Abstract
Frequently, patients with hepatitis C virus (HCV) chronic infection have high levels of serum anti-thyroperoxidase and/or anti-thyroglobulin autoantibodies, ultrasonographic signs of chronic autoimmune thyroiditis, and subclinical hypothyroidism, in female gender versus healthy controls, or hepatitis B virus infected patients. In patients with “HCV-associated mixed cryoglobulinemia” (MC + HCV), a higher prevalence of thyroid autoimmune disorders was shown not only compared to controls, but also versus HCV patients without cryoglobulinemia. Patients with MC + HCV or HCV chronic infection show a higher prevalence of papillary thyroid cancer than controls, in particular in patients with autoimmune thyroiditis. Patients with HCV chronic infection, or with MC + HCV, in presence of autoimmune thyroiditis, show higher serum levels of T-helper (Th)1 (C-X-C motif) ligand 10 (CXCL10) chemokine, but normal levels of Th2 (C-C motif) ligand 2 chemokine, than patients without thyroiditis. HCV thyroid infection could act by upregulating CXCL10 gene expression and secretion in thyrocytes recruiting Th1 lymphocytes that secrete interferon-γ and tumor necrosis factor-α. These cytokines might induce a further CXCL10 secretion by thyrocytes, thus perpetuating the immune cascade, which may lead to the appearance of autoimmune thyroid disorders in genetically predisposed subjects. A careful monitoring of thyroid function, particularly where nodules occur, is recommended in HCV patients.
1. Introduction
About 130–170 million people worldwide have been infected by hepatitis C virus (HCV) [1]. Hepatocytes represent the major site of viral replication, and the replication of HCV is present in extrahepatic tissues and peripheral blood mononuclear cells [2].
Previous studies have shown that 38–76% of patients with chronic HCV infection develop at least one extrahepatic manifestation (EHM) [3, 4].
An association between HCV and mixed cryoglobulinemia (MC) was first described; subsequently, the involvement of many organs and systems was reported (kidney, skin, eyes, joints, and nervous system). The infected extrahepatic tissues might act as a reservoir for HCV [5] and play a role in both HCV persistence and reactivation of infection. HCV, as an etiological agent replicating and expressing viral proteins in extrahepatic tissues itself, contributes to EHM associated with chronic HCV infection. An important feature of HCV is that the virus avoids immune elimination; a consequence is chronic infection and an accumulation of circulating immunocomplexes and autoimmune phenomena [6–8], as recently Cheng et al. have demonstrated in their study among 297 Chinese patients [9].
These EHM mainly include autoimmune disorders [10–12] such as MC [13, 14] and Sjogren's syndrome and endocrinological diseases as autoimmune thyroid disorders (AITD) and type 2 diabetes [15–17].
2. Autoimmune Thyroiditis
Hashimoto's thyroiditis or autoimmune chronic thyroiditis (AT) is among the most common thyroid diseases. AT is the most widespread thyroiditis form and its prevalence is definitely more frequent in female gender and in the elderly. The incidence in female gender is of 3,5 cases/1000 subjects per year, while in men it is lower (0,8 cases/1000 persons per year): there is a remarkable variability in different geographic areas [18].
AT is an organ-specific autoimmune disease, morphologically characterized by a chronic lymphocytes infiltration of thyroid and the presence of circulating autoantibodies such as antiperoxidase (AbTPO) and antithyroglobulin (AbTg). The inflammatory process leads to a follicular destruction; indeed, AT is the most common cause of hypothyroidism in areas of iodine sufficiency [19].
Occasionally, thyroid stimulating hormone (TSH) receptor blocking antibodies can be responsible of an atrophic form of AT; more rarely, anti-TSH receptor stimulating antibodies can cause a transient form of hyperthyroidism (hashitoxicosis) [20].
Risk factors associated with AT are numerous [21–24].
Age: the prevalence of disease tends to increase with age.
Genetic: a significant association between Hashimoto's thyroiditis and some histocompatibility antigens (HLA-DR, HLA-DR5, and some DQ alleles) is demonstrated. Many other susceptibility genes have been associated with AT; for example, specific CTLA4 gene polymorphisms are linked to a possible development of antithyroid antibodies [25].
Iodine: an increased AT prevalence is observed in areas of iodine sufficiency, compared with iodine-deficient areas [26, 27].
Selenium: a selenium deficit is linked to a higher AT prevalence [28].
Irradiation: AT occurs more frequently after the exposure to low doses of radiations [26].
Cytokine: the treatment with Interferon- (IFN-) α, or with Interleukin- (IL-) 2, can promote the onset of AT in predisposed patients [29].
Infections: it was seen that several viral infections can predispose to an AT in animals. Moreover, different studies tried to associate AT with viral infections in humans with conflicting results [30–33].
3. Chronic HCV (CHC) Infection and Thyroid
3.1. CHC Infection and Thyroid Autoimmunity
In a first study, Tran et al. report two cases of Hashimoto's thyroiditis associated with chronic active HCV infection, suggesting that HCV infection might be involved in the appearance of AT [34, 35].
The prevalence of HCV infection in patients with different thyroid disorders has been evaluated by several studies with conflicting results. Duclos-Vallée et al. evaluated the prevalence of HCV infection in 200 patients with thyroid diseases; among 50 patients with simple goiter, none were anti-HCV-positive; among 50 individuals with goiter, 2 were positive; among 5 individuals with myxedema, 2 were positive; among 50 patients with Hashimoto's thyroiditis, 12 were positive. These results suggested that HCV infection might be associated with AT [36]. Recently, Yang et al. compared 462 persons with positive AbTPO and/or AbTg to 360 persons with antibody negativity and no difference in the prevalence of anti-HCV positivity between the 2 groups (1.3% versus 0.53%; P > 0.05) was found [37]. In a study conducted by Marconcini et al., 66 HCV+ patients were evaluated and AbTPOs were detected in 4/54 (7.4%) of the patients, whereas AbTgs were detected in none of the patients (0/48) [38].
Conflicting results have been reported from earlier studies of patients with CHC, with some supporting an association of HCV infection with AITD [39–47] and others not [48, 49].
However, some of the earlier studies were negative because of the lack of control for factors which may affect the development of thyroid autoimmunity, such as iodine intake [50].
Indeed, the largest study about HCV and thyroiditis, in which iodine deficiency was evaluated, demonstrated that both hypothyroidism and thyroid autoimmunity were significantly more common in patients with HCV compared to controls [41].
The prevalence of thyroid disorders in 630 consecutive patients with chronic hepatitis due to HCV infection was investigated; all patients were free of cirrhosis and hepatocarcinoma and were not on interferon treatment. Three control groups were included: (a) 389 subjects from an iodine-deficient area, (b) 268 persons living in an area of iodine sufficiency, and (c) 86 patients > 40 years of age with chronic hepatitis B. Levels of thyroid-stimulating hormone (TSH), free T4 (FT4), and free T3 (FT3), as well as AbTgs and AbTPOs, were measured. Mean TSH levels were higher (P = 0.001) and FT3 and FT4 levels were lower (P < 0.0001) in patients with CHC than in all other groups. Patients with CHC were more likely to have hypothyroidism (13% (n = 82)), AbTgs (17% (n = 108)), and AbTPOs (21% (n = 132)) than were any of the other groups. The results of this study suggested that both hypothyroidism and thyroid autoimmunity are more common in patients with CHC, even in the absence of cirrhosis, hepatocellular carcinoma, or interferon treatment, than in HCV-negative controls or in patients with chronic hepatitis B infection [41].
Evidence for this association also came from a study that reported a higher prevalence of hypothyroidism and AbTgs in untreated children with CHC compared to healthy non-HCV infected controls [51]. In most studies, examining the frequency of thyroid disorders in patients with HCV, approximately 10–15% of the patients had positive thyroid antibodies before the beginning of the therapy with IFN [52–58]. Moreover, pooling of data from controlled studies on HCV infection and thyroid autoimmunity demonstrated a significant increase in the risk of thyroiditis in HCV patients [59]. A large study which included 146394 patients infected with HCV confirmed these results showing a significant increased risk for thyroiditis [60]. This was a retrospective cohort study of users of US Veterans Affairs health care facilities from 1997 to 2004, which included 146394 CHC patients who had at least 2 visits and 572293 patients uninfected with HCV. The thyroiditis risk was significantly increased in HCV patients. Since 97% of HCV patients were men and it is well known that male gender has a lower risk of thyroiditis than female, this result is particularly interesting [60].
The presence of higher risk of AT in female gender increased circulating levels of AbTPOs and increased risk of hypothyroidism in female gender and AbTPO-positive subjects characterized the pattern of thyroid disorders observed in HCV infection [59, 61, 62].
Despite their remarkable therapeutic efficacy, IFN-α adverse effects are well-known, from influenza-like symptoms to hematologic effects, neuropsychiatric symptoms, and thyroid diseases [63]. In particular, previous studies showed that female gender is one of the most common risk factors that predict the development of AITD during interferon therapy [64, 65]. An association between IFN-α and thyroid disease was recognized as early as 1985 in patients who have been treated with IFN-α for breast cancer [66]. Later, several cases have reported the possible association between thyroid disease and IFN-α [67]. Different forms of IFN induced thyroid autoimmunity have been identified, such as GD, thyroiditis, and subclinical hypothyroidism [68]. Graves' hyperthyroidism is the less common type, because only 20–25% of all patients with IFN-related thyrotoxicosis are linked to Graves' disease (GD) induced by circulating thyroid receptor antibodies (TRAb) [69, 70].
Interferon induced thyroiditis (IIT) can be divided into two main groups: autoimmune type and nonautoimmune type [71]. The former can manifest as HT and GD and sometimes may be related to the production of thyroid autoantibodies without clinical disease.
Another interesting classification of interferon induced hyperthyroidism has been proposed by Czarnywojtek et al. [72] (in comparison with amiodarone induced thyrotoxicosis (AIT)): (I) type 1, corresponding to type I amiodarone induced thyrotoxicosis (AIT): (a) GT without TAO and (b) GT with TAO (mild or severe); (II) type 2 destructive thyrotoxicosis, partially analogous to type II AIT: (a) asymptomatic: silent thyroiditis and (b) symptomatic; and (III) type 3 unknown aetiology, partial analogy to type III AIT—undefined or mixed.
The presence of thyroid autoantibodies before the initiation of IFN-α therapy is an important risk factor for the development of IIT. In HCV-positive individuals, the progression toward hypothyroidism, in thyroid autoantibodies positive patients who undergo IFN-α treatment, is often associated with an increase in antibody titers [73]. Furthermore, Prummel and Laurberg showed that positive pretreatment AbTPOs are an important risk factor for the development of thyroid dysfunction [74]. There is also an obvious link between female sex, old age, and genetic predisposition with the development of antibodies [75, 76].
3.2. Cryoglobulinemia and Thyroid Diseases
Few anecdotal studies evaluated AITD in patients with cryoglobulinemia [77, 78].
A case-control prospective study has been conducted to evaluate thyroid disorders in 93 MC + HCV patients, matched by sex and age (±2 years), to 93 patients with CHC without MC and 93 healthy (HCV-negative) controls from the local population [79, 80]. The following thyroid autoimmune abnormalities were significantly more frequent in MC + HCV patients than in HCV-negative controls: serum AbTPO levels (28% versus 9%), serum AbTPO and/or AbTg levels (31% versus 12%), AT (35% versus 16%), and subclinical hypothyroidism (11% versus 2%). Serum AbTPOs were also significantly more frequent in MC + HCV patients than in CHC controls (28% versus 14%). A higher prevalence of thyroid disorders in patients with MC + HCV not only with respect to controls, but also with respect to HCV patients without cryoglobulinemia was shown, suggesting a careful monitoring of thyroid function in these patients [80].
The presence of a higher risk of AT and hypothyroidism and increased circulating levels of AbTPO, in female gender, characterized the pattern of thyroid disorders observed in MC + HCV infection, similarly to HCV patients without MC [59, 61].
3.3. CHC Infection and Thyroid Cancer
A high prevalence of papillary thyroid cancer (PTC) was first observed in 139 HCV patients (2.2%), while no case was observed in 835 control subjects who were long-term residents of an iodine-deficient area [81], and it was subsequently confirmed in other studies [82, 83]. Montella et al. have carried out a case-controlled study on the different oncological pathologies. They screened 495 patients with different types of cancer: 114 cases of liver cancer, 41 of multiple myeloma, 111 of non-Hodgkin's lymphomas, 130 of thyroid cancers, and 63 cases of Hodgkin's disease. The controls were 226 patients with no history of cancer. The relationship between each cancer and HCV infection was assessed by means of odds ratios (OR) and corresponding 95% confidence intervals.
Risks were greater for liver cancer (OR = 32.9, 95% CI 16.5–65.4, P < 0.0001), multiple myeloma (OR = 4.5, 95% CI 1.9–10.7, P = 0.0004), and B-cell non-Hodgkin's lymphoma (OR = 3.7, 95% CI 1.9–7.4, P = 0.0001). For Hodgkin's disease, there was no significant association (P = 0.3). An association between HCV and thyroid cancer was noted (OR = 2.8, 95% CI 1.2–6.3, P = 0.01) [83].
The prevalence of thyroid cancer was also investigated in a series of unselected 94 MC + HCV patients in comparison with a gender- and age-matched control group obtained from a sample of the general population (470 subjects). The prevalence of thyroid nodules was higher in control subjects than in MC + HCV patients (65.3% versus 54.8%), even though not significantly. Two patients with PTC were found in the MC + HCV series, while no case was observed among controls (P = 0.001). Lymphocytic infiltration was observed in the thyroid tissue in both MC + HCV patients with PTC [84]. Other studies have confirmed an association between AT and thyroid cancer [85, 86]. Accordingly, features of AT were observed more frequently in HCV patients than in controls suggesting that AT may be a predisposing condition for thyroid cancer [87]. Since about 15–30% of HCV patients may show an aggressive disease, for example, lung metastases, difficult to treat [88, 89], the finding of an increased prevalence of thyroid cancer in these patients is clinically relevant [90].
3.4. Immunopathogenesis of HCV Infection and AITD
Several molecular mechanisms have been suggested for the association of CHC with AT: (a) molecular mimicry or cross-reactivity which may occur between viral antigens and thyroidal antigens [91], (b) heat shock proteins expression in thyroid gland [92], and (c) abnormal expression of MHC class II molecules by thyrocytes [93].
An increased expression of IFN-γ and IFN-γ inducible chemokines [94, 95], in particular (C-X-C motif) ligand 10 chemokine (CXCL10), has been shown in hepatocytes and in lymphocytes of HCV infected patients, directly related to the degree of inflammation and to an increase in circulating levels of IFN-γ and CXCL10 [40, 96–103]. CXCL10 is one of chemokines with C-X-C motif. IP-10 activates specifically CXCR3 receptor that is a G protein-coupled receptor with seven transmembrane domains mainly expressed in T activated lymphocytes, natural-killer cells (NKs), macrophages, and B cells [104, 105]. Recent studies showed that CXCL10 expression in serum and/or tissue levels is increased in autoimmune organ-specific diseases [106], such as type 1 diabetes [107–109], or systemic rheumatological diseases like rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis [97, 110], sarcoidosis [111, 112], and psoriatic arthritis [113, 114].
High levels of CXCL10 are present in patients with AT, in particular in the presence of hypothyroidism, and an involvement of T-helper (Th)1 immune response in the induction of AT [97, 115–117], GD, and Graves' ophthalmopathy [97–100, 118] has been demonstrated, suggesting that intrathyroidal lymphocytes and/or thyrocytes may be the source of CXCL10 [119]. Furthermore, the presence of HCV in the thyroid of chronically infected patients has been recently shown [120, 121].
On the abovementioned bases, it has been speculated that HCV thyroid infection may act by upregulating CXCL10 gene expression and secretion in thyrocytes recruiting Th1 lymphocytes that secrete IFN-γ and tumor necrosis factor- (TNF-) α. These cytokines induce CXCL10 secretion by thyrocytes, thus perpetuating the immune cascade, which may lead to the appearance of AITD in genetically predisposed subjects [111] (Figure 1).
Figure 1.
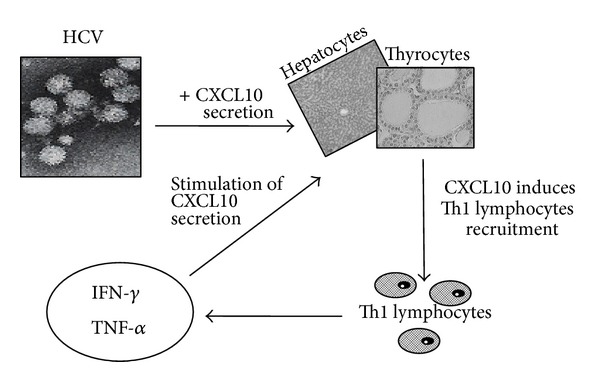
HCV thyroid infection may act by upregulating CXCL10 gene expression and secretion in thyrocytes recruiting Th1 lymphocytes that secrete IFN-γ and TNF-α. These cytokines induce CXCL10 secretion by thyrocytes, thus perpetuating the immune cascade.
Recently, the finding of high serum levels of CXCL10 but normal levels of the prototype Th2 chemokine (C-C motif) ligand 2 (CCL2) in MC + HCV patients with AT, in comparison with patients without thyroiditis, has confirmed this hypothesis. These data suggest that the Th1 CXCL10 chemokine is specifically linked to the appearance of AT in these patients [122].
Serums CXCL10 and CCL2 were assayed in 60 MC + HCV patients, in 45 patients with “MC with AT” (MC + AT), and in controls (60 without (control 1) and 45 with AT (control 2)). CXCL10 was significantly higher in control 2 than in control 1 (P < 0.001), in MC than in control 1, and in MC + AT than in controls 1 and 2 and MC (P = 0.002). A high CXCL10 level (>mean ± SD control 1; >167 pg/mL) was present in 7% of control 1, 21% of control 2, 49% of MC, and 78% of MC + AT (P < 0.0001). CCL2 was significantly higher in MC and in MC + AT than in control 1 or in control 2 (P < 0.01). A high CCL2 level (>mean ± SD control 1; >730 pg/mL) was present in 2% of control 1, 1% of control 2, 18% MC, and 21% of MC + AT (P < 0.0001) [122].
Among the proinflammatory cytokines, IL-1β and TNF-α were not associated with the presence of AT in MC + HCV patients, while IL-6 was modestly but significantly increased in patients with AT [5, 123–125].
On the whole, in agreement with what was observed in other autoimmune disorders [126–129], the above reported data underline the importance of the activation of the Th1 immunity in the initiation of AT in patients with MC + HCV.
4. Conclusion
In conclusion, the abovementioned results show a high prevalence of AITD in patients with CHC infection. The presence of a higher risk of AT in female gender, increased circulating levels of AbTPOs, and increased risk of hypothyroidism in female gender and AbTPO-positive subjects characterized the pattern of thyroid disorders observed in HCV infection. In HCV patients with thyroid cancer, thyroidectomy is required and, if appropriate, radioiodine treatment. Patients with HCV infection with AITD where nodules occurred and in fine needle aspiration biopsy without neoplastic processes do require careful observation.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Alter MJ. Epidemiology of hepatitis C virus infection. World Journal of Gastroenterology. 2007;13(17):2436–2441. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackard JT, Kemmer N, Sherman KE. Extrahepatic replication of HCV: insights into clinical manifestations and biological consequences. Hepatology. 2006;44(1):15–22. doi: 10.1002/hep.21283. [DOI] [PubMed] [Google Scholar]
- 3.Stefanova-Petrova DV, Tzvetanska AH, Naumova EJ, et al. Chronic hepatitis C virus infection: prevalence of extrahepatic manifestations and association with cryoglobulinemia in Bulgarian patients. World Journal of Gastroenterology. 2007;13(48):6518–6528. doi: 10.3748/wjg.v13.i48.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galossi A, Guarisco R, Bellis L, Puoti C. Extrahepatic manifestations of chronic HCV infection. Journal of Gastrointestinal and Liver Diseases. 2007;16(1):65–73. [PubMed] [Google Scholar]
- 5.Ferri C, Antonelli A, Mascia MT, et al. B-cells and mixed cryoglobulinemia. Autoimmunity Reviews. 2007;7(2):114–120. doi: 10.1016/j.autrev.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Ferri C, Antonelli A, Mascia MT, et al. HCV-related autoimmune and neoplastic disorders: the HCV syndrome. Digestive and Liver Disease. 2007;39(1):S13–S21. doi: 10.1016/s1590-8658(07)80005-3. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari SM, Fallahi P, Mancusi C, et al. HCV-related autoimmune disorders in HCV chronic infection. La Clinica Terapeutica. 2013;164(4):e305–e312. doi: 10.7417/CT.2013.1594. [DOI] [PubMed] [Google Scholar]
- 8.Fallahi P, Ferrari SM, Giuggioli D, et al. Mixed cryoglobulinemia and thyroid autoimmune disorders. Clinica Terapeutica. 2013;164(4):e337–e341. doi: 10.7417/CT.2013.1598. [DOI] [PubMed] [Google Scholar]
- 9.Cheng ZJ, Zhou BT, Shi XC, et al. Extrahepatic manifestations of chronic hepatitis C virus infection: 297 cases from a tertiary medical center in Beijing, China. Chinese Medical Journal. 2014;127(7):1206–1210. [PubMed] [Google Scholar]
- 10.Blackard JT, Kong L, Huber AK, Tomer Y. Hepatitis C virus infection of a thyroid cell line: implications for pathogenesis of hepatitis C virus and thyroiditis. Thyroid. 2013;23(7):863–870. doi: 10.1089/thy.2012.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palazzi C, Buskila D, D’Angelo S, D’Amico E, Olivieri I. Autoantibodies in patients with chronic hepatitis C virus infection: pitfalls for the diagnosis of rheumatic diseases. Autoimmunity Reviews. 2012;11(9):659–663. doi: 10.1016/j.autrev.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Saadoun D, Landau DA, Calabrese LH, Cacoub PP. Hepatitis C-associated mixed cryoglobulinaemia: a crossroad between autoimmunity and lymphoproliferation. Rheumatology. 2007;46(8):1234–1242. doi: 10.1093/rheumatology/kem132. [DOI] [PubMed] [Google Scholar]
- 13.Cicardi M, Cesana B, Del Ninno E, et al. Prevalence and risk factors for the presence of serum cryoglobulins in patients with chronic hepatitis C. Journal of Viral Hepatitis. 2000;7(2):138–143. doi: 10.1046/j.1365-2893.2000.00204.x. [DOI] [PubMed] [Google Scholar]
- 14.Lunel F, Musset L, Cacoub P, et al. Cryoglobulinemia in chronic liver diseases: Role of hepatitis C virus and liver damage. Gastroenterology. 1994;106(5):1291–1300. doi: 10.1016/0016-5085(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 15.Fallahi P, di Domenicantonio A, Mazzi V, et al. Hepatitis c virus and type 1 diabetes. Clinica Terapeutica. 2013;164(5):e437–e444. doi: 10.7417/CT.2013.1624. [DOI] [PubMed] [Google Scholar]
- 16.Fallahi P, Ferrari SM, Colaci M, et al. Hepatitis C virus infection and type 2 diabetes. La Clinica Terapeutica. 2013;164(5):e393–e404. doi: 10.7417/CT.2013.1620. [DOI] [PubMed] [Google Scholar]
- 17.Obermayer-Straub P, Manns MP. Hepatitis C and D, retroviruses and autoimmune manifestations. Journal of Autoimmunity. 2001;16(3):275–285. doi: 10.1006/jaut.2000.0488. [DOI] [PubMed] [Google Scholar]
- 18.McLeod DSA, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42(2):252–265. doi: 10.1007/s12020-012-9703-2. [DOI] [PubMed] [Google Scholar]
- 19.Orgiazzi J. Thyroid autoimmunity. La Presse Médicale. 2012;41(12) part 2:e611–e625. doi: 10.1016/j.lpm.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Caturegli P, de Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmunity Reviews. 2014;13(4-5):391–397. doi: 10.1016/j.autrev.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Corrado A, Ferrari SM, Ferri C, Ferrannini E, Antonelli A, Fallahi P. Type 1 diabetes and (C-X-C motif) ligand (CXCL) 10 chemokine. Clinica Terapeutica. 2014;165(2):e181–e185. doi: 10.7471/CT.2014.1706. [DOI] [PubMed] [Google Scholar]
- 22.Mancusi C, di Domenicantonio A, Politti U, et al. The alpha chemokine “Interferon gamma-induced protein 10” (IP-10) in Graves' disease. Clinica Terapeutica. 2014;165(2):e174–e180. doi: 10.7471/CT.2014.1705. [DOI] [PubMed] [Google Scholar]
- 23.Saranac L, Zivanovic S, Bjelakovic B, Stamenkovic H, Novak M, Kamenov B. Why is the thyroid so prone to autoimmune disease? Hormone Research in Paediatrics. 2011;75(3):157–165. doi: 10.1159/000324442. [DOI] [PubMed] [Google Scholar]
- 24.Brent GA. Environmental exposures and autoimmune thyroid disease. Thyroid. 2010;20(7):755–761. doi: 10.1089/thy.2010.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomer Y. Genetic susceptibility to autoimmune thyroid disease: past, present, and future. Thyroid. 2010;20(7):715–725. doi: 10.1089/thy.2010.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duntas LH. Environmental factors and thyroid autoimmunity. Annales d'Endocrinologie. 2011;72(2):108–113. doi: 10.1016/j.ando.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Martino E, Macchia E, Aghini-Lombardi F, et al. Is humoral thyroid autoimmunity relevant in amiodarone iodine-induced thyrotoxicosis (AIIT)? Clinical Endocrinology. 1986;24(6):627–633. doi: 10.1111/j.1365-2265.1986.tb01658.x. [DOI] [PubMed] [Google Scholar]
- 28.Köhrle J. Selenium and the thyroid. Current Opinion in Endocrinology, Diabetes and Obesity. 2013;20(5):441–448. doi: 10.1097/01.med.0000433066.24541.88. [DOI] [PubMed] [Google Scholar]
- 29.Antonelli A, Ferri C, Fallahi P. Hepatitis C: thyroid dysfunction in patients with hepatitis C on IFN-α therapy. Nature Reviews Gastroenterology and Hepatology. 2009;6(11):633–635. doi: 10.1038/nrgastro.2009.168. [DOI] [PubMed] [Google Scholar]
- 30.Desailloud R, Hober D. Viruses and thyroiditis: an update. Virology Journal. 2009;6, article 5 doi: 10.1186/1743-422X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akeno N, Blackard JT, Tomer Y. HCV E2 protein binds directly to thyroid cells and induces IL-8 production: a new mechanism for HCV induced thyroid autoimmunity. Journal of Autoimmunity. 2008;31(4):339–344. doi: 10.1016/j.jaut.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minelli R, Braverman LE, Giuberti T, et al. Effects of excess iodine administration on thyroid function in euthyroid patients with a previous episode of thyroid dysfunction induced by interferon-alpha treatment. Clinical Endocrinology. 1997;47(3):357–361. doi: 10.1046/j.1365-2265.1997.2721081.x. [DOI] [PubMed] [Google Scholar]
- 33.Tomer Y, Davies TF. Infection, thyroid disease, and autoimmunity. Endocrine Reviews. 1993;14(1):107–120. doi: 10.1210/edrv-14-1-107. [DOI] [PubMed] [Google Scholar]
- 34.Bartolomé J, Rodríguez-Iñigo E, Quadros P, et al. Detection of hepatitis C virus in thyroid tissue from patients with chronic HCV infection. Journal of Medical Virology. 2008;80(9):1588–1594. doi: 10.1002/jmv.21269. [DOI] [PubMed] [Google Scholar]
- 35.Agnello V, De Rosa FG. Extrahepatic disease manifestations of HCV infection: some current issues. Journal of Hepatology. 2004;40(2):341–352. doi: 10.1016/j.jhep.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Duclos-Vallée JC, Johanet C, Trinchet JC, et al. High prevalence of serum antibodies to hepatitis C virus in patients with Hashimoto's thyroiditis. British Medical Journal. 1994;309(6958):846–847. doi: 10.1136/bmj.309.6958.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang R, Shan Z, Li Y, Fan C, Li C, Teng W. Prevalence of thyroid autoantibodies in hepatitis C and hepatits B infection in China. Internal Medicine. 2011;50(8):811–815. doi: 10.2169/internalmedicine.50.4870. [DOI] [PubMed] [Google Scholar]
- 38.Marconcini ML, Fayad L, Shiozawa MBC, Dantas-Correa EB, de Lucca Schiavon L, Narciso-Schiavon JL. Autoantibody profile in individuals with chronic hepatitis C. Revista da Sociedade Brasileira de Medicina Tropical. 2013;46(2):147–153. doi: 10.1590/0037-8682-0039-2013. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Soto L, Gonzalez A, Escobar-Jimenez F, et al. Increased risk of autoimmune thyroid disease in hepatitis C vs hepatitis B before, during, and after discontinuing interferon therapy. Archives of Internal Medicine. 1998;158(13):1445–1448. doi: 10.1001/archinte.158.13.1445. [DOI] [PubMed] [Google Scholar]
- 40.Danilovic DLS, Mendes-Correa MC, Chammas MC, Zambrini H, Barros RK, Marui S. Thyroid disturbance related to chronic hepatitis C infection: role of CXCL10. Endocrine Journal. 2013;60(5):583–590. doi: 10.1507/endocrj.ej12-0321. [DOI] [PubMed] [Google Scholar]
- 41.Antonelli A, Ferri C, Pampana A, et al. Thyroid disorders in chronic hepatitis C. The American Journal of Medicine. 2004;117(1):10–13. doi: 10.1016/j.amjmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez-Torres M, Ríos-Bedoya CF, Ortiz-Lasanta G, Marxuach-Cuétara AM, Jiménez-Rivera J. Thyroid dysfunction (TD) among chronic hepatitis C patients with mild and severe hepatic fibrosis. Annals of Hepatology. 2008;7(1):72–77. [PubMed] [Google Scholar]
- 43.Ferri S, Muratori L, Lenzi M, Granito A, Bianchi FB, Vergani D. HCV and autoimmunity. Current Pharmaceutical Design. 2008;14(17):1678–1685. doi: 10.2174/138161208784746824. [DOI] [PubMed] [Google Scholar]
- 44.Menconi F, Hasham A, Tomer Y. Environmental triggers of thyroiditis: hepatitis C and interferon-α. Journal of Endocrinological Investigation. 2011;34(1):78–84. doi: 10.1007/BF03346699. [DOI] [PubMed] [Google Scholar]
- 45.Testa A, Castaldi P, Fant V, et al. Prevalence of HCV antibodies in autoimmune thyroid disease. European Review for Medical and Pharmacological Sciences. 2006;10(4):183–186. [PubMed] [Google Scholar]
- 46.Antonelli A, Ferri C, Ferrari SM, Colaci M, Sansonno D, Fallahi P. Endocrine manifestations of hepatitis C virus infection. Nature Clinical Practice Endocrinology and Metabolism. 2009;5(1):26–34. doi: 10.1038/ncpendmet1027. [DOI] [PubMed] [Google Scholar]
- 47.Metcalfe RA, Ball G, Kudesia G, Weetman AP. Failure to find an association between hepatitis C virus and thyroid autoimmunity. Thyroid. 1997;7(3):421–424. doi: 10.1089/thy.1997.7.421. [DOI] [PubMed] [Google Scholar]
- 48.Boadas J, Rodriguez-Espinosa J, Enriquez J, et al. Prevalence of thyroid autoantibodies is not increased in blood donors with hepatitis C virus infection. Journal of Hepatology. 1995;22(6):611–615. doi: 10.1016/0168-8278(95)80216-9. [DOI] [PubMed] [Google Scholar]
- 49.Ganne-Carrie N, Medini A, Coderc E, et al. Latent autoimmune thyroiditis in untreated patients with HCV chronic hepatitis: a case-control study. Journal of Autoimmunity. 2000;14(2):189–193. doi: 10.1006/jaut.1999.0360. [DOI] [PubMed] [Google Scholar]
- 50.Donati L, Antonelli A, Bertoni F, et al. Clinical picture of endemic cretinism in central Apennines (Montefeltro) Thyroid. 1992;2(4):283–290. doi: 10.1089/thy.1992.2.283. [DOI] [PubMed] [Google Scholar]
- 51.Indolfi G, Stagi S, Bartolini E, et al. Thyroid function and anti-thyroid autoantibodies in untreated children with vertically acquired chronic hepatitis C virus infection. Clinical Endocrinology. 2008;68(1):117–121. doi: 10.1111/j.1365-2265.2007.03009.x. [DOI] [PubMed] [Google Scholar]
- 52.Pateron D, Hartmann DJ, Duclas-Vallee JC, Jouanolle H, Beaugrand M. Latent autoimmune thyroid disease in patients with chronic HCV hepatitis. Journal of Hepatology. 1992;16(1-2):244–245. doi: 10.1016/s0168-8278(05)80124-2. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe U, Hashimoto E, Hisamitsu T, Obata H, Hayashi N. The risk factor for development of thyroid disease during interferon-α therapy for chronic hepatitis C. The American Journal of Gastroenterology. 1994;89(3):399–403. [PubMed] [Google Scholar]
- 54.Carella C, Amato G, Biondi B, et al. Longitudinal study of antibodies against thyroid in patients undergoing interferon-α therapy for HCV chronic hepatitis. Hormone Research. 1995;44(3):110–114. doi: 10.1159/000184606. [DOI] [PubMed] [Google Scholar]
- 55.Roti E, Minelli R, Giuberti T, et al. Multiple changes in thyroid function in patients with chronic active HCV hepatitis treated with recombinant interferon-alpha. The American Journal of Medicine. 1996;101(5):482–487. doi: 10.1016/s0002-9343(96)00259-8. [DOI] [PubMed] [Google Scholar]
- 56.Marazuela M, García-Buey L, González-Fernández B, et al. Thyroid autoimmune disorders in patients with chronic hepatitis C before and during interferon-α therapy. Clinical Endocrinology. 1996;44(6):635–642. doi: 10.1046/j.1365-2265.1996.751768.x. [DOI] [PubMed] [Google Scholar]
- 57.Carella C, Mazziotti G, Morisco F, et al. The addition of ribavirin to interferon-α therapy in patients with hepatitis C virus-related chronic hepatitis does not modify the thyroid autoantibody pattern but increases the risk of developing hypothyroidism. European Journal of Endocrinology. 2002;146(6):743–749. doi: 10.1530/eje.0.1460743. [DOI] [PubMed] [Google Scholar]
- 58.Teng ZL, Gong WJ, Zhang SQ, Sun YX, Ma XH. Clinical observation of Hashimoto thyroiditis in patients with chronic hepatitis C undergoing pegylated-interferon alpha-2a and ribavirin combination therapy. Zhonghua Ganzangbing Zazhi. 2013;21(2):101–104. doi: 10.3760/cma.j.issn.1007-3418.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Antonelli A, Ferri C, Fallahi P, et al. Thyroid disorders in chronic hepatitis C virus infection. Thyroid. 2006;16(6):563–572. doi: 10.1089/thy.2006.16.563. [DOI] [PubMed] [Google Scholar]
- 60.Giordano TP, Henderson L, Landgren O, et al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. The Journal of the American Medical Association. 2007;297(18):2010–2017. doi: 10.1001/jama.297.18.2010. [DOI] [PubMed] [Google Scholar]
- 61.Antonelli A, Ferri C, Galeazzi M, et al. HCV infection: pathogenesis, clinical manifestations and therapy. Clinical and Experimental Rheumatology. 2008;26(1, supplement 48):S39–S47. [PubMed] [Google Scholar]
- 62.Mohran ZY, Abdel Kader NA, Abdel Moez AT, Abbas AA. Subclinical autoimmune thyroid disorders in Egyptian patients with untreated chronic hepatitis C virus infection. Journal of the Egyptian Society of Parasitology. 2010;40(1):45–56. [PubMed] [Google Scholar]
- 63.Russo MW, Fried MW. Side effects of therapy for chronic hepatitis C. Gastroenterology. 2003;124(6):1711–1719. doi: 10.1016/s0016-5085(03)00394-9. [DOI] [PubMed] [Google Scholar]
- 64.Dalgard O, Bjøro K, Hellum K, et al. Thyroid dysfunction during treatment of chronic hepatitis C with interferon alpha: No association with either interferon dosage or efficacy of therapy. Journal of Internal Medicine. 2002;251(5):400–406. doi: 10.1046/j.1365-2796.2002.00974.x. [DOI] [PubMed] [Google Scholar]
- 65.Friedrich-Rust M, Theobald J, Zeuzem S, Bojunga J. Thyroid function and changes in ultrasound morphology during antiviral therapy with pegylated interferon and ribavirin in patients with chronic hepatitis C. Journal of Viral Hepatitis. 2009;16(3):168–177. doi: 10.1111/j.1365-2893.2008.01059.x. [DOI] [PubMed] [Google Scholar]
- 66.Fentiman IS, Thomas BS, Balkwill FR, Rubens RD, Hayward JL. Primary hypothyroidism associated with interferon therapy of breast cancer. The Lancet. 1985;1(8438):p. 1166. doi: 10.1016/s0140-6736(85)92475-4. [DOI] [PubMed] [Google Scholar]
- 67.de Oliveira Andrade LJ, Junior AD, Costa Silva CA, et al. A meta-analysis of patients with chronic hepatitis C treated with interferon-alpha to determine the risk of autoimmune thyroiditis. Acta Gastroenterologica Latinoamericana. 2011;41(2):104–110. [PubMed] [Google Scholar]
- 68.Tran HA, Reeves GEM. The spectrum of autoimmune thyroid disease in the short to medium term following interferon-therapy for chronic hepatitis C. International Journal of Endocrinology. 2009;2009:5 pages. doi: 10.1155/2009/241786.241786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koh LKH, Greenspan FS, Yeo PPB. Interferon-α induced thyroid dysfunction: three clinical presentations and a review of the literature. Thyroid. 1997;7(6):891–896. doi: 10.1089/thy.1997.7.891. [DOI] [PubMed] [Google Scholar]
- 70.Wong V, Fu AX-L, George J, Cheung NW. Thyrotoxicosis induced by alpha-interferon therapy in chronic viral hepatitis. Clinical Endocrinology. 2002;56(6):793–798. doi: 10.1046/j.1365-2265.2002.01553.x. [DOI] [PubMed] [Google Scholar]
- 71.Mandac JC, Chaudhry S, Sherman KE, Tomer Y. The clinical and physiological spectrum of interferon-alpha induced thyroiditis: Toward a new classification. Hepatology. 2006;43(4):661–672. doi: 10.1002/hep.21146. [DOI] [PubMed] [Google Scholar]
- 72.Czarnywojtek A, Zgorzalewicz-Stachowiak M, Waško R, et al. Patients with chronic hepatitis type C and interferon-alpha-induced hyperthyroidism in two-years clinical follow-up. Neuroendocrinology Letters. 2013;34(2):154–161. [PubMed] [Google Scholar]
- 73.Andrade LJDO, Atta AM, D'Almeida A, Jr., Paranà R. Thyroid dysfunction in hepatitis C individuals treated with interferon-alpha and ribavirin—a review. Brazilian Journal of Infectious Diseases. 2008;12(2):144–148. doi: 10.1590/s1413-86702008000200009. [DOI] [PubMed] [Google Scholar]
- 74.Prummel MF, Laurberg P. Interferon-α and autoimmune thyroid disease. Thyroid. 2003;13(6):547–551. doi: 10.1089/105072503322238809. [DOI] [PubMed] [Google Scholar]
- 75.Savvas SP, Papakostas N, Giannaris M, Malaktari S, Koskinas J, Archimandritis AJ. Interferon alpha-Induced hashimoto thyroiditis followed by transient graves disease in a patient with chronic HCV infection. Southern Medical Journal. 2010;103(6):585–588. doi: 10.1097/SMJ.0b013e3181ddd952. [DOI] [PubMed] [Google Scholar]
- 76.Mammen JS, Ghazarian SR, Rosen A, Ladenson PW. Patterns of interferon-alpha-induced thyroid dysfunction vary with ethnicity, sex, smoking status, and pretreatment thyrotropin in an international cohort of patients treated for hepatitis C. Thyroid. 2013;23(9):1151–1158. doi: 10.1089/thy.2012.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldman M, Lambert P, Tasiaux N, et al. Thyroid-stimulating immunoglobulins in mixed (type II) cryoglobulinemia associated with thyrotoxicosis. Arthritis and Rheumatism. 1987;30(11):1318–1319. doi: 10.1002/art.1780301121. [DOI] [PubMed] [Google Scholar]
- 78.Castellano Higuera A, González Reimers E, Alarcó Hernández B, Santolaria Fernández F, Rodríguez Gaspar M. Hypotiroidism, hemolytic anemia and cryoglobulinemia in a patient with hepatitis C virus infection: efficacy of treatment with alpha-interferon. Anales de Medicina Interna. 2003;20(7):391–392. [PubMed] [Google Scholar]
- 79.Antonelli A, Ferri C, Fallahi P, et al. High values of CXCL10 serum levels in mixed cryoglobulinemia associated with hepatitis C infection. The American Journal of Gastroenterology. 2008;103(10):2488–2494. doi: 10.1111/j.1572-0241.2008.02040.x. [DOI] [PubMed] [Google Scholar]
- 80.Antonelli A, Ferri C, Fallahi P, et al. Thyroid involvement in patients with HCV-related mixed cryoglobulinaemia. QJM. 2004;97(8):499–506. doi: 10.1093/qjmed/hch088. [DOI] [PubMed] [Google Scholar]
- 81.Antonelli A, Ferri C, Fallahi P. Thyroid cancer in patients with hepatitis C infection. Journal of the American Medical Association. 1999;281(17):p. 1588. doi: 10.1001/jama.281.17.1588. [DOI] [PubMed] [Google Scholar]
- 82.Montella M, Crispo A, Pezzullo L, et al. Is hepatitis C virus infection associated with thyroid cancer? A case-control study. International Journal of Cancer. 2000;87(4):611–612. doi: 10.1002/1097-0215(20000815)87:4<611::aid-ijc24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 83.Montella M, Crispo A, de Bellis G, et al. HCV and cancer: a case-control study in a high-endemic area. Liver. 2001;21(5):335–341. doi: 10.1034/j.1600-0676.2001.210506.x. [DOI] [PubMed] [Google Scholar]
- 84.Antonelli A, Ferri C, Fallahi P, Nesti C, Zignego AL, Maccheroni M. Thyroid cancer in HCV-related mixed cryoglobulinemia patients. Clinical and Experimental Rheumatology. 2002;20(5):693–696. [PubMed] [Google Scholar]
- 85.Fiore E, Rago T, Latrofa F, et al. Hashimoto's thyroiditis is associated with papillary thyroid carcinoma: role of TSH and of treatment with L-thyroxine. Endocrine-Related Cancer. 2011;18(4):429–437. doi: 10.1530/ERC-11-0028. [DOI] [PubMed] [Google Scholar]
- 86.Lee M-H, Yang H-I, Lu S-N, et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. The Journal of Infectious Diseases. 2012;206(4):469–477. doi: 10.1093/infdis/jis385. [DOI] [PubMed] [Google Scholar]
- 87.Antonelli A, Ferri C, Fallahi P, et al. Thyroid cancer in HCV-related chronic hepatitis patients: a case-control study. Thyroid. 2007;17(5):447–451. doi: 10.1089/thy.2006.0194. [DOI] [PubMed] [Google Scholar]
- 88.Neri S, Boraschi P, Antonelli A, Falaschi F, Baschieri L. Pulmonary function, smoking habits, and high resolution computed tomography (HRCT) early abnormalities of lung and pleural fibrosis in shipyard workers exposed to asbestos. The American Journal of Industrial Medicine. 1996;30(5):588–595. doi: 10.1002/(SICI)1097-0274(199611)30:5<588::AID-AJIM6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 89.Antonelli A, Fallahi P, Ferrari SM, et al. Dedifferentiated thyroid cancer: a therapeutic challenge. Biomedicine and Pharmacotherapy. 2008;62(8):559–563. doi: 10.1016/j.biopha.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 90.Antonelli A, Bocci G, La Motta C, et al. Novel pyrazolopyrimidine derivatives as tyrosine kinase inhibitors with antitumoral activity in vitro and in vivo in papillary dedifferentiated thyroid cancer. The Journal of Clinical Endocrinology and Metabolism. 2011;96(2):E288–E296. doi: 10.1210/jc.2010-1905. [DOI] [PubMed] [Google Scholar]
- 91.Martocchia A, Falaschi P. Amino acid sequence homologies between HCV polyprotein and thyroid antigens. Internal and Emergency Medicine. 2007;2(1):65–67. doi: 10.1007/s11739-007-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tomer Y, Villanueva R. Hepatitis C and thyroid autoimmunity: is there a link? The American Journal of Medicine. 2004;117(1):60–61. doi: 10.1016/j.amjmed.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 93.Tomer Y, Blackard JT, Akeno N. Interferon alpha treatment and thyroid dysfunction. Endocrinology and Metabolism Clinics of North America. 2007;36(4):1051–1066. doi: 10.1016/j.ecl.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Patzwahl R, Meire V, Ramadori G, Mihm S. Enhanced expression of interferon-regulated genes in the liver of patients with chronic hepatitis C virus infection: detection by suppression-subtractive hybridization. Journal of Virology. 2001;75(3):1332–1338. doi: 10.1128/JVI.75.3.1332-1338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mihm S, Schweyer S, Ramadori G. Expression of the chemokine IP-10 correlates with the accumulation of hepatic IFN-γ and IL-18 mRNA in chronic hepatitis C but not in hepatitis B. Journal of Medical Virology. 2003;70(4):562–570. doi: 10.1002/jmv.10431. [DOI] [PubMed] [Google Scholar]
- 96.Antonelli A, Ferrari SM, Giuggioli D, Ferrannini E, Ferri C, Fallahi P. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmunity Reviews. 2014;13(3):272–280. doi: 10.1016/j.autrev.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 97.Antonelli A, Ferrari SM, Corrado A, Ferrannini E, Fallahi P. CXCR3, CXCL10 and type 1 diabetes. Cytokine & Growth Factor Reviews. 2014;25(1):57–65. doi: 10.1016/j.cytogfr.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 98.Antonelli A, Rotondi M, Fallahi P, et al. Increase of interferon-γ-inducible CXC chemokine CXCL10 serum levels in patients with active Graves' disease, and modulation by methimazole therapy. Clinical Endocrinology. 2006;64(2):189–195. doi: 10.1111/j.1365-2265.2006.02447.x. [DOI] [PubMed] [Google Scholar]
- 99.Antonelli A, Rotondi M, Fallahi P, et al. Iodine-131 given for therapeutic purposes modulates differently interferon-γ-inducible α-chemokine CXCL10 serum levels in patients with active Graves' disease or toxic nodular goiter. The Journal of Clinical Endocrinology and Metabolism. 2007;92(4):1485–1490. doi: 10.1210/jc.2006-1571. [DOI] [PubMed] [Google Scholar]
- 100.Antonelli A, Fallahi P, Rotondi M, Ferrari SM, Serio M, Miccoli P. Serum levels of the interferon-γ-inducible α chemokine CXCL10 in patients with active Graves’ disease, and modulation by methimazole therapy and thyroidectomy. British Journal of Surgery. 2006;93(10):1226–1231. doi: 10.1002/bjs.5401. [DOI] [PubMed] [Google Scholar]
- 101.Antonelli A, Rotondi M, Fallahi P, et al. Increase of CXC chemokine CXCL10 and CC chemokine CCL2 serum levels in normal ageing. Cytokine. 2006;34(1-2):32–38. doi: 10.1016/j.cyto.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 102.Matskevich AA, Strayer DS. Exploiting hepatitis C virus activation of NFκB to deliver HCV-responsive expression of interferons α and γ . Gene Therapy. 2003;10(22):1861–1873. doi: 10.1038/sj.gt.3302091. [DOI] [PubMed] [Google Scholar]
- 103.Murata M, Nabeshima S, Maeda N, Nakashima H, Kashiwagi S, Hayashi J. Increased frequency of IFN-γ-producing peripheral CD8+ T cells with memory-phenotype in patients with chronic hepatitis C. Journal of Medical Virology. 2002;67(2):162–170. doi: 10.1002/jmv.2205. [DOI] [PubMed] [Google Scholar]
- 104.Loetscher M, Loetscher P, Brass N, Meese E, Moser B. Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. European Journal of Immunology. 1998;28(11):3696–3705. doi: 10.1002/(SICI)1521-4141(199811)28:11<3696::AID-IMMU3696>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 105.Qin S, Rottman JB, Myers P, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. The Journal of Clinical Investigation. 1998;101(4):746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rotondi M, Rosati A, Buonamano A, et al. High pretransplant serum levels of CXCL10/IP-10 are related to increased risk of renal allograft failure. The American Journal of Transplantation. 2004;4(9):1466–1474. doi: 10.1111/j.1600-6143.2004.00525.x. [DOI] [PubMed] [Google Scholar]
- 107.Pupilli C, Giannini S, Marchetti P, et al. Autoantibodies to CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase) in Caucasian patients with diabetes: effects on insulin release from human islets. Diabetes. 1999;48(12):2309–2315. doi: 10.2337/diabetes.48.12.2309. [DOI] [PubMed] [Google Scholar]
- 108.Antonelli A, Tuomi T, Nannipieri M, et al. Autoimmunity to CD38 and GAD in type I and type II diabetes: CD38 and HLA genotypes and clinical phenotypes. Diabetologia. 2002;45(9):1298–1306. doi: 10.1007/s00125-002-0886-6. [DOI] [PubMed] [Google Scholar]
- 109.Antonelli A, Baj G, Marchetti P, et al. Human anti-CD38 autoantibodies raise intracellular calcium and stimulate insulin release in human pancreatic islets. Diabetes. 2001;50(5):985–991. doi: 10.2337/diabetes.50.5.985. [DOI] [PubMed] [Google Scholar]
- 110.Antonelli A, Ferri C, Fallahi P, et al. CXCL10 (α) and CCL2 (β) chemokines in systemic sclerosis—a longitudinal study. Rheumatology. 2008;47(1):45–49. doi: 10.1093/rheumatology/kem313. [DOI] [PubMed] [Google Scholar]
- 111.Antonelli A, Fazzi P, Fallahi P, Ferrari SM, Ferrannini E. Prevalence of hypothyroidism and Graves disease in sarcoidosis. Chest. 2006;130(2):526–532. doi: 10.1378/chest.130.2.526. [DOI] [PubMed] [Google Scholar]
- 112.Takeuchi M, Oh-I K, Suzuki J, et al. Elevated serum levels of CXCL9/monokine induced by interferon-γ and CXCL10/interferon-γ-inducible protein-10 in ocular sarcoidosis. Investigative Ophthalmology and Visual Science. 2006;47(3):1063–1068. doi: 10.1167/iovs.05-0966. [DOI] [PubMed] [Google Scholar]
- 113.Antonelli A, Delle Sedie A, Fallahi P, et al. High prevalence of thyroid autoimmunity and hypothyroidism in patients with psoriatic arthritis. Journal of Rheumatology. 2006;33(10):2026–2028. [PubMed] [Google Scholar]
- 114.Antonelli A, Fallahi P, Sedie AD, et al. High values of alpha (CXCL10) and beta (CCL2) circulating chemokines in patients with psoriatic arthritis, in presence or absence of autoimmune thyroiditis. Autoimmunity. 2008;41(7):537–542. doi: 10.1080/08916930802170401. [DOI] [PubMed] [Google Scholar]
- 115.Antonelli A, Ferrari SM, Fallahi P, et al. Monokine induced by interferon γ (IFNγ) (CXCL9) and IFNγ inducible T-cell α-chemoattractant (CXCL11) involvement in Graves' disease and ophthalmopathy: modulation by peroxisome proliferator-activated receptor-γ agonists. The Journal of Clinical Endocrinology and Metabolism. 2009;94(5):1803–1809. doi: 10.1210/jc.2008-2450. [DOI] [PubMed] [Google Scholar]
- 116.Antonelli A, Fallahi P, Nesti C, et al. Anti-CD38 autoimmunity in patients with chronic autoimmune thyroiditis or Graves' disease. Clinical and Experimental Immunology. 2001;126(3):426–431. doi: 10.1046/j.1365-2249.2001.01683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Antonelli A, Fallahi P, Rotondi M, et al. Increased serum CXCL10 in Graves’ disease or autoimmune thyroiditis is not associated with hyper- or hypothyroidism per se, but is specifically sustained by the autoimmune, inflammatory process. European Journal of Endocrinology. 2006;154(5):651–658. doi: 10.1530/eje.1.02137. [DOI] [PubMed] [Google Scholar]
- 118.Hiromatsu Y, Yang D, Bednarczuk T, Miyake I, Nonaka K, Inoue Y. Cytokine profiles in eye muscle tissue and orbital fat tissue from patients with thyroid-associated ophthalmopathy. Journal of Clinical Endocrinology and Metabolism. 2000;85(3):1194–1199. doi: 10.1210/jcem.85.3.6433. [DOI] [PubMed] [Google Scholar]
- 119.Romagnani P, Rotondi M, Lazzeri E, et al. Expression of IP-10/CXCL10 and MIG/CXCL9 in the thyroid and increased levels of IP-10/CXCL10 in the serum of patients with recent-onset Graves' Disease. The American Journal of Pathology. 2002;161(1):195–206. doi: 10.1016/S0002-9440(10)64171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Antonelli A, Rotondi M, Ferrari SM, et al. Interferon-γ-inducible α-chemokine CXCL10 involvement in Graves' ophthalmopathy: modulation by peroxisome proliferator-activated receptor-γ agonists. The Journal of Clinical Endocrinology and Metabolism. 2006;91(2):614–620. doi: 10.1210/jc.2005-1689. [DOI] [PubMed] [Google Scholar]
- 121.Gowans EJ. Distribution of markers of hepatitis C virus infection throughout the body. Seminars in Liver Disease. 2000;20(1):85–102. doi: 10.1055/s-2000-9503. [DOI] [PubMed] [Google Scholar]
- 122.Antonelli A, Ferri C, Fallahi P, et al. α-Chemokine CXCL10 and β-chemokine CCL2 serum levels in patients with hepatitis C-associated cryoglobulinemia in the presence or absence of autoimmune thyroiditis. Metabolism: Clinical and Experimental. 2008;57(9):1270–1277. doi: 10.1016/j.metabol.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 123.Antonelli A, Ferri C, Ferrari SM, et al. Interleukin-1β, C-X-C motif ligand 10, and interferon-gamma serum levels in mixed cryoglobulinemia with or without autoimmune thyroiditis. Journal of Interferon and Cytokine Research. 2010;30(11):835–842. doi: 10.1089/jir.2010.0024. [DOI] [PubMed] [Google Scholar]
- 124.Antonelli A, Ferri C, Ferrari SM, et al. The presence of autoimmune thyroiditis in mixed cryoglobulinemia patients is associated with high levels of circulating interleukin-6, but not of tumour necrosis factor-alpha. Clinical and Experimental Rheumatology. 2011;29(1, supplement 64):S17–S22. [PubMed] [Google Scholar]
- 125.Villa E, Karampatou A, Camm C, et al. Early menopause is associated with lack of response to antiviral therapy in women with chronic hepatitis C. Gastroenterology. 2011;140(3):818–829. doi: 10.1053/j.gastro.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 126.Müller M, Carter SL, Hofer MJ, et al. CXCR3 signaling reduces the severity of experimental autoimmune encephalomyelitis by controlling the parenchymal distribution of effector and regulatory T cells in the central nervous system. Journal of Immunology. 2007;179(5):2774–2786. doi: 10.4049/jimmunol.179.5.2774. [DOI] [PubMed] [Google Scholar]
- 127.Putheti P, Morris M, Stawiarz L, et al. Multiple sclerosis: a study of chemokine receptors and regulatory T cells in relation to MRI variables. European Journal of Neurology. 2003;10(5):529–535. doi: 10.1046/j.1468-1331.2003.00638.x. [DOI] [PubMed] [Google Scholar]
- 128.Stiles LN, Liu MT, Kane JC, Lane TE. CXCL10 and trafficking of virus-specific T cells during coronavirus-induced demyelination. Autoimmunity. 2009;42(6):484–491. doi: 10.1080/08916930902810708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wildner G, Kaufmann U. What causes relapses of autoimmune diseases? The etiological role of autoreactive T cells. Autoimmunity Reviews. 2013;12(11):1070–1075. doi: 10.1016/j.autrev.2013.04.001. [DOI] [PubMed] [Google Scholar]


