Abstract
The histological analysis of peripheral nerve regeneration is one of the most used methods to demonstrate the success of the regeneration through nerve conduits. Nowadays, it is possible to evaluate different parameters of nerve regeneration by using histological, histochemical, immunohistochemical and ultrastructural techniques. The histochemical methods are very sensible and are useful tools to evaluate the extracellular matrix remodeling and the myelin sheath, but they are poorly specific. In contrast, the immunohistochemical methods are highly specific and are frequently used for the identification of the regenerated axons, Schwann cells and proteins associated to nerve regeneration or neural linage. The ultrastructural techniques offer the possibility to perform a high resolution morphological and quantitative analysis of the nerve regeneration. However, the use of a single histological method may not be enough to assess the degree of regeneration, and the combination of different histological techniques could be necessary.
Keywords: peripheral nerve regeneration; histology, histochemistry; immunohistochemistry; quantitative histology
Introduction
Peripheral nerves (PN) are specialized organs that form a highly complex network throughout the body providing the motor and/or sensory innervations to target organs. Histologically, PN are composed by two main elements, a functional unit or parenchyma and the stroma. The parenchyma consists of the nerve fibers, formed by axons and the surrounding Schwann cells (SC), while the stroma is formed by three layers of specialized connective tissues. The SC can myelinate a single axon forming the myelinated nerve fibers, or a single SC can interact with a small group of thinner axons forming the unmyelinated nerve fibers. In the case of the stroma, it regulates the compartmentalization of these organs. In transversal sections of PN, it is possible to observe how nerve fibers are immersed in the endoneurial connective tissue and it is surrounded by the perineurial layer that forms individual fascicles which are immersed in a vascularized connective tissue called epineurium (Geuna et al., 2009; Mills, 2012).
The normal function of the PN is often affected by traumatic injuries with serious physical and/or psychological consequences for these patients (Daly et al., 2012; Carriel et al., 2014a). Direct nerve repair is the preferred treatment for short nerve gaps, and nerve autografting is the gold standard treatment for critical nerve defects, although it has well-known disadvantages. Nerve conduits (tubulization) are often used with variable success rates (Daly et al., 2012; Carriel et al., 2014b). Due to the clinical limitations associated to the autograft and the unsatisfactory results obtained with the tubulization technique, current research is focused on the development of novel tissue engineering alternatives to repair critical nerve gaps. In these sense, a range of strategies were achieved or are under investigation, highlighting the use of micropatterned structures, aligned biomaterials and the use of conduits filled with cellular scaffolds (Daly et al., 2012; Carriel et al., 2013, 2014b).
Peripheral nerve tissue engineering (PNTE) is focused on the development of successful strategies to promote nerve regeneration from the clinical, functional and histological point of view. In this sense, the sciatic nerve functional index (walking track and toe spread), the toe spread and the pinch test of sensory recovery are frequently used to measure clinical and functional parameters (Vleggeert-Lankamp, 2007; Carriel et al., 2013). Currently, one of the most used and reliable method to evaluate nerve regeneration is the electrophysiological assessment of the distal muscles (innervated by the injured nerve), which allows determining the degree of muscle denervation or reinnervation (Vleggeert-Lankamp, 2007; Carriel et al., 2013). In addition, the evaluation of the muscle weight and volume is another simple and useful method to assess the degree of muscle recovery (Vleggeert-Lankamp, 2007; Xie et al., 2008).
Regarding to the histological analysis, there are several histological, histochemical, immunohistochemical and also ultrastructural methods with specific applications. First, it is necessary to understand the basic concepts of the PN histology in normal conditions and during regeneration. This process is characterized by the proliferation and migration of the local cells, specially the SC, which progressively synthesize a new extracellular matrix (ECM) and form the bands of Büngner that guide the axonal growth from the proximal to the distal nerve stump (Geuna et al., 2009; Daly et al., 2012; Carriel et al., 2013, 2014). These cellular and molecular processes are essential for the success of nerve regeneration. In addition, the most reliable way to evaluate them with high accuracy is the histological and ultrastructural analysis, making the histological analysis one of the most useful quality controls in PNTE.
The first and more critical step in histology is the fixation of the tissues, whose main objective is to maintain clear and consistent morphological features, to inhibit the metabolic processes and post-mortem bacterial degradation (Kiernan, 2008). Currently, cryofixation and chemical fixation (coagulant and non-coagulant) are used in PNTE (Kalbermatten et al., 2009; Carriel et al., 2011a, 2013; di Summa et al., 2014). Cryofixation is a good method to preserve the enzymatic activity and tissue antigens. For this reason, it is a suitable method for histochemical and immunohistochemical techniques. Regarding to the chemical fixation, the aldehyde-based fixatives are the most used for light and electron microscopy. Once the tissues are fixed, they can be embedded in OCT (cryofixation), paraffin (light microscopy) or resin (electron microscopy), and sectioned for their staining (Kiernan, 2008).
Staining procedure in light microscopy
Hematoxylin and eosin (HE) are the most used staining agents for light microscopy in pathology and research. HE staining is commonly non-specific for many tissue elements due to its electropolar nature (Carriel et al., 2011a, b). Using this method, it is not possible to observe the myelin sheath and the ECM is nonspecifically stained. Therefore, HE is not an ideal method allowing an accurate analysis of the nerve regeneration process (Di Scipio et al., 2008; Raimondo et al., 2009; Carriel et al., 2011a).
Trichrome staining offers the possibility to differentiate the collagen fibers of the ECM from the other types of structures (epithelial tissue, muscles, parenchyma, etc.). Masson's trichrome is the most common trichrome method in pathology and muscle histology, but it is infrequently used for the histological analysis of PN. The quality of the histology of PN with this method is considerably better than HE (Raimondo et al., 2009). However, it is not possible to identify the myelin sheath with high accuracy with this method (Figure 1A).
Figure 1.
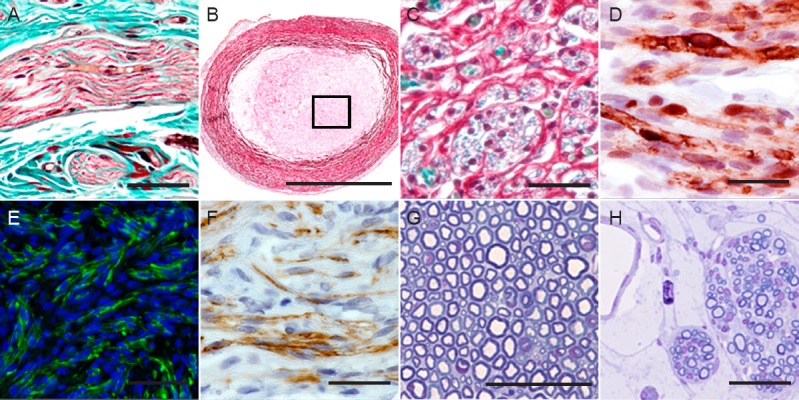
Histological method as quality control in PNTE.
Longitudinal section of a peripheral nerve stained with Masson's trichrome method (A). Transversal section of a NeuraGen® collagen conduit stained with the MCOLL histochemical method with low (B) and high magnification (C), where it is possible to observe the collagen fibers in red, the myelin in blue and the nucleus darkly stained. Identification of Schwann cells with S-100, with the characteristic nuclear and cytoplasmatic positive reaction (D). Immunofluorescence and immunohistochemical identification of regenerated axons by using neurofilament (green) and GAP-43 (brown), respectively (E, F). Semithin transversal sections of native nerve (G) and regenerated nerve tissue (H) stained with toluidine blue. PNTE: Peripheral nerve tissue engineering; GAP-43: growth associated protein-43. Scale bar: 50 μm (A, D, F, G, and H), 100 μm (C, E) and 1 mm (B).
Regarding the myelin, it is composed by several types of lipids and lipoproteins. Unfortunately, a significant amount of these elements are dissolved by organic solvents during tissue processing, but some of them are preserved (associated to structural proteins), thus allowing their identification. Interestingly, pre-embedding myelin sheath staining with OsO4 is a useful alternative to identify myelin with high accuracy in light microscopy (Di Scipio et al., 2008; Li et al., 2013). This method can be combined with other histochemical methods including Masson's method or immunohistochemical procedures.
In formalin-fixed tissues myelin is frequently stained with luxol fast Blue (LFB) method, which specifically stains myelin in blue. However, LFB only allows the specific identification of myelin, and it is not possible to make a comprehensive assessment of the morphologic parameters and ECM remodeling associated to the PN regeneration process (Carriel et al., 2011a). In this sense, the technical limitations associated to the LFB were improved with the new integrated histochemical method called MCOLL. This method combines the specificity and sensibility of conventional LFB (myelin), picrosirius staining (collagen fibers) and Harris haematoxylin (cellular nucleus) allowing the identification of different parameters simultaneously (Figure 1B, C). This method was successfully used in PNTE, allowing an accurate histological and histochemical evaluation of the nerve regeneration (Carriel et al., 2011a, 2013).
Finally, another useful technique is the histochemical identification of the acetylcholinesterase activity in skeletal muscle cryosections. This method was used to identify with high specificity the newly-formed endplate during the reinnervation of the distal skeletal muscles (Jiao et al., 2009).
Immunostaining in PNTE
The immunohistochemical and immunofluorescence methods allow the identification of highly specific proteins in tissue sections. Both methods are suitable for cryosections or formalin-fixed tissues. Currently, there are several antibodies that recognize specific proteins related to cell lineage (surface or intracellular markers), cytoskeletal proteins, ECM molecules, growth factors, etc., and their correct application is essential to establish the degree of PN regeneration in PNTE (Vleggeert-Lankamp, 2007; Raimondo et al., 2009; Mills, 2012; Carriel et al., 2013, 2014b).
SCs play a key role during PN regeneration, and their identification with conventional histological techniques is extremely difficult, but they can be identified with high accuracy with antibodies that recognize the glial fibrillar acid protein (GFAP) and the S-100 protein (Figure 1D). Due to the importance of SC during nerve regeneration, high levels of SC immunostaining associated to an organized pattern of regeneration is considered as a positive indicator of nerve regeneration in PNTE (Kalbermatten et al., 2009; Siemionow et al., 2011; Carriel et al., 2013; di Summa et al., 2014).
Demonstration of axonal regrowth is the most important indicator of PN regeneration. Axons are mainly composed by neurotubules and neurofilaments and both cytoskeletal proteins can be easily evaluated by the immunohistochemical identification of β-III tubulin (Huang et al., 2012), neurofilaments (Ma et al., 2011; Huang et al., 2012; Carriel et al., 2013) and growth associated protein-43 (GAP-43), which is specific of newly-formed axons that do not express neurofilaments (Figure 1E, F) (Carriel et al., 2014b). Another commonly used marker to evaluate the axonal growth is the protein gene product 9.5 (PGP9.5), which is positive in neurons and neuroendocrine cells (Kalbermatten et al., 2008).
Regarding the ECM, it plays a crucial role in guiding the complex process of PN regeneration. Laminin is a normal component of the basal membrane of nerve fibers and this glycoprotein supports SC proliferation and migration during nerve regeneration (Chernousov et al., 2008; Carriel et al., 2013). Despite the important role of laminin in this process, it is infrequently evaluated in PNTE. However, its expression is a valid parameter to determine the degree of PN regeneration (Siemionow et al., 2011; Carriel et al., 2013). In the case of collagen fibers, these may be evaluated by histochemical methods such as MCOLL and Masson's trichrome.
Myelin can also be detected by immunohistochemistry and immunofluorescence using antibodies that specifically recognize the myelin basic proteins (Carriel et al., 2011a; Mills, 2012). This also applies to the muscular endplate, which can be evaluated with high accuracy and resolution by using a rhodamine-conjugated α–bungarotoxin that irreversibly binds to the postsynaptic acetylcholine receptor site (Ma et al., 2011; Li et al., 2013).
Quantitative histology
Histology is classically considered as a purely descriptive method. However, over the recent years, several studies demonstrate that it is possible to perform morphometric or quantitative analyse in histological sections (Raimondo et al., 2009). Regarding the morphometric analysis, it is possible to determine the number of cells in a specific area, the diameters of cells or structures, the area occupied by regeneration tissue or injury etc.
More recently, it is possible to determine with accuracy the intensity and area fraction of the positive reaction for histochemical (Carriel et al., 2011b, 2013; Oliveira et al., 2013), immunohistochemical or immunofluorescence techniques (Carriel et al., 2013, 2014b). The intensity is related to the increase of the expression or synthesis of some proteins (not all of them). Neurofilament intensity is significantly higher in native nerves as compared to the newly-formed axons during nerve regeneration. However, the experimental groups with high levels of regeneration (functional and histological) showed similar intensity values than native nerves in contrast to other experimental groups (Carriel et al., 2014b). The same was performed for the intensity of S-100 and laminin (Carriel et al., 2013). In relation with the area fraction, this allows determining the percentage of positive reaction in a specific histological area. This method was successfully applied to the quantitative determination of the area fraction occupied by myelinated nerve fibers with the MCOLL histochemical method. In addition, quantitative analysis was applied to immunohistochemical methods for determination of S-100 area fraction, laminin, neurofilament (Carriel et al., 2013) and, more recently GAP-43 (Carriel et al., 2014b). Additionally, this quantitative approach was also used as histological quality control of the ECM following a chemical and physical decellularization of small intestine for tissue engineering applications (Oliveira et al., 2013).
Semithin and ultrathin sections in PNTE
For more researchers, the gold standard in PN histology is toluidine blue staining of resin-embedded semithin sections (Raimondo et al., 2009; Carriel et al., 2011a; Mills, 2012). By using this method, most of the myelinated axons can be clearly identified and myelin sheaths are sharply delimited due to post-fixation and staining with OsO4 (Figure 1G, H). The high morphological quality of this method makes it a suitable method to perform a morphometric analysis of PN regeneration. In this sense, the most important parameters that can be used as quality control are: number of fibers, density of fibers, diameter of fibers and axons, cross-sectional area of fibers and axons, perimeter of fibers and axons, myelin thickness, myelin thickness/axon-diameter ratio, and fiber-diameter/axon-diameter ratio or axon-diameter/fiber-diameter (g-ratio) (Vleggeert-Lankamp, 2007; Raimondo et al., 2009; Bozkurt et al., 2012).
The ultrathin sections are often used to evaluate ultrastructural changes associated to the axonal growth and myelination process by transmission electron microscopy. In addition, it is a suitable method to perform morphometric analyses and also to identify and evaluate the unmyelinated nerve fibers (Hirano, 2005).
Conclusions
In order to determine the degree of PN regeneration with high accuracy in PNTE, it is necessary to combine clinical, functional, electrophysiological and histological analyses. Regarding the histology, there are several technical alternatives to evaluate different important parameters associated to the success of failure of nerve regeneration. In all cases, a proper knowledge of the PN histology in normal and regenerative conditions is essential to perform an adequate histological description and to apply the most adequate histological method. Currently, there are useful histochemical techniques available for assessment of the nerve regeneration process such as OsO4 technique, the MCOLL histochemical method and the gold standard toluidine blue in semithin sections. In relation to the immunohistochemical markers, there are a wide range of antibodies available to evaluate PN regeneration, especially S-100, GFAP, neurofilament, β-III tubulin, GAP-43, and laminin.
In conclusion, PN regeneration is a complex process and the use of a single descriptive histological method may not be enough to assess the degree of regeneration. In this sense, it is and it will be necessary to combine different histochemical and immunohistochemical techniques with morphometric or quantitative parameters in order to find statistical differences between the different models of nerve regeneration. Finally, histological assessment of the PN regeneration is one of the most reliable methods available to demonstrate the success or failure of the PN regeneration in tissue engineering.
Acknowledgments
We are grateful to Ms. Ariane Ruyffelaert from the Department of Linguistics, Faculty of Arts and Philosophy, Ghent University, Belgium, for revising the English version.
Footnotes
Conflicts of interest: None declared.
References
- 1.Bozkurt A, Lassner F, O’Dey D, Deumens R, Bocker A, Schwendt T, Janzen C, Suschek CV, Tolba R, Kobayashi E, Sellhaus B, Tholl S, Eummelen L, Schugner F, Damink LO, Weis J, Brook GA, Pallua N. The role of microstructured and interconnected pore channels in a collagen-based nerve guide on axonal regeneration in peripheral nerves. Biomaterials. 2012;33:1363–1375. doi: 10.1016/j.biomaterials.2011.10.069. [DOI] [PubMed] [Google Scholar]
- 2.Carriel V, Garzon I, Alaminos M, Campos A. Evaluation of myelin sheath and collagen reorganization pattern in a model of peripheral nerve regeneration using an integrated histochemical approach. Histochem Cell Biol. 2011a;136:709–717. doi: 10.1007/s00418-011-0874-3. [DOI] [PubMed] [Google Scholar]
- 3.Carriel V, Garrido-Gomez J, Hernandez-Cortes P, Garzon I, Garcia-Garcia S, Saez-Moreno JA, Del Carmen Sanchez-Quevedo M, Campos A, Alaminos M. Combination of fibrin-agarose hydrogels and adipose-derived mesenchymal stem cells for peripheral nerve regeneration. J Neural Eng. 2013;10:026022. doi: 10.1088/1741-2560/10/2/026022. [DOI] [PubMed] [Google Scholar]
- 4.Carriel V, Alaminos M, Garzon I, Campos A, Cornelissen M. Tissue engineering of the peripheral nervous system. Expert Rev Neurother. 2014a, b;14:301–318. doi: 10.1586/14737175.2014.887444. [DOI] [PubMed] [Google Scholar]
- 5.Carriel V, Garzón I, Campos A, Cornelissen M, Alaminos M. Differential expression of GAP-43 and neurofilament during peripheral nerve regeneration through bioartificial conduits. J Tissue Eng Regen Med (in press) 2014a, b doi: 10.1002/term.1949. [DOI] [PubMed] [Google Scholar]
- 6.Carriel VS, Aneiros-Fernandez J, Arias-Santiago S, Garzon IJ, Alaminos M, Campos A. A novel histochemical method for a simultaneous staining of melanin and collagen fibers. J Histochem Cytochem. 2011b;59:270–277. doi: 10.1369/0022155410398001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chernousov MA, Yu WM, Chen ZL, Carey DJ, Strickland S. Regulation of Schwann cell function by the extracellular matrix. Glia. 2008;56:1498–1507. doi: 10.1002/glia.20740. [DOI] [PubMed] [Google Scholar]
- 8.Daly W, Yao L, Zeugolis D, Windebank A, Pandit A. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface. 2012;9:202–221. doi: 10.1098/rsif.2011.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Scipio F, Raimondo S, Tos P, Geuna S. A simple protocol for paraffin-embedded myelin sheath staining with osmium tetroxide for light microscope observation. Microsc Res Tech. 2008;71:497–502. doi: 10.1002/jemt.20577. [DOI] [PubMed] [Google Scholar]
- 10.di Summa PG, Kingham PJ, Campisi CC, Raffoul W, Kalbermatten DF. Collagen (NeuraGen((R))) nerve conduits and stem cells for peripheral nerve gap repair. Neurosci Lett. 2014;572:26–31. doi: 10.1016/j.neulet.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 11.Geuna S, Raimondo S, Ronchi G, Di Scipio F, Tos P, Czaja K, Fornaro M. Chapter 3: Histology of the peripheral nerve and changes occurring during nerve regeneration. Int Rev Neurobiol. 2009;87:27–46. doi: 10.1016/S0074-7742(09)87003-7. [DOI] [PubMed] [Google Scholar]
- 12.Hirano A. The role of electron microscopy in neuropathology. Acta Neuropathol. 2005;109:115–123. doi: 10.1007/s00401-004-0960-x. [DOI] [PubMed] [Google Scholar]
- 13.Huang W, Begum R, Barber T, Ibba V, Tee NC, Hussain M, Arastoo M, Yang Q, Robson LG, Lesage S, Gheysens T, Skaer NJ, Knight DP, Priestley JV. Regenerative potential of silk conduits in repair of peripheral nerve injury in adult rats. Biomaterials. 2012;33:59–71. doi: 10.1016/j.biomaterials.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 14.Jiao H, Yao J, Yang Y, Chen X, Lin W, Li Y, Gu X, Wang X. Chitosan/polyglycolic acid nerve grafts for axon regeneration from prolonged axotomized neurons to chronically denervated segments. Biomaterials. 2009;30:5004–5018. doi: 10.1016/j.biomaterials.2009.05.059. [DOI] [PubMed] [Google Scholar]
- 15.Kalbermatten DF, Kingham PJ, Mahay D, Mantovani C, Pettersson J, Raffoul W, Balcin H, Pierer G, Terenghi G. Fibrin matrix for suspension of regenerative cells in an artificial nerve conduit. J Plast Reconstr Aesthet Surg. 2008;61:669–675. doi: 10.1016/j.bjps.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Kalbermatten DF, Pettersson J, Kingham PJ, Pierer G, Wiberg M, Terenghi G. New fibrin conduit for peripheral nerve repair. J Reconstr Microsurg. 2009;25:27–33. doi: 10.1055/s-0028-1090619. [DOI] [PubMed] [Google Scholar]
- 17.Kiernan JA. Histological and Histochemical Methods: Theory and Practice: Scion Publishing Limited. 2008 [Google Scholar]
- 18.Li QT, Zhang PX, Yin XF, Han N, Kou YH, Deng JX, Jiang BG. Functional recovery of denervated skeletal muscle with sensory or mixed nerve protection: a pilot study. PLoS One. 2013;8:e79746. doi: 10.1371/journal.pone.0079746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma CH, Omura T, Cobos EJ, Latremoliere A, Ghasemlou N, Brenner GJ, van Veen E, Barrett L, Sawada T, Gao F, Coppola G, Gertler F, Costigan M, Geschwind D, Woolf CJ. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J Clin Invest. 2011;121:4332–4347. doi: 10.1172/JCI58675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mills SE. 4th Edition. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2012. Histology for pathologists. [Google Scholar]
- 21.Oliveira AC, Garzon I, Ionescu AM, Carriel V, Cardona Jde L, Gonzalez-Andrades M, Perez Mdel M, Alaminos M, Campos A. Evaluation of small intestine grafts decellularization methods for corneal tissue engineering. PLoS One. 2013;8:e66538. doi: 10.1371/journal.pone.0066538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raimondo S, Fornaro M, Di Scipio F, Ronchi G, Giacobini-Robecchi MG, Geuna S. Chapter 5: Methods and protocols in peripheral nerve regeneration experimental research: part II-morphological techniques. Int Rev Neurobiol. 2009;87:81–103. doi: 10.1016/S0074-7742(09)87005-0. [DOI] [PubMed] [Google Scholar]
- 23.Siemionow M, Duggan W, Brzezicki G, Klimczak A, Grykien C, Gatherwright J, Nair D. Peripheral nerve defect repair with epineural tubes supported with bone marrow stromal cells: a preliminary report. Ann Plast Surg. 2011;67:73–84. doi: 10.1097/SAP.0b013e318223c2db. [DOI] [PubMed] [Google Scholar]
- 24.Vleggeert-Lankamp CL. The role of evaluation methods in the assessment of peripheral nerve regeneration through synthetic conduits: a systematic review. Laboratory investigation. J Neurosurg. 2007;107:1168–1189. doi: 10.3171/JNS-07/12/1168. [DOI] [PubMed] [Google Scholar]
- 25.Xie F, Li QF, Gu B, Liu K, Shen GX. In vitro and in vivo evaluation of a biodegradable chitosan-PLA composite peripheral nerve guide conduit material. Microsurgery. 2008;28:471–479. doi: 10.1002/micr.20514. [DOI] [PubMed] [Google Scholar]


