Abstract
Ginsenoside Rd has a clear neuroprotective effect against ischemic stroke. We aimed to verify the neuroprotective effect of ginsenoside Rd in spinal cord ischemia/reperfusion injury and explore its anti-apoptotic mechanisms. We established a spinal cord ischemia/reperfusion injury model in rats through the occlusion of the abdominal aorta below the level of the renal artery for 1 hour. Successfully established models were injected intraperitoneally with 6.25, 12.5, 25 or 50 mg/kg per day ginsenoside Rd. Spinal cord morphology was observed at 1, 3, 5 and 7 days after spinal cord ischemia/reperfusion injury. Intraperitoneal injection of ginsenoside Rd in ischemia/reperfusion injury rats not only improved hindlimb motor function and the morphology of motor neurons in the anterior horn of the spinal cord, but it also reduced neuronal apoptosis. The optimal dose of ginsenoside Rd was 25 mg/kg per day and the optimal time point was 5 days after ischemia/reperfusion. Immunohistochemistry and western blot analysis showed ginsenoside Rd dose-dependently inhibited expression of pro-apoptotic Caspase 3 and down-regulated the expression of the apoptotic proteins ASK1 and JNK in the spinal cord of rats with spinal cord ischemia/reperfusion injury. These findings indicate that ginsenoside Rd exerts neuroprotective effects against spinal cord ischemia/reperfusion injury and the underlying mechanisms are achieved through the inhibition of ASK1-JNK pathway and the down-regulation of Caspase 3 expression.
Keywords: nerve regeneration, spinal cord injury, ginsenoside Rd, ischemia/reperfusion injury, apoptosis, ASK1, JNK, Caspase 3, neural regeneration
Introduction
Delayed paralysis that occurs after spinal cord ischemia/reperfusion injury may cause neuronal apoptosis (Mattsom, 2000; Kimberly et al., 2007; Zhang et al., 2012). The ASK1-JNK/P38MAPK pathway is important in regulating cell apoptosis, and inhibition of ASK1 signaling can significantly reduce apoptosis (Sekine et al., 2006; Shiizaki et al., 2013). ASK1 is a member of the MAPKKK family and has been widely studied. It can be activated by a variety of inflammatory cytokines and various stresses, such as oxidative stress, reactive oxygen determinants, tumor necrosis factor, lipopolysaccharide, endoplasmic reticulum stress and calcium influx (Nagai et al., 2007). ASK1 may selectively activate JNK and P38MAPK pathways. ASK1 activation not only leads to apoptosis but has a variety of functions that determine cell fate, survival and differentiation. Kim et al. (2011) demonstrated that the down-regulation of ASK1 expression significantly reduced apoptosis of neuronal cells following ischemic stroke.
Ginsenoside Rd is one of the main ingredients of Radix Ginseng and has been shown to have neuroprotective effects against ischemic stroke in clinical trials (Ye et al., 2011a, b, c). Ma et al. (2010) found that the effective dose of ginsenoside Rd in rabbits with spinal cord ischemic injury was 5–40 mg/kg, and that the protective effect was dose-dependent. However, the optimal dose and time point of the effectiveness of ginsenoside Rd remain unclear, and the correlation between the protective effect and neuronal apoptosis has not been reported.
We used rat models of spinal cord ischemia/reperfusion injury to investigate the effect of intraperitoneal injection of ginsenoside Rd on hindlimb motor function, the pathological changes that occur in spinal cord tissue, and apoptosis in nerve cells.
Materials and Methods
Experimental animals
Seventy-four healthy female Sprague-Dawley rats of clean grade aged 10–11 weeks and weighing 250 ± 20 g, were provided by Beijing HFK Bioscience Co., Ltd., Beijing, China (license No. SCXK (Beijing) 2009-0015). Animal experiments were approved by the Ethics Committee of Jilin University in China.
Ginsenoside Rd
Ginseng stem-leaf saponins were precipitated with alcohol and alkaline to obtain ginsenoside stem-leaf panaxadiol saponins. The obtained saponins were detected with normal-phase silica gel column chromatography and C18 reverse-phase column chromatography. After combining the identical phase, solvents were dried and ginsenoside Rd (> 98% purity) prepared. Ginsenoside Rd is the white powder observed at a melting point of 208–209°C, has a molecular weight of 946, and a molecular formula of C48H82O18. Ginsenoside Rd is soluble in organic solvents such as methanol and ethanol, and it is also water-soluble. In this study, ginsenoside Rd was dissolved in 0.9% sodium chloride solution. The above reagents were provided by Professor Chen YP from School of Chemistry, Jilin University, China.
Modeling and interventions
Thirty Sprague-Dawley rats were randomly divided into sham group, ischemia/reperfusion injury group (I/R group), and 6.25, 12.5, 25, 50 mg/kg ginsenoside Rd groups. Each group contained five rats. In the I/R group, rats were anesthetized with 10% chloral hydrate (3 mL/kg) via intraperitoneal injection, and fixed in the arm recumbent position. The surgical area was disinfected with 75% ethanol and a 3 cm incision was made along the midline of the inferior border in the left ribs and the left kidney and the abdominal aorta were located. The abdominal aorta below the level of the renal artery was occluded for 60 minutes using a 10 g aortic clamp, causing spinal cord ischemic injury. The wounds were covered with saline gauze and the arterial clamp was removed 60 minutes later. The abdominal cavity was closed after applying and spreading penicillin powder. After modeling, the defects were assessed using the Basso, Beattie, Bresnahan (BBB) scale, a lower BBB score indicated severer dyskinesia and a deterioration of injury. The models were defined a success upon the appearance of hindlimb motor function defects, lack of coordination of movement and abnormal gait. In the I/R group and four ginsenoside Rd groups, spinal cord ischemia/reperfusion injury model was established and rats were then given an intraperitoneal injection of either 6.25, 12.5, 25 or 50 mg/kg per day of ginsenoside Rd (1.0 mg/mL); Organic Chemistry Laboratory, Department of Chemistry, Jilin University, China) (Ma et al., 2010). In the sham group, only the abdominal cavity was opened and the abdominal aorta was not occluded.
Determination of the optimal therapeutic dose
The hindlimb and tail movement of the thirty rats were observed daily and graded using the BBB scores. Rats in all six groups were sacrificed 5 days after injury. In brief, rats were anesthetized with 10% chloral hydrate (3 mL/kg) via intraperitoneal injection and then bilateral ribs and the diaphragmatic muscle below the xiphoid process were cut exposing the heart. A 3 mm incision was made in the right atrial appendage. A fine needle was then inserted into the left ventricle and a rapid injection of PBS solution was given until clear liquid flowed out of the right atrial appendage. The heart was then perfused and fixed with 150 mL of 10% neutral formalin. The pedicle of the vertebral arch was sheared using an ophthalmic scissor, lifting the lamina and spinous process, and carefully peeling spinal cord tissue below T13 level. The tissue was fixed in 10% neutral formalin at 4°C for 24 hours, and taking L2 as the center, trimmed into 3 mm segments. The segments were embedded, fixed, and made into paraffin slices at a thickness of 5 μm. The slices were stained with hematoxylin-eosin to determine the optimal therapeutic dose of ginsenoside Rd.
Determination of the optimal treatment time
Twenty rats were randomly selected to establish spinal cord ischemia/reperfusion injury model and receive intraperitoneal injection of the optimal therapeutic dose of ginsenoside Rd every day for up to 7 days. Five rats were evaluated using the BBB scores at 1, 3, 5 and 7 days after injury and L2 spinal segment was stained with hematoxylin-eosin. The optimal treatment time was determined according to the BBB scores and staining results.
Morphological changes
The sections of spinal cord were observed under BX53 microscope (Olympus, Tokyo, Japan) and CellSens Dimension camera system (Olympus). A scoring system was established on the basis of the microscope images, previous scoring methods (Xue et al., 2012; Deng et al., 2013), the number and size of neurons in rat spinal cord tissue, and changes in the nucleoli. Scoring was determined as follows:
-
(1)
Under 4 × magnification microscope, the cross-section of the spinal cord was divided into four quadrants; taking the central canal as the origin, the line between anterior median fissure and posterior median sulcus as the longitudinal axis, and the straight line passing through the origin and being perpendicular to the longitudinal axis as the transverse axis. The number of motor neurons in the left anterior quadrant and the right anterior quadrant was calculated and averaged. The following scores were allocated based on the number of neurons: 3 points: ≤ 25 neurons; 2 points: 25–28 neurons; 1 point: 29–32 neurons; 0 point: ≥ 32 neurons.
-
(2)
Under 20 × magnification microscope, the scoring was: 0 point if the cell nucleus was round, with clearly visible nucleoli and abundant mass-like substances in cytoplasm; 1 point if the cell nucleus was round, with visible nucleoli and few mass-like substances in the cytoplasm; and 2 points if the cell volume was reduced, nuclear condensation was found, the nucleolus was not visible, and nuclear structure was unclear.
-
(3)
Under 40 × magnification microscope, the scoring was: 0 point if motor neurons were enlarged (the maximum long-axis diameter ≥ 25 μm, the maximum transverse-axis diameter ≥ 20 μm), the cell nuclei were round, the nucleolus was clearly visible, and there were abundant mass-like substances in the cytoplasm; 1 point if motor neurons showed normal morphology (the maximum long-axis diameter 15–25 μm, the maximum transverse-axis diameter 10–20 μm), no nuclear condensation and no apoptotic bodies found; and 2 points if the size of motor neurons was reduced, nuclear condensation was found, and apoptotic bodies were occasionally seen.
-
(4)
The final score was the sum of the scores in step 1, 2, 3.
Nissl staining of rat spinal cord slices
After the optimal therapeutic dose and the optimal treatment time had been determined, sections of lumbar spinal cord were deparaffinized and rinsed with distilled water three times then stained with 0.25% toluidine blue at 50°C for 3 hours, and bleached using 95% ethanol. Following 100% ethanol dehydration, xylene transparency and neutral gum mounting, sections were observed under a BX53 microscope (Olympus) and CellSens Dimension camera system (Olympus). The images were analyzed using Image-Pro Plus 6.0 software (Media Cybernetics, USA), and the gray value was measured.
Caspase 3 expression in rat spinal cord tissue detected by immunohistochemical staining
Rat spinal cord slices were deparaffinized, hydrated, and washed with PBS three times for 3 minutes each. Each slice was incubated with 50 μL of peroxidase blocking solution (solution A in immunohistochemistry kit; Maixin, Fujian Province, China) at room temperature for 10 minutes to eliminate endogenous peroxidase activity. After another series of PBS washes, each slice was incubated with 50 μL of normal non-immune animal serum (solution B in immunohistochemistry kit; Maixin) at room temperature for 10 minutes. The serum was removed and each slice was incubated with 50 μL of goat anti-rat Caspase 3 antibody (1:250; Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) at room temperature for 60 minutes. Following three PBS washes and removal of PBS solution, each slice was incubated with 50 μL of biotinylated anti-goat immunoglobulin reagent (solution C in immunohistochemistry kit; Maixin) at room temperature for 10 minutes and with 50 μL of streptavidin-peroxidase solution (solution D in immunohistochemistry kit; Maixin) at room temperature for 10 minutes, followed by PBS washing and removal of PBS solution. The slices were developed with 100 μL DAB solution and observed under the microscope for 10 minutes then rinsed with tap water and stained with hematoxylin. After gradient ethanol dehydration, xylene transparency and neutral gum mounting, the slices were observed under a BX53 microscope (Olympus). Previous immunohistochemical studies (Xue et al., 2012; Deng et al., 2013) showed Caspase 3 positive nerve cells contained brown particles in the cytoplasm, while no brown particles were found in non-positive nerve cells. The number of positive nerve cells and total nerve cells in the spinal cord gray matter was measured and the positive rate was calculated as follows: Caspase 3 positive expression rate = number of positive nerve cells/total nerve cells × 100%. Caspase 3 expression in immunohistochemical specimens is determined by the percentage of positive cells and the expression intensity of positive cells, the final score of Caspase 3 expression is the sum of the scores of the positive cell expression rate and expression intensity. The percentage of positive cells was scored as follows: 0 (0%), 1 (0–10%), 2 (10–50%), 3 (51–80%), 4 (> 80%); the expression intensity was scored as 1 (mild), 2 (moderate), 3 (strong). The final total scores were divided into the following groups: no expression (score < 2), low expression (score = 2–3), the medium expression (score = 4–5), high expression (score = 6–7).
Western blot analysis
The remaining 24 rats were randomly divided into control group, sham group, I/R group, and treatment group. Each group contained six rats. In the control group, rats were anesthetized with intraperitoneal injection of 10% chloral hydrate, and the abdominal cavity was not exposed. In the sham group, an incision was made along the left ribs under anesthesia; the abdominal cavity was opened and then closed after local spreading of penicillin powder. In the I/R group, spinal cord ischemia/reperfusion injury was carried out as previously described. In the above three groups, rats were given intraperitoneal injections of saline every day after modeling. In the treatment group, spinal cord ischemia/reperfusion injury was produced with the aforementioned methods and the group was then treated with intraperitoneal injection of ginsenoside Rd at the optimal therapeutic dose and time described previously.
After rats were anesthetized with intraperitoneal injection of 10% chloral hydrate, an incision was made on the back skin at the thoracolumbar junction, exposing the spine. The vertebral column below thoracic 11 segment was transected and the vertebral pedicle was carefully cut using ophthalmic scissors. 50 g spinal cord tissue was harvested from each Sprague-Dawley rat and longitudinally cut and stored at −80°C for subsequent western blot analysis. The samples were incubated with rabbit anti-rat GAPDH, ASK1, p38MAPK, JNK antibodies (1:100; BIOSS, Beijing, China) at 4°C overnight, and with goat anti-rabbit HRP (1:2,000; Beijing ComWin Biotech Co., Ltd., Beijing, China) at room temperature for 45 minutes. Blots were developed with chemiluminescence and images were analyzed using Tanon Gel Image System ID 4.1.2 System (Shanghai Tanon Technology Co., Ltd., Shanghai, China).
Statistical analysis
Measurement data are expressed as the mean ± SD and analyzed using SPSS 11.0 software (SPSS, Chicago, IL, USA). The difference among the groups was compared using one-way or repeated measures analysis of variance, while the difference between groups was compared using Student-Newman-Keuls test. A P < 0.05 value was considered statistically significant.
Results
Optimal therapeutic dose of ginsenoside Rd determined by BBB scores and hematoxylin-eosin staining
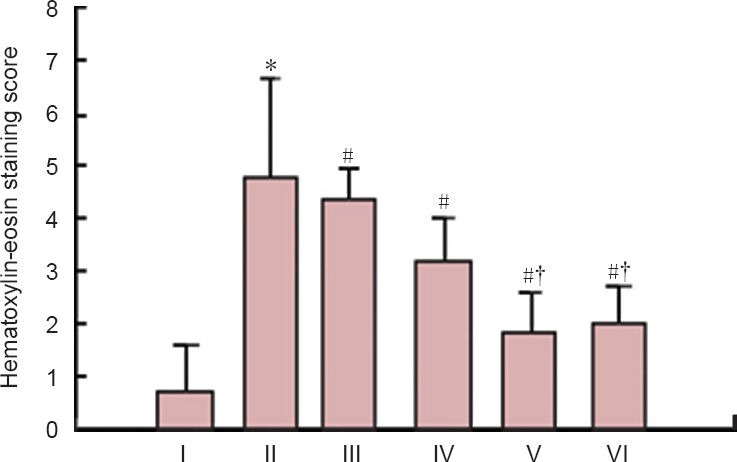
The BBB scores are shown in Table 1. The lowest BBB score was observed in the I/R group (P < 0.05), indicating loss of motor function following spinal cord injury was the most severe in the I/R group when compared with the other four groups. There was no significant difference in BBB scores between 6.25 mg/kg and 12.5 mg/kg ginsenoside Rd group, or between 25 mg/kg and 50 mg/kg ginsenoside Rd groups (P > 0.05), however the treatment groups (6.25, 12.5, 25, 50 mg/kg ginsenoside Rd) showed significant differences (P < 0.05). BBB scores gradually increased as the injection dose of ginsenoside Rd increased, indicating ginsenoside Rd dose-dependently improved BBB scores. The treatment groups showed significant differences in the BBB scores at 1–4 days after modeling (P < 0.05), except for 6.25 mg/kg ginsenoside Rd group at 1 and 2 days (P > 0.05). In each treatment group, no significant difference was found between 4 and 5 days (P > 0.05), suggesting BBB scores remained the same after 4 days.
Table 1.
Effect of intraperitoneal injection of ginsenoside Rd on lower limb motor function (Basso, Beattie, Bresnahan (BBB) score) of rats with spinal cord ischemia/reperfusion injury
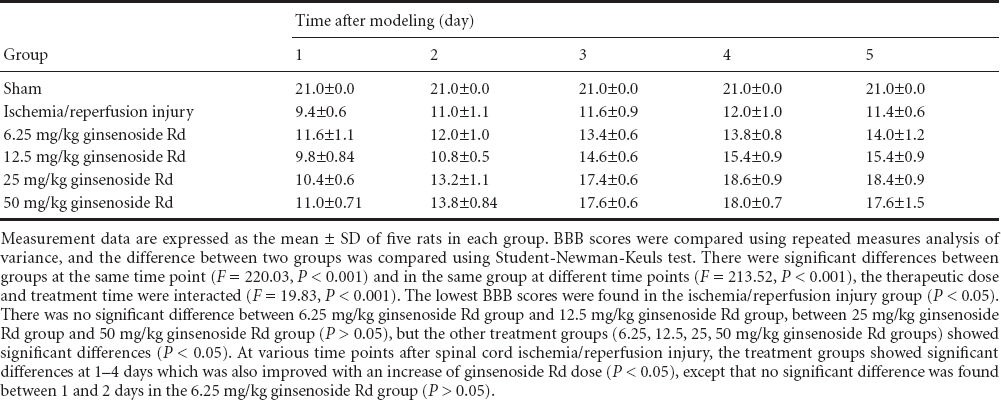
Hematoxylin-eosin staining showed that spinal cord injury was the most severe in the I/R group; in the 6.25, 12.5, 25, 50 mg/kg ginsenoside Rd treatment groups, microscopic pathologic changes in the spinal cord were also gradually reduced with increased ginsenoside Rd dose, and there was no significant difference between 25 and 50 mg/kg ginsenoside Rd groups (P > 0.05; Figures 1, 2).
Figure 1.
Effect of intraperitoneal injection of ginsenoside Rd on the morphology of L2 spinal cord cross-sections in rats 5 days after spinal cord ischemia/reperfusion injury (hematoxylin-eosin staining).
(a, A) Sham group; (b, B) ischemia/reperfusion in-jury group (I/R group); (c, C) 6.25 mg/kg ginseno-side Rd group; (d, D) 12.5 mg/kg Rd group; (e, E) 25 mg/kg ginsenoside Rd group; (f, F) 50 mg/kg ginsenoside Rd group. (A) Large neurons, slightly stained nuclei, clearly visible nucleolus, and patchy Nissl bodies (arrows) were found in the cytoplasm. (B) Large neurons and slightly stained nuclei were found, the nucleolus was visible. Nissl body content (arrows) was significantly lower than that in the sham group. (C) The neurons were large; Nissl body content (arrows) was higher than that in the I/R group. (D) The neurons were large, with a large amount of cytoplasmic Nissl bodies (arrows). (E) The neurons were large and round, with slightly stained nuclei and thick nucleolus, there were a large number of Nissl bodies (arrows) in the cytoplasm. (F) The neurons were large and round, with slightly stained nuclei and thick nucleolus, and there were a large number of Nissl bodies (arrows) in the cytoplasm. Original magnification, a–f: × 40, A–F: × 400; A–F are the magnified images of the boxes in a–f, respectively.
Figure 2.
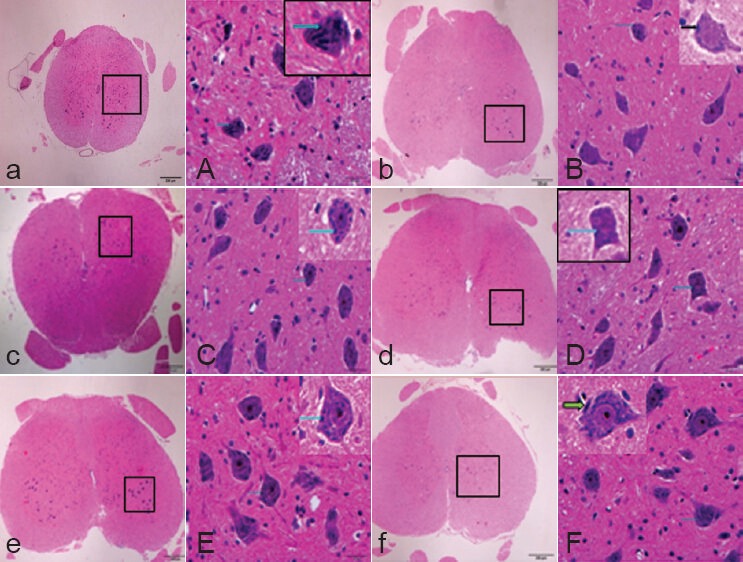
Hematoxylin-eosin staining score in rats with spinal cord ischemia/reperfusion injury to determine the optimal dose of ginsenoside Rd.
*P < 0.05, vs. sham group; #P < 0.05, vs. ischemia/reperfusion group; †P < 0.05, vs. 12.5 mg/kg Rd group; P < 0.05, 12.5, 25 and 50 mg/kg ginsenoside Rd group vs. 6.25 mg/kg ginsenoside Rd group. Measure-ment data are expressed as the mean ± SD of five rats in each group, one-way analysis of variance and Student-Newman-Keuls test were used. I–VI are sham, ischemic/reperfusion, 6.25, 12.5, 25, and 50 mg/kg ginsenoside Rd groups, respectively.
Based on the BBB scores and hematoxylin-eosin staining results, the optimal therapeutic dose was determined as 25 mg/kg ginsenoside Rd and we subsequently selected and used 25 mg/kg per day in this study (Table 1, Figures 1, 2).
The optimal treatment time of ginsenoside Rd detected by BBB scores and hematoxylin-eosin staining
Each group was treated with 25 mg/kg per day ginsenoside Rd for 1, 3, 5 and 7 days after modeling. BBB scores gradually increased with prolonged treatment time (P < 0.05, the lowest at 1 day), indicating that ginsenoside Rd treatment was effective in neuroprotection. Hematoxylin-eosin staining showed microscopic structural changes consistent with improvement in the function of movement, ischemia/reperfusion injury in spinal cord tissue was significantly attenuated, the size of neurons was increased, and the nucleolus was clearly visible. BBB scores showed no significant difference at 4–7 days after modeling, indicating optimal treatment effects had been achieved. Hematoxylin-eosin staining scores were lowest at 5 days after modeling, which were lower than that at 1 and 3 days (P < 0.05), so the optimal treatment time was determined as 5 days (Table 2, Figures 3, 4).
Table 2.
Basso, Beattie, Bresnahan (BBB) scores of rats 7 days after spinal cord ischemia/reperfusion injury
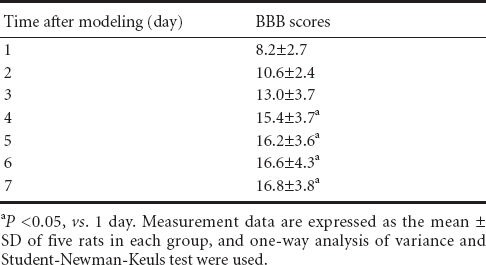
Figure 3.
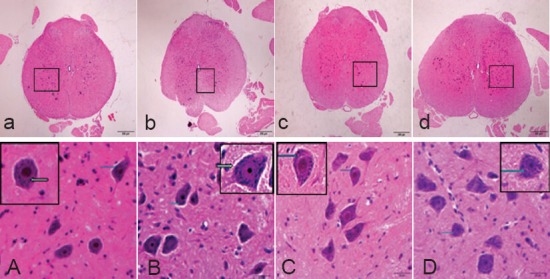
Morphology of spinal cord in Sprague-Dawley rats for determination of the optimal treatment time of ginsenoside Rd (hematoxylin-eosin staining).
(a, A) 1 day after modeling; (b, B) 3 days after mod-eling; (c, C) 5 days after modeling; (d, D) 7 days after modeling. (a, A) There were large and round neurons, slightly stained nuclei, large nucleolus, and many Nissl bodies (arrows) in the cytoplasm. (b, B) The nuclei were slightly stained, nucleoli were clearly visible, and many Nissl bodies (arrows) were found in the cytoplasm. (c, C) The nuclei were slightly stained and many Nissl bodies (arrows) were found, there were more large neurons than A and B. (d, D) The nuclei were slightly stained and many Nissl bodies (arrows) were found, there were more large neurons than A and B. Original magnifi-cation, a–d: × 40, A–D: × 400, A–D are the magni-fied images of the boxes in a–d, respectively.
Figure 4.
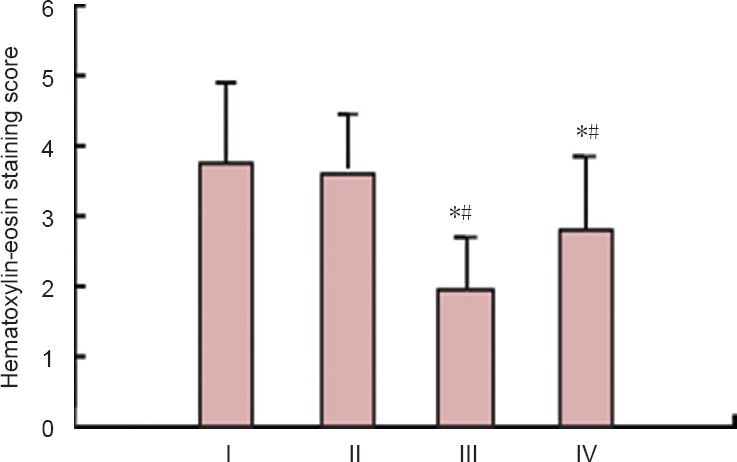
Hematoxylin-eosin staining scorein rats with spinal cord ischemia/reperfusion injury for the determination of the optimal treatment time of ginsenoside Rd.
*P < 0.05, vs. 1 day; #P < 0.05, vs. 3 days. Measurement data are ex-pressed as the mean ± SD of five rats in each group, one-way analysis of variance and Student-Newman-Keuls test were used. I–IV represent 1, 3, 5 and 7 days after treatment with ginsenoside Rd, respectively.
Nissl staining and scoring results in rats with spinal cord ischemia/reperfusion injury after ginsenoside Rd intervention
Nissl staining of spinal cord tissue is shown and described in Figures 5, 6 and Tables 3, 4.
Figure 5.
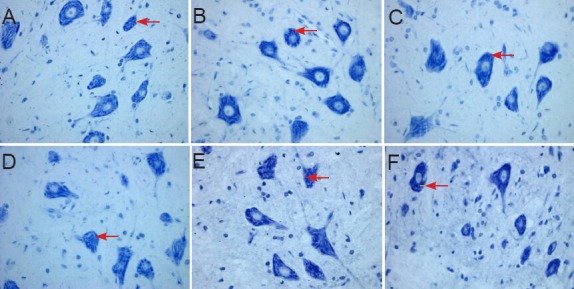
Morphology of spinal cord sections in rats 5 days after spinal cord ischemia/reperfusion injury for the determination of the optimal dose of ginsenoside Rd (Nissl staining, × 400).
(A) Sham group; (B) ischemia/reperfusion injury group (I/R group); (C–F) 6.25, 12.5, 25, 50 mg/kg ginsenoside Rd groups. (A) In the sham group, spinal anterior horn moto-neurons were large; the nuclei were lightly stained, with a clearly visible nucleolus and a large amount of Nissl bodies in the cyto-plasm. (B) In the I/R group, there were large anterior horn motoneurons, lightly stained nuclei and clearly visible nucleoli, Nissl bod-ies that accumulated in the cytoplasm and were darkly stained. (C) 6.25 mg/kg ginse-noside Rd group: spinal anterior horn mo-toneurons were lightly stained and showed clear nucleoli, the content of Nissl bodies was high in the cytoplasm, which was lower than that in the I/R group, and the intensity of staining was lighter than that in the I/R group. (D–F) 12.5, 25, 50 mg/kg ginsenoside Rd groups: spinal anterior horn motor neu-rons showed light staining, clear nucleolus, and high content of Nissl bodies. Arrows indicate Nissl bodies.
Figure 6.
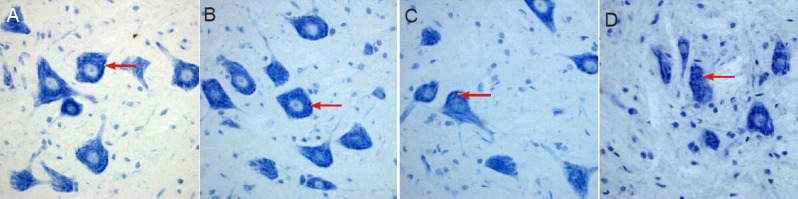
Morphology of spinal cord sections in rats with spinal cord ischemia/reperfusion injury for the determination of the optimal treatment time of ginsenoside Rd (Nissl staining, × 400).
(A–D) 1, 3, 5, 7 days after spinal cord ischemia/reperfusion injury. In all groups, spinal cord anterior horn motor neurons showed lightly stained cell nuclei, clearly visible nucleolus, and high content of Nissl bodies in the cytoplasm. Arrows indicate Nissl bodies.
Table 3.
Gray values of Nissl stained sections for the determination of the optimal dose of ginsenoside Rd
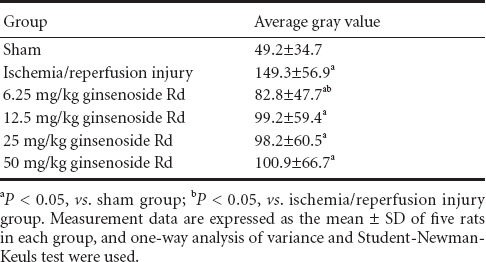
Table 4.
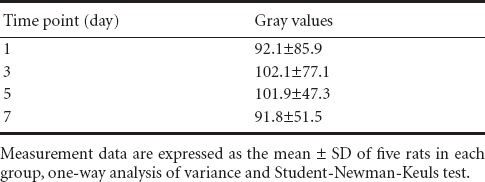
Gray values of Nissl stained sections for the determination of the optimal treatment time of ginsenoside Rd
Determination of the optimal dose
In the sham group, spinal motoneurons in the anterior horn were large, the nuclei were lightly stained with a clearly visible nucleolus and a large amount of Nissl bodies were present in the cytoplasm. In the I/R group, there were large motoneurons in the anterior horn, slightly stained nuclei and clearly visible nucleoli. Nissl bodies accumulated in the cytoplasm and were darkly stained, so the gray values were significantly higher compared with the sham group (P < 0.05). In the 6.25 mg/kg ginsenoside Rd group, anterior horn spinal motoneurons were slightly stained and showed a clear nucleolus, the content of Nissl bodies was high in the cytoplasm which was lower compared with the I/R group, the intensity of staining was lighter than that in the I/R group. 12.5, 25, 50 mg/kg ginsenoside Rd groups: spinal anterior horn motor neurons showed light staining, a clear nucleolus, and a high content of Nissl bodies. The gray values were measured and showed no significant difference.
Determination of the optimal treatment time
Nissl staining in each group (1, 3, 5 and 7 day groups) revealed light staining of cell nuclei, a clearly visible nucleolus, and high Nissl content in the cytoplasm of spinal motoneurons in the anterior horn. The gray values showed no significant difference at any of the treatment time points. Under the microscope, the neurons in sham group were normal; Nissl staining in the cytoplasm became dark in the I/R group; in all treatment groups, Nissl staining became light and Nissl bodies were plaque-like with the increase of ginsenoside Rd concentration and treatment time (1, 3, 5 and 7 days after modeling).
Effect of ginsenoside Rd on Caspase 3 expression in spinal cord of spinal ischemia/reperfusion injury rat detected by immunohistochemistry
Determination of the optimal dose
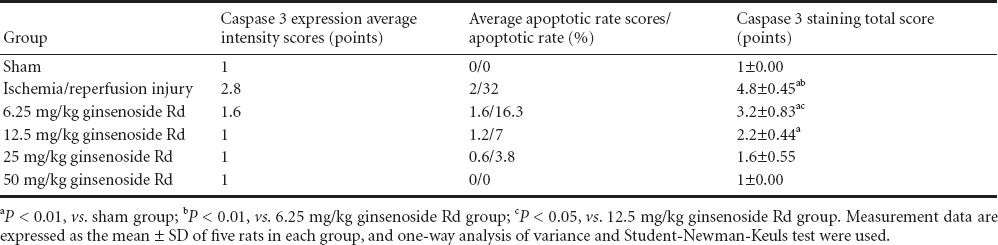
Immunohistochemical staining showed that in the sham group, neurons were large, nuclei were round and lightly stained, nucleoli were clearly visible and no Caspase 3 positive particles were found in the cytoplasm, indicating no Caspase 3 positive cells. In the I/R group, the neurons were large and the nuclei were round, a large amount of Caspase 3 positive particles were found in the cytoplasm of some neurons, indicating the presence of Caspase 3 positive cells. In 6.25 mg/kg ginsenoside Rd group, large neurons and round nuclei were observed, Caspase 3 positive particles were found in the cytoplasm of some neurons, but the number of particles and cells was significantly lower than that in the I/R group. In the 12.5 mg/kg ginsenoside Rd group, there were large neurons, round nuclei, and Caspase 3 positive particles in a small number of neurons. In the 25 and 50 mg/kg ginsenoside Rd groups, the neurons were large and the nuclei were round, Caspase 3 positive particles were found in a small amount of nerve cells, with no significant difference between the two groups. The scoring system can reflect the percentage of Caspase 3 positive cells and the intensity of positive expression simultaneously. The I/R group had a higher score than the sham group (P < 0.01), indicating that apoptosis of some neurons began following the ischemia/reperfusion injury of spinal cord in rats; intraperitoneal injection of 6.25 mg/kg ginsenoside Rd led to reduced scores comparing with I/R group (P < 0.01), meaning that this intervention was effective; the scores were significantly lower in 12.5 mg/kg ginsenoside Rd group than that in 6.25 mg/kg ginsenoside Rd group (P < 0.05), suggesting that the inhibition effect of ginsenoside Rd on Caspase 3 expression was mediated in a dose-dependent manner; however, there was no significant difference between 25 and 50 mg/kg ginsenoside Rd groups (Figure 7, Table 5). After the intervention of ginsenoside Rd, the expression of Caspase 3 became rare and disappeared.
Figure 7.
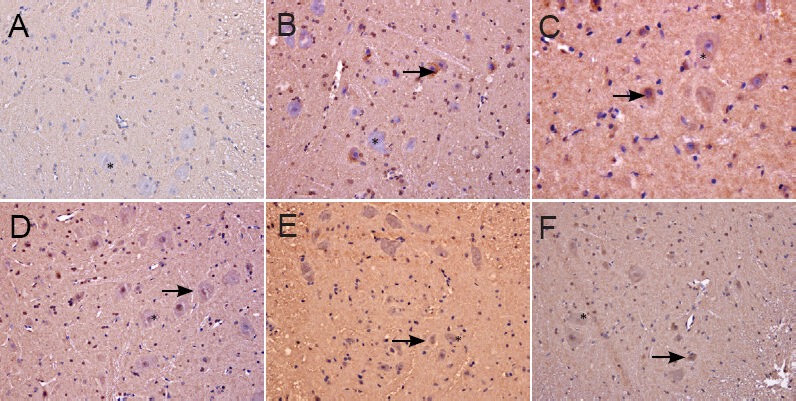
Caspase 3 immunoreactivity in the spinal cord of rats at 5 days after spinal cord ischemia/reperfusion injuny for the determination of the optimal dose of ginsenoside Rd (immunohistochemistry, × 400).
(A) Sham group; (B) ischemia/reperfusion injury group; (C–F) 6.25, 12.5, 25, 50 mg/kg ginsenoside Rd groups. (A) In the sham group, the neurons were large, the nuclei were round and lightly stained, the nucleolus was clearly visible, and no Caspase 3 posi-tive particles were found in the cytoplasm. (B) In the ischemia/reperfusion injury group, a large amount of Caspase 3 positive particles were found in the cytoplasm of some neurons. (C) In the 6.25 mg/kg ginsenoside Rd group, Caspase 3 positive particles were found in the cytoplasm of some neurons, but the number of particles and cells was significantly lower than that in the ischemia/reperfusion injury group. (D) In the 12.5 mg/kg ginsenoside Rd group, Caspase 3 positive particles were visible in a small number of neurons. (E, F) In the 25 and 50 mg/kg ginsenoside Rd groups, the neurons were large, a small amount of Caspase 3 positive particles were found in nerve cells. Arrows refer to apoptotic nerve cells.
Table 5.
Comparison of Caspase 3 staining scores of rats with spinal cord ischemia/reperfusion injury for the determination of the optimal dose of ginsenoside Rd
Determination of the optimal treatment time
Immunohistochemical staining demonstrated large neurons and round nuclei at 1, 3, 5, 7 days after spinal cord ischemia/reperfusion induction, with no Caspase 3 positivity in the cytoplasm of nerve cells. Immunohistochemical scores showed no significant difference in each group (Figure 8). Caspase 3 positive cells had no significant difference among various time points with ginsenoside Rd treatment in a particular dose. This evidence suggested that the effect of ginsenoside Rd on the expression of Caspase 3 was sensitive to the dose of ginsenoside Rd, but insensitive to the time of treatment.
Figure 8.
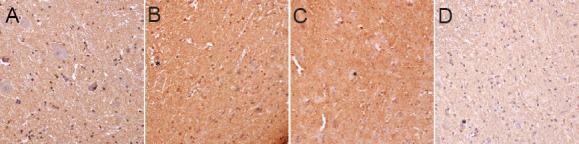
Caspase 3 immunoreactivity of the spinal cord in rats for the determination of the optimal treatment time of ginsenoside Rd (immunohistochemistry, × 400).
(A–D) 1, 3, 5, 7 days after spinal cord isch-emia/reperfusion injury. In all groups, the neurons were large and the nuclei were round, no Caspase 3 positive cells formed. Stars refer to normal nerve cells.
Effect of ginsenoside Rd on ASK1-JNK pathway in spinal cord of spinal ischemia/reperfusion injury rats
Western blot analysis showed that the expression of ASK1, JNK and p38 MAPK protein in the I/R group was higher than that in the sham group (P < 0.01), indicating the success of establishing the models. The expression of ASK1 and JNK protein was reduced by the treatment of ginsenoside Rd, compared with the I/R group (P < 0.05), suggesting the treatment of intraperitoneal injection of ginsenoside Rd was effective. There were no significant differences in the expression of p38 MAPK between the treatment groups and I/R group (P > 0.05; Figure 9). Above all, we concluded that ginsenoside Rd prevents neuronal apoptosis following ischemia/reperfusion injury in spinal cord of rats by suppressing the ASK1-JNK pathway, rather than p38 MAPK.
Figure 9.
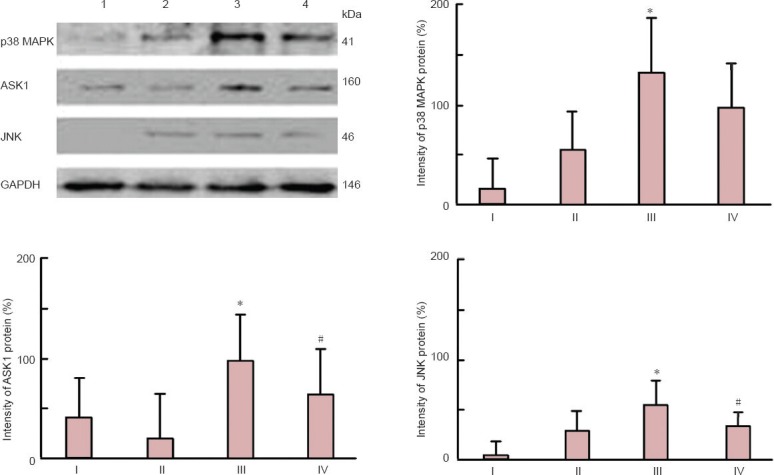
Effect of ginsenoside Rd treatment on ASK1, JNK, p38 MAPK protein expression in spinal cord of rats with ischemia/reperfusion injury (western blot analysis).
Measurement data are expressed as the mean ± SD and the experiment was repeated three times, one-way analysis of variance and Student-Newman-Keuls test. *P < 0.01, vs. sham group; #P < 0.05, vs. ischemia/reperfusion group. I: Control group; II: sham group; III: ischemia/reperfusion group; IV: ginsenoside-treated group.
Discussion
Ginsenoside Rd is one of the main active ingredients in ginseng root extract; it is fat-soluble and can cross through the blood-brain barrier and has a half-life of up to 19.29 hours in human plasma (Yang et al., 2007). Ginsenoside Rd, the derivative of triterpenoids, can reduce peroxide production, stabilize mitochondrial membrane potential, and protect mitochondrial respiratory chain complexes and aconitase activity in an in vitro study (Ye et al., 2011a, b, c). Clinical studies have shown that ginsenoside Rd exerts clear neuroprotective effects when administered before and after onset of ischemic stroke (Ye et al., 2011a, b, c; Hu et al., 2013). Meteria medica studies have shown that ginsenoside Rd is a voltage independent calcium channel blocker (Li et al., 2010). Increasing evidence has highlighted the contribution of ginsenoside Rd in the treatment of tumors (Pokharel et al., 2010), transplant rejection (Wang et al., 2012), atherosclerosis (Li et al., 2011) and articular cartilage degeneration (Shin et al., 2009). Previous research and clinical trials have emphasized the relationship between ginsenoside Rd and ischemic stroke, but not the role of ginsenoside Rd in spinal cord ischemia/reperfusion injury. This study aimed to verify whether ginsenoside Rd is a feasible treatment for spinal cord injury.
Lu et al. (2000) found that neuronal apoptosis plays a crucial role in spinal cord injury. Previous studies have shown apoptosis to be the main pattern of neuronal injury in patients suffering from secondary paralysis after spinal cord ischemia/reperfusion injury (Beattie et al., 2000; Chu et al., 2002; Lin et al., 2003). Neuronal apoptosis caused by the delayed paralysis following spinal cord ischemia/reperfusion injury differs from apoptosis in mechanical injury of spinal cord in both time and the location of the cortex. In terms of location, apoptotic cells are mainly distributed in the spinal cord gray matter, especially in the motor neurons of the anterior horn, whilst mechanical spinal cord injury causes neuronal apoptosis in the white matter, mainly glial cells. In terms of time, apoptosis occurs 72 hours after ischemia/reperfusion, whilst neuronal apoptosis caused by mechanical spinal cord injury was detected 24 hours post-injury (McEwen et al., 1997). Caspase 3 is the most important protease in spinal cord nerve apoptosis (Liu et al., 2005), and a common downstream effector of a variety of apoptosis pathways. The upregulation of Caspase 3 expression contributes to spinal cord secondary injury, and selective inhibition of Caspase 3 effectively prevented spinal cord secondary injury (Knoblach et al., 2005).
We have shown that ginsenoside Rd reduces Caspase 3 expression in spinal cord tissue, improves motor function, and exerts neuroprotective effects in rats with spinal cord ischemia/reperfusion injury. When spinal cord ischemia/reperfusion injury occurred, the number of motor neurons in the anterior horn of the spinal cord was decreased, apoptotic bodies were visible and protein synthesis function was also affected to adapt intracellular environment changes. As the concentration of ginsenoside Rd increased and treatment time was prolonged, the number of surviving neurons following spinal cord ischemia/reperfusion injury gradually increased and cellular microstructures were improved; there were larger neurons, clearer nucleoli and abundant Nissl bodies. These changes promote the restoration of spinal cord function. The morphology of motoneurons, as detected by Nissl staining and Caspase 3 staining, did not change after treatment. This indicated that the inhibitory effect of ginsenoside Rd on Caspase 3 was mediated in a dose-dependent manner. Although the difference in morphology was not significant, there was a significant difference in function. Hindlimb activity coordination and stability were improved significantly along with prolonged treatment time; BBB scores showed no significant difference in 4–7 days after modeling, indicating ginsenoside Rd treatment entered a plateau phase and a longer treatment time could not produce more pronounced effects. We speculate that initial treatment of high-dose ginsenoside Rd contribute to improve motor functions. Taken together, conventional staining (hematoxylin-eosin staining), special staining (Nissl staining), immunohistochemistry (Caspase 3 staining) and behavioral evaluation confirmed that ginsenoside Rd dose-dependently downregulated the expression of Caspase 3, reduced the apoptosis of spinal anterior horn motor neurons, and effectively increased BBB scores in rats with spinal cord ischemia/reperfusion injury. Additionally, microscopic changes were consistent with behavioral evaluations.
If the ASK1-JNK pathway is activated, apoptosis occurs. Activation of the ASK1-P38 pathway (in cases where JNK is either not activated or weakly activated) leads to the differentiation of neuronal cells (Matsuzawa et al., 2001). ASK1 can adjust the level of JNK activation. The mechanism associated with activated JNK regulates apoptosis and includes transcription-dependent pathways. For example, JNK activates transcription factor AP1. It also includes transcription-independent pathways. For example, JNK promotes phosphorylation and activation of Bim and Bmf, members of the pro-apoptotic Bcl-2 family (Shao et al., 2010). Co-expression of ASK1 and JNK contributes to the deactivation of phosphorylated Bcl-2, and decreased resistance to apoptosis (Matsuzawa et al., 2001). The activation of ASK1 plays an important role in spinal cord ischemia/reperfusion injury of nerve cells (Wang et al., 2007; Yang et al., 2008). Our findings suggest that ginsenoside Rd inhibit the ASK1-JNK signaling pathway and reduce Caspase 3 expression, thus attenuating apoptosis of spinal anterior horn motor neurons.
The limitation of this study is that it relies on experimental conditions for the determination of optimal therapeutic dose and the optimal treatment time in a broader attempt to explore the neuroprotective effects of ginsenoside Rd in spinal cord ischemia/reperfusion injury, and the correlation between ginsenoside Rd dose and motor function recovery after injury. A larger sample size study is needed to further ascertain optimal dose and time.
Ginsenoside Rd could be a potential therapeutic drug in the treatment of spinal cord ischemia/reperfusion injury. However, spinal cord ischemic injury is an extremely complex process involving a variety of factors, and additional underlying mechanisms need to be explored. For example, whether other signaling pathways in addition to the ASK1-JNK are involved in the regulation of apoptosis of spinal cord neurons remains unclear.
Acknowledgments
We would like to thank all the staffs from Department of Toxicology, School of Public Health, Jilin University, China and Department of Histology and Embryology, School of Basic Medicine, Jilin University, China for providing technical support and guidance.
Footnotes
Funding: This study was financially supported by a grant from the Jilin Provincial Science and Technology Development Program Foundation of China, No. 20110915.
Conflicts of interest: None declared.
Copyedited by Paul P, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
References
- 1.Beattie MS, Farooqui AA, Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma. 2000;17:915–925. doi: 10.1089/neu.2000.17.915. [DOI] [PubMed] [Google Scholar]
- 2.Byrnes KR, Stoica BA, Fricke S, Di Giovanni S, Faden AI. Cell cycle activation contributes to post-mitotic cell death and secondary damage after spinal cord injury. Brain. 2007;130:2977–2992. doi: 10.1093/brain/awm179. [DOI] [PubMed] [Google Scholar]
- 3.Chu D, Qiu J, Grafe M, Fabian R, Kent TA, Rassin D, Nesic O, Werrbach-Perez K, Perez-Polo R. Delayed cell death signaling in traumatized central nervous system: hypoxia. Neurochem Res. 2002;27:97–106. doi: 10.1023/a:1014858707218. [DOI] [PubMed] [Google Scholar]
- 4.Deng Z, Niu G, Cai L, Wei R, Zhao X. The prognostic significance of CD44V6, CDH11, and β-Catenin E xpression in patients with osteosarcoma. Biol Med Res Int 2013. 2013:496193. doi: 10.1155/2013/496193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu G, Wu Z, Yang F, Zhao H, Liu X, Deng Y, Shi M, Zhao G. Ginsenoside Rd blocks mitochondriol-NF-KB nuclear accumulation by inhibiting Poly (ADP-ribose) Polymerase-1 after focal cerebral ischemia in rats. Neurol Sci. 2013;34:2101–2106. doi: 10.1007/s10072-013-1344-6. [DOI] [PubMed] [Google Scholar]
- 6.Kim HW, Cho KJ, Lee SK, Kim GW. Apoptosis signal-regulating kinase 1 (Ask1) targeted small interfering RNA on ischemic neuronal cell death. Brain Res. 2011;1412:73–78. doi: 10.1016/j.brainres.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Knoblach SM, Huang X, VanGelderen J, Calva-Cerqueira D, Faden AI. Selective caspase activation may contribute to neurological dysfunction after experimental spinal cord trauma. J Neurosci Res. 2005;80:369–880. doi: 10.1002/jnr.20465. [DOI] [PubMed] [Google Scholar]
- 8.Ma L, Chen Y, Sang HF, Xiong LZ. The dose-response relationship between ginsenoside Rd injection and spinal cord ischemic injury in rabbit. Zhonghua Shenjing Waike Jibing Yanjiu Zazhi. 2010;9:422–426. [Google Scholar]
- 9.Li J, Xie ZZ, Tang YB, Zhou JG, Guan YY. Ginsenoside-Rd, a purified component from panax notoginseng saponins, prevents atherosclerosis in apoE knockout mice. Eur J Pharmacol. 2011;652:104–110. doi: 10.1016/j.ejphar.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Li XY, Liang J, Tang YB, Zhou JG, Guan YY. Ginsenoside Rd prevents glutamate-induced apoptosis in rat cortical neurons. Clin Exp Pharmacol Physiol. 2010;37:199–204. doi: 10.1111/j.1440-1681.2009.05286.x. [DOI] [PubMed] [Google Scholar]
- 11.Lin R, Roseborough G, Dong Y, Williams GM, Wei C. DNA damage and repair system in spinal cord ischemia. J Vas Surg. 2003;37:847–858. doi: 10.1067/mva.2003.150. [DOI] [PubMed] [Google Scholar]
- 12.Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu J, Ashwell KW, Waite P. Advances in secondary spinal cord injury: role of apoptosis. Spine. 2000;25:1859–1866. doi: 10.1097/00007632-200007150-00022. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzawa A, Ichijo H. Molecular mechanisms of the decision between life and death: regulation of apoptosis by apoptosis signal-regulating kinase 1. J Biochem. 2001;130:1–8. doi: 10.1093/oxfordjournals.jbchem.a002947. [DOI] [PubMed] [Google Scholar]
- 15.Mattsom MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 16.McEwen ML, Springer JE. A mapping study of caspase-3 activation following acute spinal cord contusion in rats. J Histochem Cytochem. 2005;53:809–819. doi: 10.1369/jhc.4A6467.2005. [DOI] [PubMed] [Google Scholar]
- 17.Nagai H, Noguchi T, Takeda K, Ichijo H. Pathophysiological roles of ASK1-MAP kinase signaling pathways. J Biochem Mol Biol. 2007;40:1–6. doi: 10.5483/bmbrep.2007.40.1.001. [DOI] [PubMed] [Google Scholar]
- 18.Peng X, Wu Z, Yu L, Li J, Xu W, Chan HC, Zhang Y, Hu L. Overexpression of cysticfibrosis transmembrane conductance regulator (CFTR) is associated with human cervical cancer malignancy progression and prognosis. Gynecol Oncol. 2012;125:470–476. doi: 10.1016/j.ygyno.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Pokharel YR, Kim ND, Han HK, Oh WK, Kang KW. Increased ubiquitination of multidrug resistance 1 by ginsenoside Rd. Nutr Cancer. 2010;62:252–259. doi: 10.1080/01635580903407171. [DOI] [PubMed] [Google Scholar]
- 20.Sekine Y, Takeda K, Ichijo H. The ASK1-MAP kinase signaling in ER stress and neurodegenerative diseases. Curr Mol Med. 2006;6:87–97. doi: 10.2174/156652406775574541. [DOI] [PubMed] [Google Scholar]
- 21.Shao L, Goronzy JJ, Weyand CM. DNA-dependent protein kinase catalytic subunit mediates T-cell loss in rheumatoid arthritis. EMBO Mol Med. 2010;2:415–427. doi: 10.1002/emmm.201000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiizaki S, Naguro I, Ichijo H. Activation mechanisms of ASK1 in response to various stresses and its significance in intracellular signaling. Adv Biol Regul. 2013;53:135–144. doi: 10.1016/j.jbior.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Shin JS, Park N, Ra J, Kim Y, Shin M, Hong M, Kim SH, Kwon HJ, Hong SP, Kim J, Bae H. Panax ginseng C. A. Meyer modulates the levels of MMP3 in S12 murine articular cartilage cell line. J Ethnopharmacol. 2009;124:397–403. doi: 10.1016/j.jep.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Zhang Y, Chen J, Li S, Wang Y, Hu L, Wang L, Wu Y. Immunosuppressive effects of ginsenoside-Rd on skin allograft rejection in rat. J Surg Res. 2012;176:267–274. doi: 10.1016/j.jss.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 25.Wang P, Cao X, Nagel DJ, Yin G. Activation of ASK1 during reperfusion of ischemic spinal cord. Neurosci lett. 2007;415:248–252. doi: 10.1016/j.neulet.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 26.Yang C, Ren Y, Liu F, Cai W, Zhang N, Nagel DJ, Yin G. Ischemic preconditioning suppresses apoptosis of rabbit spinal neurocytes by inhibiting ASK1-14-3-3 dissociation. Neurosci Lett. 2008;441:267–271. doi: 10.1016/j.neulet.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Deng Y, Xu S, Zeng X. In vivo pharmacokinetic and metabolism Studies of ginsenoside Rd. J chromatogr B Analyt Technol Biomed Life Sci. 2007;854:77–84. doi: 10.1016/j.jchromb.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Ye R, Kong X, Yang Q, Zhang Y, Han J, Zhao G. Ginsenoside Rd attenuates redox imbalance and improve stroke outcome after focal cerebral ischemia in aged mice. Neuropharmacology. 2011a;61:815–824. doi: 10.1016/j.neuropharm.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 29.Ye R, Zhang X, Kong X, Han J, Yang Q, Zhang Y, Chen Y, Li P, Liu J, Shi M, Xiong L, Zhao G. Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia. Neuroscience. 2011b;178:169–180. doi: 10.1016/j.neuroscience.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Ye R, Zhang X, Kong X, Han J, Yang Q, Zhang Y, Chen Y, Li P, Liu J, Shi M, Xiong L, Zhao G. Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia. Neuroscience. 2011c;178:169–180. doi: 10.1016/j.neuroscience.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Zhang N, Yin Yi, Xu SJ, Wu YP, Chen WS. Inflammation & apoptosis in spinal cord injury. Indian J Med Res. 2012;135:287–296. [PMC free article] [PubMed] [Google Scholar]


