Sinha MS, McGowan MA, Reese MR, Harpp KS. Curr. Org. Chem. 2005;9:1493.. Evano G, Blanchard N, Toumi M. Chem. Rev. 2008;108:3054. doi: 10.1021/cr8002505.. Isambert N, Lavilla R. Chem.—Eur. J. 2008;14:8444. doi: 10.1002/chem.200800473.. Chinchilla R, Nájera C, Yus M. Chem. Rev. 2004;104:2667. doi: 10.1021/cr020101a..
- (3).Brandi A, Cicchi S, Cordero FM, Goti A. Chem. Rev. 2003;103:1213. doi: 10.1021/cr010005u. [DOI] [PubMed] [Google Scholar]
- (4).Deiters A, Martin SF. Chem. Rev. 2004;104:2199. doi: 10.1021/cr0200872. [DOI] [PubMed] [Google Scholar]
- (5).Alonso F, Beletskaya IP, Yus M. Chem. Rev. 2004;104:3079. doi: 10.1021/cr0201068. [DOI] [PubMed] [Google Scholar]
- (6).Nakamura I, Yamamoto Y. Chem. Rev. 2004;104:2127. doi: 10.1021/cr020095i. [DOI] [PubMed] [Google Scholar]
- (7).Zeni G, Larock RC. Chem. Rev. 2004;104:2285. doi: 10.1021/cr020085h. [DOI] [PubMed] [Google Scholar]
- (8).Maes BUW. In: Microwave-Assisted Synthesis of Heterocycles. Eycken E, Kappe CO, Maes BUW, editors. Vol. 1. Springer-Verlag; Berlin: 2006. pp. 155–211. (Topics in Heterocyclic Chemistry Series). [Google Scholar]
- (9).Zeni G, Larock RC. Chem. Rev. 2006;106:4644. doi: 10.1021/cr0683966. [DOI] [PubMed] [Google Scholar]
- (10).D'Souza DM, Müller TJJ. Chem. Soc. Rev. 2007;36:1095. doi: 10.1039/b608235c. [DOI] [PubMed] [Google Scholar]
- (11).Chemler SR, Fuller PH. Chem. Soc. Rev. 2007;36:1153. doi: 10.1039/b607819m. [DOI] [PubMed] [Google Scholar]
- (12).Álvarez-Corral M, Muñoz-Dorado M, Rodríguez-García I. Chem. Rev. 2008;108:3174. doi: 10.1021/cr078361l. [DOI] [PubMed] [Google Scholar]
- (13).Patil NT, Yamamoto Y. Chem. Rev. 2008;108:3395. doi: 10.1021/cr050041j. [DOI] [PubMed] [Google Scholar]
- (14).Weibel J-M, Blanc A, Pale P. Chem. Rev. 2008;108:3149. doi: 10.1021/cr078365q. [DOI] [PubMed] [Google Scholar]
- (15).Kirsch SF. Synthesis. 2008;3183 [Google Scholar]
- (16).Beletskaya IP, Cheprakov AV. Coord. Chem. Rev. 2004;248:2337. [Google Scholar]
- (17).Csende F, Stájer G. Curr. Org. Chem. 2005;9:1737. [Google Scholar]
- (18).Ley SV, Thomas AW. Angew. Chem., Int. Ed. 2003;42:5400. doi: 10.1002/anie.200300594. [DOI] [PubMed] [Google Scholar]
- (19).Monnier F, Taillefer M. Angew. Chem., Int. Ed. 2008;47:3096. doi: 10.1002/anie.200703209. [DOI] [PubMed] [Google Scholar]
- (20).Arcadi A. Chem. Rev. 2008;108:3266. doi: 10.1021/cr068435d. [DOI] [PubMed] [Google Scholar]
- (21).Shen HC. Tetrahedron. 2008;64:7847. [Google Scholar]
- (22).Shen HC. Tetrahedron. 2008;64:3885. [Google Scholar]
- (23).Li Z, Brouwer C, He C. Chem. Rev. 2008;108:3239. doi: 10.1021/cr068434l. [DOI] [PubMed] [Google Scholar]
- (24).Jiménez-Núñez E, Echavarren AM. Chem. Commun. 2007;333 doi: 10.1039/b612008c. [DOI] [PubMed] [Google Scholar]
- (25).Hashmi ASK, Hutchings GJ. Angew. Chem., Int. Ed. 2006;45:7896. doi: 10.1002/anie.200602454. [DOI] [PubMed] [Google Scholar]
- (26).Hashmi ASK. Chem. Rev. 2007;107:3180. doi: 10.1021/cr000436x. [DOI] [PubMed] [Google Scholar]
- (27).Fürstner A, Davies PW. Angew. Chem., Int. Ed. 2007;46:3410. doi: 10.1002/anie.200604335. [DOI] [PubMed] [Google Scholar]
- (28).Belmont P, Parker E. Eur. J. Org. Chem. 2009;6075 [Google Scholar]
- (29).Mihovilovic MD, Stanetty P. Angew. Chem., Int. Ed. 2007;46:3612. doi: 10.1002/anie.200604743. [DOI] [PubMed] [Google Scholar]
- (30).Balme G. Angew. Chem., Int. Ed. 2004;43:6238. doi: 10.1002/anie.200461073. [DOI] [PubMed] [Google Scholar]
- (31).Heilbron I, Jones ERH, Sondheimer F. J. Chem. Soc. 1947;1586 [Google Scholar]
- (32).Castro CE, Gaughan EJ, Owsley DC. J. Org. Chem. 1966;31:4071. [Google Scholar]
- (33).Miller D. J. Chem. Soc. C. 1969;12 [Google Scholar]
- (34).Sheng H, Lin S, Huang YZ. Tetrahedron Lett. 1986;27:4893. [Google Scholar]
- (35).Sheng H, Lin S, Huang YZ. Synthesis. 1987;1022 [Google Scholar]
- (36).Marshall JA, Robinson ED. J. Org. Chem. 1990;55:3450. [Google Scholar]
- (37).Sromek AW, Gevorgyan V. In: Sulfur-Mediated Rearrangements I. Schaumann E, Hunter CA, et al., editors. Vol. 274. Springer; Berlin: 2007. pp. 77–124. (Topics in Current Chemistry Series). [Google Scholar]
- (38).For select examples of the transition-metal-catalyzed 1,2-sulfur shift, see: Shapiro ND, Toste FD. J. Am. Chem. Soc. 2007;129:4160. doi: 10.1021/ja071231+.. Peng L, Zhang X, Zhang S, Wang J. J. Org. Chem. 007;72:1192. doi: 10.1021/jo0618674.. Xu F, Shi W, Wang J. J. Org. Chem. 2005;70:4191. doi: 10.1021/jo050109v..
- (39).For selected reviews on furan synthesis, see: Kirsch SF. Org. Biomol. Chem. 2006;4:2076. doi: 10.1039/b602596j.. Patil NT, Yamamoto Y. ARKIVOC. 2007;(x):121..
- (40).Kim JT, Kel'in AV, Gevorgyan V. Angew. Chem., Int. Ed. 2003;42:98. doi: 10.1002/anie.200390064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Kel'in AV, Sromek AW, Gevorgyan V. J. Am. Chem. Soc. 2001;123:2074. doi: 10.1021/ja0058684. [DOI] [PubMed] [Google Scholar]
- (42).Kim JT, Gevorgyan V. Org. Lett. 2002;4:4697. doi: 10.1021/ol027129t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Kel'in AV, Gevorgyan V. J. Org. Chem. 2002;67:95. doi: 10.1021/jo010832v. [DOI] [PubMed] [Google Scholar]
- (44).For select reviews on pyrrole synthesis, see: Agarwal S, Cämmerer S, Filali S, Fröhner W, Knöll J, Krahl MP, Reddy KR, Knölker H-J. Curr. Org. Chem. 2005;9:1601.. Balme G, Bouyssi D, Monteiro N. Heterocycles. 2007;73:87.. Bellina F, Rossi R. Tetrahedron. 2006;62:7213..
- (45).Fan H, Peng J, Hamann MT, Hu J-F. Chem. Rev. 2008;108:264. doi: 10.1021/cr078199m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Dudnik AS, Sromek AW, Rubina M, Kim JT, Kel'in AV, Gevorgyan V. J. Am. Chem. Soc. 2008;130:1440. doi: 10.1021/ja0773507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).For examples of 1,4-sulfur migration in the synthesis of furans, see Peng L, Zhang X, Ma M, Wang J. Angew. Chem., Int. Ed. 2007;46:1905. doi: 10.1002/anie.200604299..
- (48).Peng L, Zhang X, Ma J, Zhong Z, Wang J. Org. Lett. 2007;9:1445. doi: 10.1021/ol070205d. [DOI] [PubMed] [Google Scholar]
- (49).For selected reviews on indole synthesis, see: Cacchi S, Fabrizi G. Chem. Rev. 2005;105:2873. doi: 10.1021/cr040639b.. Humphrey GR, Kuethe JT. Chem. Rev. 2006;106:2875. doi: 10.1021/cr0505270.. Krüger K, Tillack A, Beller M. Adv. Synth. Catal. 2008;350:2153..
- (50).Nakamura I, Yamagishi U, Song D, Konta S, Yamamoto Y. Angew. Chem., Int. Ed. 2007;46:2284. doi: 10.1002/anie.200604038. [DOI] [PubMed] [Google Scholar]
- (51).For select examples of 1,2-halogen shift in transition-metal-catalyzed transformations, see: Tobisu M, Nakai H, Chatani N. J. Org. Chem. 2009;74:5471. doi: 10.1021/jo901045g.. Miura T, Iwasawa N. J. Am. Chem. Soc. 2002;124:518. doi: 10.1021/ja0113091.. Mamane V, Hannen P, Fürstner A. Chem.—Eur. J. 2004;10:4556. doi: 10.1002/chem.200400220.. Shen H-C, Pal S, Lian J-J, Liu R-S. J. Am. Chem. Soc. 2003;125:15762. doi: 10.1021/ja0379159..
- (52).Sromek AW, Rubina M, Gevorgyan V. J. Am. Chem. Soc. 2005;127:10500. doi: 10.1021/ja053290y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Schröter S, Stock C, Bach T. Tetrahedron. 2005;61:2245. [Google Scholar]
- (54).Xia Y, Dudnik AS, Gevorgyan V, Li Y. J. Am. Chem. Soc. 2008;130:6940. doi: 10.1021/ja802144t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Gorin DJ, Sherry BD, Toste FD. Chem. Rev. 2008;108:3351. doi: 10.1021/cr068430g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Crone B, Kirsch SF. Chem.—Eur. J. 2008;14:3514. doi: 10.1002/chem.200701985. [DOI] [PubMed] [Google Scholar]
- (57).For select examples of 1,2-alkyl shifts in transition-metal-catalyzed transformations, see: Funami H, Kusama H, Iwasawa N. Angew. Chem., Int. Ed. 2007;46:909. doi: 10.1002/anie.200603986.. Lee JH, Toste FD. Angew. Chem., Int. Ed. 2007;46:912. doi: 10.1002/anie.200604006.. Kirsch SF, Binder JT, Liébert C, Menz H. Angew. Chem., Int. Ed. 2006;45:5878. doi: 10.1002/anie.200601836.. Shen M, Leslie BE, Driver TG. Angew. Chem., Int. Ed. 2008;47:5056. doi: 10.1002/anie.200800689.. Gao H, Zhao X, Yu Y, Zhang J. Chem.—Eur. J. 2010;16:456. doi: 10.1002/chem.200901813.. Jiménez-Núñez E, Claverie CK, Nieto-Oberhuber C, Echavarren AM. Angew. Chem., Int. Ed. 2006;45:5452. doi: 10.1002/anie.200601575.. Binder JT, Crone B, Kirsch SF, Liébert C, Menz H. Eur. J. Org. Chem. 2007;1636.
- (58).Gorin DJ, Davis NR, Toste FD. J. Am. Chem. Soc. 2005;127:11260. doi: 10.1021/ja053804t. [DOI] [PubMed] [Google Scholar]
- (59).Dudnik AS, Gevorgyan V. Angew. Chem., Int. Ed. 2007;46:5195. doi: 10.1002/anie.200701128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Kusama H, Miyashita Y, Takaya J, Iwasawa N. Org. Lett. 2006;8:289. doi: 10.1021/ol052610f. [DOI] [PubMed] [Google Scholar]
- (61).See also: Kusama H, Takaya J, Iwasawa N. J. Am. Chem. Soc. 2002;124:11592. doi: 10.1021/ja027589h.. Takaya J, Udagawa S, Kusama H, Iwasawa N. Angew. Chem., Int. Ed. 2008;47:4906. doi: 10.1002/anie.200705517.. Kusama H, Suzuki Y, Takaya J, Iwasawa N. Org. Lett. 2006;8:895. doi: 10.1021/ol0529795..
- (62).Davies PW, Martin N. Org. Lett. 2009;11:2293. doi: 10.1021/ol900609f. [DOI] [PubMed] [Google Scholar]
- (63).Zhao X, Zhang E, Tu Y-Q, Zhang Y-Q, Yuan D-Y, Cao K, Fan C-A, Zhang F-M. Org. Lett. 2009;11:4002. doi: 10.1021/ol901649p. [DOI] [PubMed] [Google Scholar]
- (64).Fürstner A, Davies PW. J. Am. Chem. Soc. 2005;127:15024. doi: 10.1021/ja055659p. [DOI] [PubMed] [Google Scholar]
- (65).Nakamura I, Mizushima Y, Yamamoto Y. J. Am. Chem. Soc. 2005;127:15022. doi: 10.1021/ja055202f. [DOI] [PubMed] [Google Scholar]
- (66).Fürstner A, Heilmann EK, Davies PW. Angew. Chem., Int. Ed. 2007;46:4760. doi: 10.1002/anie.200700895. [DOI] [PubMed] [Google Scholar]
- (67).Liang Z, Ma S, Yu J, Xu R. J. Org. Chem. 2007;72:9219. doi: 10.1021/jo7016263. [DOI] [PubMed] [Google Scholar]
- (68).Nakamura I, Mizushima Y, Yamagishi U, Yamamoto Y. Tetrahedron. 2007;63:8670. [Google Scholar]
- (69).Shimada T, Nakamura I, Yamamoto Y. J. Am. Chem. Soc. 2004;126:10546. doi: 10.1021/ja047542r. [DOI] [PubMed] [Google Scholar]
- (70).Nakamura I, Sato T, Yamamoto Y. Angew. Chem., Int. Ed. 2006;45:4473. doi: 10.1002/anie.200601178. [DOI] [PubMed] [Google Scholar]
- (71).For 1,3-alkyl-group migrations in the Pt-catalyzed synthesis of benzo[b]selenophenes, see Sato T, Nakamura I, Terada M. Eur. J. Org. Chem. 2009;5509.
- (72).For 1,3-methyl-group migrations in the Au-catalyzed synthesis of indoles, see Zeng X, Kinjo R, Donnadieu B, Bertrand G. Angew. Chem., Int. Ed. 2010;49:942. doi: 10.1002/anie.200905341..
- (73).Nakamura I, Sato T, Terada M, Yamamoto Y. Org. Lett. 2008;10:2649. doi: 10.1021/ol8007556. [DOI] [PubMed] [Google Scholar]
- (74).Oh CH, Karmakar S, Park H, Ahn Y, Kim JW. J. Am. Chem. Soc. 2010;132:1792. doi: 10.1021/ja9106226. [DOI] [PubMed] [Google Scholar]
- (75).Istrate FM, Gagosz F. Org. Lett. 2007;9:3181. doi: 10.1021/ol0713032. [DOI] [PubMed] [Google Scholar]
- (76).Cariou K, Ronan B, Mignani S, Fensterbank L, Malacria M. Angew. Chem., Int. Ed. 2007;46:1881. doi: 10.1002/anie.200604026. [DOI] [PubMed] [Google Scholar]
- (77).Heugebaert TSA, Stevens CV. Org. Lett. 2009;11:5018. doi: 10.1021/ol902031f. [DOI] [PubMed] [Google Scholar]
- (78).Peng Y, Yu M, Zhang L. Org. Lett. 2008;10:5187. doi: 10.1021/ol802159v. [DOI] [PubMed] [Google Scholar]
- (79).Li G, Huang X, Zhang L. Angew. Chem., Int. Ed. 2008;47:346. doi: 10.1002/anie.200702931. [DOI] [PubMed] [Google Scholar]
- (80).Suhre MH, Reif M, Kirsch SF. Org. Lett. 2005;7:3925. doi: 10.1021/ol0514101. [DOI] [PubMed] [Google Scholar]
- (81).For a two-component synthesis of furans that utilizes a Ag-catalyzed isomerization of vinyl propargyl ethers, see Cao H, Jiang H, Mai R, Zhu S, Qi C. Adv. Synth. Catal. 2010;352:143..
- (82).Sherry BD, Toste FD. J. Am. Chem. Soc. 2004;126:15978. doi: 10.1021/ja044602k. [DOI] [PubMed] [Google Scholar]
- (83).Binder JT, Kirsch SF. Org. Lett. 2006;8:2151. doi: 10.1021/ol060664z. [DOI] [PubMed] [Google Scholar]
- (84).For reviews on NHC complexes of Ag, Cu, and Au, see: Díez-González S, Nolan SP. Aldrichimica Acta. 2008;41:43.. Marion N, Nolan SP. Chem. Soc. Rev. 2008;37:1776. doi: 10.1039/b711132k.. Díez-González S, Marion N, Nolan SP. Chem. Rev. 2009;109:3612. doi: 10.1021/cr900074m.. Lin JCY, Huang RTW, Lee CS, Bhattacharyya A, Hwang WS, Lin IJB. Chem. Rev. 2009;109:3561. doi: 10.1021/cr8005153.. Garrison JC, Youngs WJ. Chem. Rev. 2005;105:3978. doi: 10.1021/cr050004s..
- (85).Saito A, Konishi T, Hanzawa Y. Org. Lett. 2010;12:372. doi: 10.1021/ol902716n. [DOI] [PubMed] [Google Scholar]
- (86).Seregin IV, Gevorgyan V. J. Am. Chem. Soc. 2006;128:12050. doi: 10.1021/ja063278l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Seregin IV, Schammel AW, Gevorgyan V. Tetrahedron. 2008;64:6876. doi: 10.1016/j.tet.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).For reviews, see: Wakatsuki Y. J. Organomet. Chem. 2004;689:4092.. Bruce MI. Chem. Rev. 1991;91:197..
- (89).Nakamura I, Sato T, Terada M, Yamamoto Y. Org. Lett. 2007;9:4081. doi: 10.1021/ol701951n. [DOI] [PubMed] [Google Scholar]
- (90).For 1,3 migrations in propargyl esters, see Marion N, Nolan SP. Angew. Chem., Int. Ed. 2007;46:2750. doi: 10.1002/anie.200604773..
- (91).For 1,3 migrations in propargyl esters, see Marco-Contelles J, Soriano E. Chem.—Eur. J. 2007;13:1350. doi: 10.1002/chem.200601522..
- (92).For 1,3 migrations in propargyl esters, see Correa A, Marion N, Fensterbank L, Malacria M, Nolan SP, Cavallo L. Angew. Chem., Int. Ed. 2008;47:718. doi: 10.1002/anie.200703769..
- (93).Sromek AW, Kel'in AV, Gevorgyan V. Angew. Chem., Int. Ed. 2004;43:2280. doi: 10.1002/anie.200353535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).For select examples of 1,2-acyloxy-group migrations, see: Witham CA, Mauleon P, Shapiro ND, Sherry BD, Toste FD. J. Am. Chem. Soc. 2007;129:5838. doi: 10.1021/ja071231+.. Li G, Zhang G, Zhang L. J. Am. Chem. Soc. 2008;130:3740. doi: 10.1021/ja800001h.. Harrak Y, Blaszykowski C, Bernard M, Cariou K, Mainetti E, Mouriès V, Dhimane A-L, Fensterbank L, Malacria M. J. Am. Chem. Soc. 2004;126:8656. doi: 10.1021/ja0474695.. Mamane V, Gress T, Krause H, Fürstner A. J. Am. Chem. Soc. 2004;126:8654. doi: 10.1021/ja048094q.. Johansson MJ, Gorin DJ, Staben ST, Toste FD. J. Am. Chem. Soc. 2005;127:18002. doi: 10.1021/ja0552500.. Amijs CHM, López-Carrillo V, Echavarren AM. Org. Lett. 2007;9:4021. doi: 10.1021/ol701706d..
- (95).For select examples of 1,3-acyloxy-group migrations, see: Dudnik AS, Schwier T, Gevorgyan V. J. Organomet. Chem. 2009;694:482. doi: 10.1016/j.jorganchem.2008.08.010.. Dudnik AS, Schwier T, Gevorgyan V. Tetrahedron. 2009;65:1859.. Zhang L, Wang S. J. Am. Chem. Soc. 2006;128:1442. doi: 10.1021/ja057327q.. Buzas AK, Istrate FM, Gagosz F. Org. Lett. 2007;9:985. doi: 10.1021/ol063031t.. Dudnik AS, Schwier T, Gevorgyan V. Org. Lett. 2008;10:1465. doi: 10.1021/ol800229h.. Buzas A, Gagosz F. J. Am. Chem. Soc. 2006;128:12614. doi: 10.1021/ja064223m.. Marion N, Díez-González S, de Frémont P, Noble AR, Nolan SP. Angew. Chem., Int. Ed. 2006;45:3647. doi: 10.1002/anie.200600571..
- (96).Schwier T, Sromek AW, Yap DML, Chernyak D, Gevorgyan V. J. Am. Chem. Soc. 2007;129:9868. doi: 10.1021/ja072446m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).For a related work, see Hardin AR, Sarpong R. Org. Lett. 2007;9:4547. doi: 10.1021/ol701973s..
- (98).Rautenstrauch V. J. Org. Chem. 1984;49:950. [Google Scholar]