Abstract
Background
Lung cancer is the leading cancer cause of mortality worldwide; large-scale trials have failed to improve clinical outcomes of patients with chemorefractory non-small-cell lung cancer (NSCLC).
Methods
Following an initial equal randomization period, BATTLE adaptively randomized patients with chemorefractory NSCLC to erlotinib, vandetanib, erlotinib plus bexarotene, or sorafenib based on molecular biomarkers of NSCLC pathogenesis in fresh core needle biopsy specimens. The primary end point was disease control rate (DCR) at 8 weeks.
Results
Of 255 patients randomly assigned to erlotinib (59 patients), vandetanib (54), erlotinib plus bexarotene (37), and sorafenib (105), 244 were eligible for the DCR analysis. Pneumothorax after lung biopsy occurred in 11.5% and treatment-related toxicities grade 3–4 in 6.5% of patients. Overall results were a 46% 8-week DCR, 1.9-month median progression-free survival, 9-month median overall survival, and 35% 1-year survival. Individual markers predicting a significantly superior DCR for a treatment included: epidermal growth factor receptor (EGFR) mutation (P=0.04) for erlotinib; cyclin D1 positivity (P=0.01) or EGFR amplification (P=0.006) for erlotinib plus bexarotene; vascular endothelial growth factor receptor 2 positivity (P=0.05) for vandetanib; and absence of EGFR mutation (P=0.01) or of EGFR high polysomy (P=0.05) for sorafenib. A better 8-week DCR occurred with sorafenib versus all other regimens (64% versus 33%; P<0.001) among EGFR wild-type patients and versus all other regimens (61% versus 32%; P=0.11) among mutant-KRAS patients. The prespecified biomarker groups were less predictive than the individual biomarkers analyzed in this study.
Conclusions
The first completed biopsy-mandated study in pretreated NSCLC, BATTLE confirmed our pre-specified hypotheses regarding biomarker and targeted treatment interactions, establishing a new paradigm for personalizing therapy for patients with NSCLC. (ClinicalTrials.gov numbers, NCT00409968, NCT00411671, NCT00411632, NCT00410059, NCT00410189.)
The leading cause of cancer-related mortality, lung cancer accounts for more U.S. deaths each year than do breast, colon, prostate, liver, and kidney cancers and melanoma combined.1 Systemic chemotherapy is the mainstay for metastatic lung cancer. Although approved therapies in this setting include a few biologic agents, subjective physician preference based on clinical characteristics such as age, gender, or performance status largely drives treatment decisions.2-4
Tumor biomarker evaluations emerged as an important factor in treatment decisions for non-small cell lung cancer (NSCLC) after recently improved outcomes with the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) erlotinib and gefitinib in patients with NSCLC harboring EGFR mutations.5-8 Notwithstanding this success, biologic agents have not been effective in many randomized trials in NSCLC. There is a paucity of effective predictive markers (of drug sensitivity or resistance), due in large part to difficulties in prospectively obtaining baseline tumor tissue in patients with metastatic NSCLC.
In the novel phase II Biomarker-integrated Approaches of Targeted Therapy for Lung cancer Elimination (BATTLE) program of personalized medicine (ClinicalTrials.gov numbers, NCT00409968, NCT00411671, NCT00411632, NCT00410059, NCT00410189) reported here, we prospectively biopsied tumors and, based on tumor markers, used adaptive randomization to assign NSCLC patients to the treatment with the greatest potential benefit.
METHODS
Patient Population
We recruited patients with chemorefractory NSCLC at M. D. Anderson Cancer Center who agreed to a baseline tumor biopsy procedure. Eligibility also included age of 18 years or older and adequate performance status (Eastern Cooperative Oncology Group grade 0–2). Prior treatment with erlotinib was allowed, but such patients were excluded from the erlotinib-containing study arms, and stable (for at least 4 weeks) or treated brain metastases were allowed. Patients with multiple lines of prior therapy were eligible if they had adequate performance status. Radiographic imaging of tumors was reviewed to determine suitability for biopsy. All participants provided written informed consent. Other eligibility criteria appear in the Supplementary Appendix.
Study Design
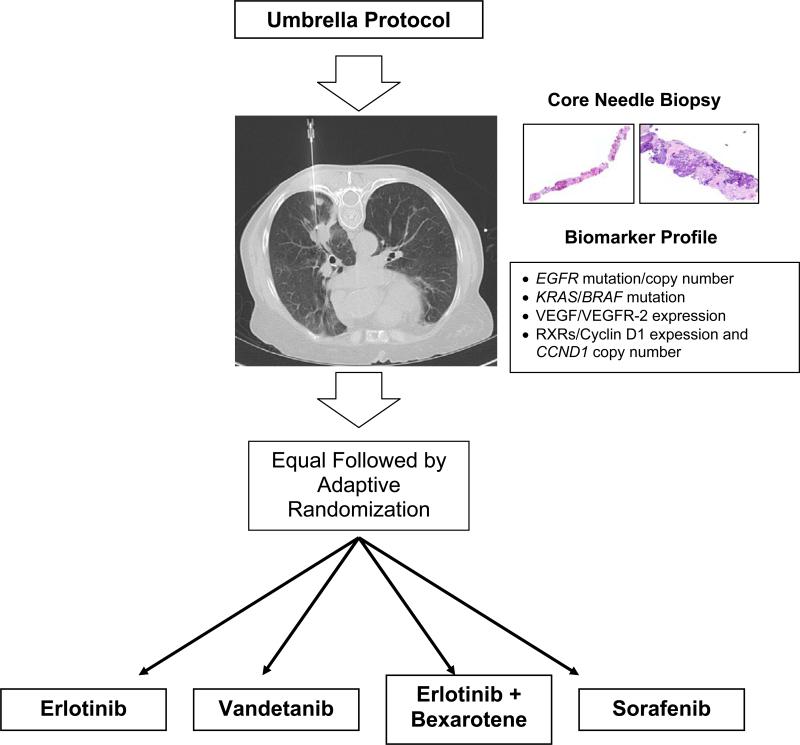
BATTLE was a randomized phase II, single-center, open-label study in patients with advanced NSCLC refractory to prior chemotherapy (Fig. 1). Following molecular tumor-biomarker assessments, patients were randomly assigned to oral treatment with erlotinib (150 mg once daily; Tarceva, OSIP/Genentech), vandetanib (300 mg once daily; Zactima, AstraZeneca), erlotinib (150 mg once daily) plus bexarotene (400 mg/m2 once daily; Targretin, Eisai), or sorafenib (400 mg twice daily; Nexavar, Bayer/Onyx). The primary end point was the disease control rate (DCR) at eight weeks. Secondary end points included response rate, progression-free survival (PFS), overall survival (OS), and toxicity. Planned exploratory objectives were each treatment's efficacy in relation to patient biomarker profiles.
Figure 1.
BATTLE Schema.
The Institutional Review boards of M. D. Anderson Cancer Center and the U.S. Department of Defense approved the study, which was monitored by an independent Data and Safety Monitoring Board.
Biopsy, Molecular Analysis, and Biomarker Grouping
An interventional radiologist used computed tomography or ultrasound guidance to obtain fresh core needle biopsy (CNB) tumor specimens from each patient (Supplementary Appendix). The procedure yielded 1–3 tissue cores approximately 1 mm in diameter and 1.2 cm– 1.8 cm long (average length, 1.5 cm). Each CNB specimen was divided at collection into two portions: 1) tissue for clinical-trial biomarker analysis (at least 1 core), and 2) tissue for future gene expression and proteomic biomarker analysis (at least 1 core). A critical study aspect was the concurrent collection of additional CNB tissue samples, which were prepared simultaneously with the study specimens, for future discovery of novel biomarkers.
The CNB tissue specimens designated for clinical-trial biomarker analysis were formalin-fixed immediately in the interventional radiology suite and transported to the research laboratory for processing and subsequent histological and biomarker analyses. Molecular pathologist I. Wistuba reviewed formalin-fixed, paraffin-embedded, and hematoxylin-and-eosin–stained histological sections within 24 hours of collection to assess the presence, quantity, quality, and histological type of tumor tissue. Each histology section considered adequate for biomarker analysis had at least 200 malignant cells.
We tested the tumor specimens from the mandatory CNB procedure (minimum 2 cores; see Supplementary Appendix) for the following 11 prespecified biomarkers: Mutations of EGFR, KRAS, and BRAF; copy numbers (by fluorescence in situ hybridization) of EGFR and the cyclin D1 gene (CCND1); and protein expression levels of vascular endothelial growth factor (VEGF), VEGF receptor 2 (VEGFR-2), retinoid X receptors (RXRs) α, β, and γ, and cyclin D1. The MD Anderson Thoracic Molecular Pathology Research Laboratory performed these biomarker tests (Supplementary Appendix), reporting results within 2 weeks of each biopsy procedure. Biomarker choices and criteria for classifying each biomarker test as positive or negative were prespecified (see definitions in Supplementary Appendix) prior to starting this study based on data available in 2005.9-14
We established 5 biomarker groups ranked for predictive value (based on evidence available at trial initiation) from 1 (highest) to 5 (lowest), as follows: 1) EGFR mutation and/or EGFR amplification/high polysomy; 2) KRAS or BRAF mutation; 3) VEGF and/or VEGFR-2 overexpression; 4) RXR α, β, or γ overexpression and/or cyclin D1 overexpression and/or CCND1 amplification; or 5) no study biomarkers. Each patient was assigned to one of these groups; patients with biomarkers in more than one group were assigned to the group with the highest ranking.
Randomization
After categorization into marker groups, patients were randomly assigned to one of the four treatment arms. The initial cohort of eligible patients was randomly assigned to the four arms without regard to their respective marker groups (except for patients who had prior treatment with erlotinib, who were excluded from the two erlotinib-containing arms). These patients were assessed for associations between their marker groups and DC, giving a “prior” probability of the DCR for a given treatment in a given marker group. Patients enrolled after the initial cohort were randomly assigned to treatment according to a Bayesian adaptive algorithm, which incorporated the prior probability and outcome (DC) data into a “posterior” probability of the DCR for a given treatment; the resulting posterior probability was continually adjusted per accumulating data on the associations between the DC and biomarkers of patients.
Clinical Assessments
Patients were evaluated clinically at the end of each treatment cycle (defined as lasting 4 weeks), and underwent imaging studies every two cycles, or every 8 weeks. Patients who progressed could re-enter the clinical trial and be re-assigned randomly to treatment if still eligible and agreeable to a new biopsy.
A radiologist independently assessed DC, which was defined as a complete or partial response or stable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST15) at the end of 8 weeks (start of treatment to end of second treatment cycle). PFS was assessed from the date of randomization to the earliest sign of disease progression or death from any cause. OS was assessed from the date of randomization until death from any cause. Tumor response was assessed every 8 weeks until disease progression. We assessed toxicity in accord with the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
Statistical Analysis
The accrual goal was 250 randomized patients to achieve a sample size of 200 patients with complete marker profiles, which would allow an 80% power, with a 20% type-I error rate, to identify effective treatments within each marker group. A high type-I error rate prevented missing any potentially effective treatments that could be confirmed in larger, future studies.16
The primary end point was the 8-week DCR (complete or partial response or stable disease via RECIST criteria), which we compared with the historical 30% DCR estimate in similar patients.17 Treatment efficacy (a positive finding) was defined as a greater than 0.80 probability of achieving greater than a 30% DCR.
We based the statistical design on adaptive randomization under a Bayesian hierarchical model that would increasingly assign patients into treatments with the greatest potential for efficacy based on individual biomarker profiles.16 We planned to randomly assign at least the initial 80 patients equally to the four treatments to allow at least one patient in each marker group to complete treatment, thus providing sufficient data to estimate the prior probability of DC for subsequent patients. Subsequent randomization “switched” to an adaptive algorithm, which incorporated the data of each patient evaluated at the 8-week time point (treatment, biomarker group, and 8-week DCR) into recalculations of the posterior probability of efficacy for treatments in relation to biomarker groups. This scheme adapts randomization probabilities for each of the four treatments from an equal chance, i.e., 25% per treatment, to chances determined by biomarkers of >25% (high predicted DC) or <25% (low predicted DC).
Bayesian adaptive randomization bases treatment assignments on accumulating data within the trial, allowing more patients to be assigned to more effective therapies and fewer patients to be assigned to less effective therapies. This “learn-as-we-go” approach leveraged accumulating patient data to improve the treatment outcome. This trial design also allows the suspension of underperforming treatments in marker groups, as stipulated for our trial if the probability of a DCR of greater than 50% was less than 0.1 (detailed statistical assumptions can be found in Zhou et al., 2008 16).
Standard statistical methods included the Fisher's exact test for contingency tables and log-rank test for survival data, in addition to calculating the Bayesian posterior probability. Each randomized patient represented a unit of the analysis. Time-to-event end points (e.g., OS) were censored at the time of a subsequent randomization for patients randomly assigned more than once.
RESULTS
Patient Characteristics
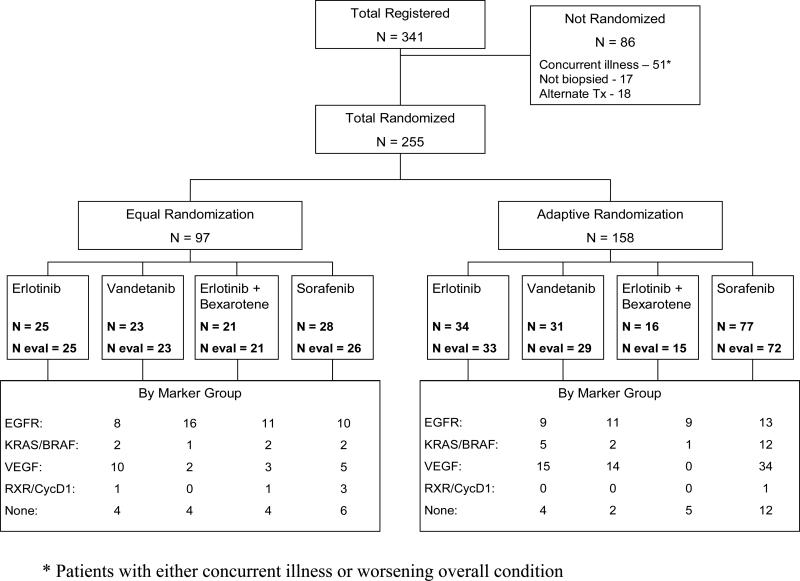
We enrolled 341 patients between November 30, 2006, and October 28, 2009, with equally random assignments for the first 97 patients and adaptive for the remaining 158. The numbers of randomized patients per treatment arm were 59 (erlotinib), 54 (vandetanib), 37 (erlotinib plus bexarotene), and 105 (sorafenib). Seventeen patients were randomly assigned twice, and 1 patient 3 times. Eighty-six patients could not be randomly assigned because of concurrent illnesses or deteriorating performance status (51), conditions preventing a biopsy (17), or choice of an alternative treatment (18) (Fig. 2).
Figure 2.
CONSORT Diagram of BATTLE.
Notable patient characteristics (Table 1) included 83 patients (33%) with prior brain metastases, 116 (45%) with prior treatment with an EGFR TKI, and a median of two prior chemotherapies. Supplementary Table 1 lists the distribution of individual biomarkers. The prevalence of mutations in our study population included 15% EGFR and 20% KRAS. Forty-two patients had inadequate tissue for biomarker analysis, and 2 patients were negative for all study biomarkers.
Table 1.
Patient Characteristics
| Variable | N | (%) | |
|---|---|---|---|
| Age (mean = 62; range=26-84) | <=50 | 41 | (16%) |
| 51~60 | 73 | (29%) | |
| 61~70 | 86 | (34%) | |
| >70 | 55 | (22%) | |
| Gender | Female | 118 | (46%) |
| Male | 137 | (54%) | |
| Ethnicity | Caucasian | 209 | (82%) |
| Hispanic | 16 | (6%) | |
| African American | 16 | (6%) | |
| Asian | 14 | (5%) | |
| Smoker | Current | 23 | (9%) |
| Former | 177 | (69%) | |
| Never | 55 | (22%) | |
| Histology | Adenocacinoma | 160 | (63%) |
| Squamous | 46 | (18%) | |
| Others | 49 | (19%) | |
| Prior erlotinib therapy | No | 139 | (55%) |
| Yes | 116 | (46%) | |
| ECOG performance status | 0 | 22 | (9%) |
| 1 | 197 | (77%) | |
| 2 | 36 | (14%) | |
| Prior cytotoxic chemo therapy (median = 2; range=1-6) | 1 | 95 | (37%) |
| 2 | 84 | (33%) | |
| 3 | 40 | (16%) | |
| 4 | 24 | (9%) | |
| 5 | 9 | (4%) | |
| 6 | 3 | (1%) | |
Efficacy
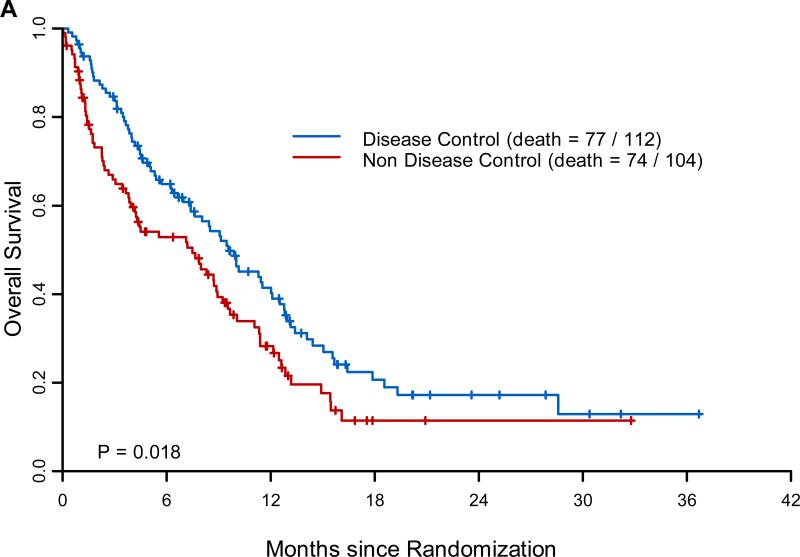
The overall 8-week DCR (in 244 patients eligible for this analysis) was 46% (Table 2); median PFS was 1.9 months (95% confidence interval [CI], 1.8–2.4); median OS was 8.8 months (95% CI, 6.4–10.6); and one-year survival was 35% (Supplementary Fig. 1). There were no complete responses and only 9 partial responses in our heavily pretreated patients. In an 8-week landmark analysis, the median survival of patients with 8-week DC was 9.6 months (95% CI, 7.4–12.5), compared with 7.5 months (95% CI, 4.2–9.2) for patients without 8-week DC (Fig. 3A; P=0.018).The overall 8-week DCRs were 34% (erlotinib), 33% (vandetanib), 50% (erlotinib plus bexarotene), and 58% (sorafenib). Effective treatment–marker-group pairings, defined as having a 0.8 posterior probability of exceeding a DCR of 30%, were as follows: erlotinib in the VEGF/VEGFR-2 group; vandetanib in the EGFR group; erlotinib plus bexarotene in the EGFR, RXR/cyclin D1, and no-marker groups; and sorafenib in the KRAS/BRAF, VEGF/VEGFR-2, and no-marker groups (Table 2).
Table 2.
Efficacy Result by Marker Groups
| Disease Control by Treatment and Marker Groups Number of DC / Total Number of Patients (%) | |||||
|---|---|---|---|---|---|
| Marker Group | Treatment | Total | |||
| Erlotinib | Vandetanib | Erlotinib + Bexarotene | Sorafenib | ||
| EGFR | 6/17 (35%) | 11/27 (41%)* | 11/20 (55%)* | 9/23 (39%) | 37/87 (43%) |
| KRAS/BRAF | 1/7 (14%) | 0/3 (0%) | 1/3 (33%) | 11/14 (79%)* | 13/27 (48%) |
| VEGF/VEGFR-2 | 10/25 (40%)* | 6/16 (38%) | 0/3 (0%) | 25/39 (64%)* | 41/83 (49%) |
| RXR/Cyclin D1 | 0/1 (0%) | 0/0 ( NA ) | 1/1 (100%)* | 1/4 (25%) | 2/6 (33%) |
| None | 3/8 (38%) | 0/6 (0%) | 5/9 (56%)* | 11/18 (61%)* | 19/41 (46%) |
| Total | 20/58 (34%) | 17/52 (33%) | 18/36 (50%) | 57/98 (58%) | 112/244 (46%) |
Cells with an asterisk show the effective treatments within specific marker groups defined as Probability (Disease control rate > 30% | data) is 80% or greater. Only one patient in the RXR/CycD1 marker group received erlotinib+bexarotene.
Figure 3. Major Efficacy Results of BATTLE.
Panel A shows the landmark analysis of overall survival for patients with or without 8-week disease control. The landmark time point is set at 8 weeks, i.e., time 0 is at 8 weeks after randomization. Panel B shows the 8-week disease control rates (in %) by treatment in patients with tumors harboring epidermal growth factor receptor (EGFR) and KRAS mutations.
In addition to analysis of prespecified marker groups, we also analyzed effects of individual markers on treatment efficacy. Confirming our prespecified study hypotheses, individual markers that predicted a better 8-week DC of treatment (versus the marker's opposite status [absence or presence]) were EGFR mutations for erlotinib (P=0.04) and high VEGFR-2 expression for vandetanib (P=0.05). Novel (from exploratory analyses) predictive results were as follows: A better 8-week DC with high cyclin D1 expression for erlotinib plus bexarotene (P=0.01) and with EGFR amplification for erlotinib plus bexarotene (P=0.006); a worse DC with EGFR mutation (P=0.01) or EGFR high polysomy (P=0.05) for sorafenib; and, compared with the combined other treatments, sorafenib had a higher DCR (64% versus 33%) in EGFR-wild-type patients (P<0.001) and a non-statistical trend towards better DCR (61% versus 32%) in mutant-KRAS patients (P=0.11, Figure 3B).
Toxicity
All four treatments were well tolerated, each having toxicity consistent with prior reports. Treatment-related grade 3–4 toxicity was 6.5% (Supplementary Table 2). Average compliance in each arm was over 95%. Sorafenib produced the most toxicity, which caused discontinuation of treatment in 19% and dose reductions in 21% of sorafenib patients (Supplementary Table 3). The lung biopsy procedure was well tolerated overall by the 139 patients undergoing one, with pneumothorax in 11.5%, and only one grade-3 event that needed an overnight hospitalization.
DISCUSSION
The phase II randomized BATTLE trial made important clinical discoveries and demonstrated the feasibility of its novel design for advancing personalized treatment of NSCLC. BATTLE is the first completed, prospective, biopsy–mandated clinical trial in pretreated, advanced lung-cancer patients. The trial data validated prespecified hypotheses regarding predictive biomarkers for targeted agents and identified new predictive markers.
EGFR mutations have been adopted as a predictive biomarker for directing NSCLC patient treatment with EGFR TKIs but are present in only 10%–15% of the lung cancer population. The vast majority of chemotherapy-based clinical trials in NSCLC, which continue to treat NSCLC as a homogeneous disease, have been disheartening, and personalized trials targeting molecular NSCLC characteristics of individual patients may be a viable option for improving treatment outcomes.
We showed that 8-week DC status is a good surrogate for OS in previously treated patients, as also reported by the Southwest Oncology Group during BATTLE.18 This clinically relevant, short-term, surrogate end point facilitated the rapid integration of outcome data into adaptive randomization, confirming its utility for personalizing treatment assignments. In addition, the short-term nature of the 8-week DC end point was not considered to be affected by patients who had received multiple prior treatments before or during BATTLE.
Results of the BATTLE trial support the potential of various biomarkers to predict the sensitivity or resistance of patients to targeted agents. Sorafenib was active against tumors with mutated or wild-type KRAS, but had a worse DCR (compared with other study agents) in patients with EGFR mutations. As expected, 5–7, 10, 19 erlotinib was beneficial in patients with mutated-EGFR tumors. Erlotinib plus bexarotene improved DC in patients with a higher expression of cyclin D1, suggesting a potential role for bexarotene in lung cancer treatment; similar to sorafenib, the combination also improved DC in the KRAS-mutant patient population. Future randomized, controlled studies are needed to further confirm the predictive value of these biomarkers.
Biomarker profiles may differ between early-stage and advanced lung tumors (I. Wistuba, personal communication, 2010). In current practice, biomarker profiles are determined from the original diagnostic tissue and may not reflect tumor biomarker status when treatment decisions are made, thus hampering decision-making for personalized treatment. The present study performed “real-time” biopsies for assessing the current status of tumor biomarkers in patients, thus validating the feasibility of this paradigm-shifting approach.
The BATTLE approach could be expanded to develop personalized cytotoxic therapy. ERCC1 or RRM1 protein can help direct cytotoxic therapy, but only in certain patient subgroups such as patients with a poor performance status; other cytotoxic-therapy markers need further elucidation.20, 21 We mandated at least two CNBs in BATTLE and collected additional tissue and blood for discovering new biomarkers, including gene signatures, which may help further define patient populations sensitive to specific cytotoxic and biologic treatments.
Our study had two important limitations. First and probably most important, biomarker groups were less predictive than were individual biomarkers, which diluted the impact of strong predictors in determining treatment probabilities. For example, EGFR mutations were far more predictive than was the overall EGFR marker group. Also, several of the prespecified markers (e.g., RXR) had little if any predictive value in optimizing treatment selections. This limitation will be addressed in future studies by not grouping or prespecifying biomarkers prior to initiating these biopsy-mandated trials. Allowing prior use of erlotinib was another limitation and biased treatment assignments; in fact, the percentage of patients previously treated with erlotinib steadily increased during trial enrollment. Overall, 45% of our patients were excluded from the two erlotinib-containing arms because of prior EGFR TKI treatment; as erlotinib is a standard of care therapy in NSCLC second-line, maintenance, and front-line settings, the number of patients receiving this targeted agent will likely continue to increase.
BATTLE is the first completed, prospective study in heavily pretreated NSCLC patients that mandated tumor profiling with “real-time” CNBs, demonstrating the feasibility of this approach and created a new paradigm for translational research. This trial took a substantial step toward realizing personalized lung cancer therapy by integrating “real-time” molecular laboratory findings in delineating specific patient populations for individualized treatment. BATTLE accumulated increasing probabilities of a positive treatment outcome and showed the potential of its mandatory-biopsy paradigm for developing specific predictive biomarkers and associated treatments for subsequent definitive clinical testing. This approach will be important for future evaluations of new molecular targets and predictive biomarkers.22, 23 The successful completion of BATTLE reported here will potentially facilitate the implementation of new such personalized trials in lung and other cancers with even more efficient designs, as a forerunner in the quest for discovery of novel cancer treatments.
Supplementary Material
Acknowledgments
This research was supported in part by the Department of Defense grant W81XWH-6-1-0303 and by the National Institutes of Health through M. D. Anderson's Cancer Center Support Grant CA016672.
We gratefully acknowledge the patients who participated in the study, as well as Jeffrey Lewis for programming and database management support, Dawn Chalaire and Sunita Patterson for editorial assistance, the Faith, Family and Friends Foundation, Rexanna's Foundation, Mayberry Memorial Foundation, Stading Family Foundation, Hewett Foundation, Cohen-Reinach Foundation, and the National Foundation for Cancer Research (NFCR). We also thank the MD Anderson Data Safety and Monitoring Board chaired by Dr. Donald A. Berry for monitoring the trial.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010 Jul 7; doi: 10.3322/caac.20073. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. PMID: 17167137. [DOI] [PubMed] [Google Scholar]
- 4.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. PMID: 18506025. [DOI] [PubMed] [Google Scholar]
- 5.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small cell lung cancer (INTEREST): a randomized Phase III trial. Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. PMID: 19027483. [DOI] [PubMed] [Google Scholar]
- 6.Douillard JY, Shepherd FA, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized Phase III INTEREST trial. J Clin Oncol. 2009;28:744–752. doi: 10.1200/JCO.2009.24.3030. PMID 20038723. [DOI] [PubMed] [Google Scholar]
- 7.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. PMID: 19692680. [DOI] [PubMed] [Google Scholar]
- 8.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. PMID: 19692684. [DOI] [PubMed] [Google Scholar]
- 9.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 10.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in Lung Cancer: Molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–44. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch FR, Dziadziuszko R, Thatcher N, et al. Epidermal growth factor receptor immunohistochemistry: comparison of antibodies and cutoff points to predict benefit from gefitinib in a phase 3 placebo-controlled study in advanced nonsmall-cell lung cancer. Cancer. 2008;112:1114–21. doi: 10.1002/cncr.23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dragnev KH, Petty WJ, Shah S, et al. Bexarotene and erlotinib for aerodigestive tract cancer. J Clin Oncol. 2005;34:8757–64. doi: 10.1200/JCO.2005.01.9521. [DOI] [PubMed] [Google Scholar]
- 13.Edelman MJ, Smith R, Hausner P, et al. Phase II trial of the novel retinoid, bexarotene, and gemcitabine plus carboplatin in advanced non-small cell lung cancer. J Clin Oncol. 2005;24:5774–78. doi: 10.1200/JCO.2005.14.373. [DOI] [PubMed] [Google Scholar]
- 14.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X, Liu S, Kim ES. Bayesian adaptive design for targeted therapy development in lung cancer--a step toward personalized medicine. Clin Trials. 2008;5:181–93. doi: 10.1177/1740774508091815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–62. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 18.Lara PN, Redman MW, Kelly K, et al. Disease control rate at 8 weeks predicts clinical benefit in advanced non-small-cell lung cancer: results from southwest oncology group randomized trials. J Clin Oncol. 2009;3:463–7. doi: 10.1200/JCO.2007.13.0344. [DOI] [PubMed] [Google Scholar]
- 19.Douillard JY, Shepherd FA, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol. 2010;28:744–52. doi: 10.1200/JCO.2009.24.3030. [DOI] [PubMed] [Google Scholar]
- 20.Simon G, Sharma A, Li X, et al. Feasibility and efficacy of molecular analysis-directed individualized therapy in advanced non-small-cell lung cancer. J Clin Oncol. 2007;19:2741–46. doi: 10.1200/JCO.2006.08.2099. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds C, Obasaju C, Schell MJ, et al. Randomized phase III trial of gemcitabine-based chemotherapy with in situ RRM1 and ERCC1 protein levels for response prediction in non-small cell lung cancer. J Clin Oncol. 2009;34:5808–15. doi: 10.1200/JCO.2009.21.9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw AT, Yeap BY, Mino-Kenudson M, et al. J Clin Oncol. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. 2009;27:4247–53. doi: 10.1200/JCO.2009.22.6993. Epub 2009 Aug 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turke AB, Zejnullahu K, Wu YL, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.