Abstract
Microtubule-associated protein 1B plays an important role in axon guidance and neuronal migration. In the present study, we sought to discover the mechanisms underlying microtubule-associated protein 1B mediation of axon guidance and neuronal migration. We exposed bone marrow mesenchymal stem cells to okadaic acid or N-acetyl-D-erythro-sphingosine (an inhibitor and stimulator, respectively, of protein phosphatase 2A) for 24 hours. The expression of the phosphorylated form of type I microtubule-associated protein 1B in the cells was greater after exposure to okadaic acid and lower after N-acetyl-D-erythro-sphingosine. We then injected the bone marrow mesenchymal stem cells through the ear vein into rabbit models of spinal cord contusion. The migration of bone marrow mesenchymal stem cells towards the injured spinal cord was poorer in cells exposed to okadaic acid- and N-acetyl-D-erythro-sphingosine than in non-treated bone marrow mesenchymal stem cells. Finally, we blocked phosphatidylinositol 3-kinase (PI3K) and extracellular signal-regulated kinase 1/2 (ERK1/2) pathways in rabbit bone marrow mesenchymal stem cells using the inhibitors LY294002 and U0126, respectively. LY294002 resulted in an elevated expression of phosphorylated type I microtubule-associated protein 1B, whereas U0126 caused a reduction in expression. The present data indicate that PI3K and ERK1/2 in bone marrow mesenchymal stem cells modulate the phosphorylation of microtubule-associated protein 1B via a cross-signaling network, and affect the migratory efficiency of bone marrow mesenchymal stem cells towards injured spinal cord.
Keywords: nerve regeneration, bone marrow mesenchymal stem cells, spinal cord injury, microtubule-associated protein 1B, protein phosphatase 2A, cell transplantation, phosphorylation, signal transduction, NSFC grant, neural regeneration
Introduction
Bone marrow mesenchymal stem cells transplanted into the spinal cord promote nerve repair and regeneration (Keirstead et al., 2005; Oudega and Xu, 2006; Bottai et al., 2008; Tetzlaff et al., 2011; Ahn et al., 2013; Ren et al., 2013; Zhang et al., 2014a). Bone marrow mesenchymal stem cells migrate to the injured site and penetrate into the spinal cord parenchyma. Bone marrow mesenchymal stem cell proliferation and differentiation reduce spinal cord injury and promote the recovery of nerve function (Tetzlaff et al., 2011; Ren et al., 2013). The immunogenicity of bone marrow mesenchymal stem cells is very low; indeed, they exhibit immunosuppressive effects (Zhang et al., 2008). Moreover, bone marrow mesenchymal stem cells have many advantages, such as being readily transfected with exogenous genes, their ability to be used in autologous transplantation, and being simple to isolate and extract (Fehlings and Vawda, 2011). Bone marrow mesenchymal stem cells suppress local glial scar formation, contributing to the recovery of nerve function (Hu et al., 2010). They also regulate the local microenvironment of the injured spinal cord and promote its repair by neuroprotection and axonal growth stimulation (Urdzíková et al., 2014). The decisive factor for the outcome of bone marrow mesenchymal stem cell transplantation is not the number of transplants, but the number of bone marrow mesenchymal stem cells that successfully migrate to the injured region (Pal et al., 2010). Furthermore, Li et al. (2010) showed that multiple transplantations elevate the number of effective bone marrow mesenchymal stem cells and strengthen the recovery of nerve function in the injured spinal cord. Therefore, the migration of bone marrow mesenchymal stem cells to the injured site is an important factor in the treatment of spinal cord injury using bone marrow mesenchymal stem cells.
The expression of various cytokines, chemotactic factors and corresponding receptors, such as tumor necrosis factor-α, vascular endothelial growth factor, hepatocyte growth factor and platelet-derived growth factor, is upregulated in the injured spinal cord (Caplan and Correa, 2011). These factors and many signaling molecules in the microenvironment are involved in the targeted transport of bone marrow mesenchymal stem cells, which form a signal network inducing directional movement of bone marrow mesenchymal stem cells in vivo (Caplan and Correa, 2011). Interactions of chemokines and related receptors cause the migration of bone marrow mesenchymal stem cells to the site with craniocerebral injury (Tamama et al., 2010). However, it remains to be determined which chemokines regulate this targeted migration.
Microtubule-associated protein 1B (MAP1B), is first expressed during the development phase of the central nervous system and contains more than 33 phosphorylation sites, each producing a different biological effect. Phosphorylated MAP1B can be divided into type I (P1-MAP1B) and type II (P2-MAP1B), which are catalyzed respectively by proline-mediated protein kinase and casein kinase. Dephosphorylation is regulated by protein phosphatases 1 and 2A (Do et al., 2013). MAP1B and P1-MAP1B induce cytoskeletal rearrangement and promote axon growth, branching and regeneration through the regulation of actin and microtubules (Kuo et al., 2009). Meixner et al. (2000) confirmed that MAP1B exerts a crucial effect on axon guidance and neuronal migration. As a downstream signaling molecule, MAP1B is also regulated by many signaling molecules that are associated with axon regeneration, axon guidance and neuronal migration. The dynamic equilibrium of MAP1B and phosphorylated MAP1B after spinal cord injury plays a key role in axon regeneration and neuronal migration (Meixner et al., 2000). Vein transplantation of bone marrow mesenchymal stem cells induces their migration to the injured spinal cord, promoting recovery (Urdzíková et al., 2006). Such targeted migration may be involved in the regulatory effects of various factors on bone marrow mesenchymal stem cells and the activation of related signal transduction pathways. After spinal cord injury, upregulated expression of MAP1B specifically controls axon guidance and neuronal migration. We therefore hypothesized that the dynamic equilibrium between MAP1B and P1-MAP1B is associated with targeted migration to the injury site during bone marrow mesenchymal stem cell transplantation. In the present study, we disrupted this dynamic equilibrium to observe the effect on the targeted migration of bone marrow mesenchymal stem cells to the injured spinal cord, and investigated MAP1B-related signaling pathways in bone marrow mesenchymal stem cells and the relationship between MAP1B and P1-MAP1B.
Materials and methods
Subculture and identification of bone marrow mesenchymal stem cells
OriCell™ rabbit bone marrow mesenchymal stem cells (Cyagen Biosciences, Guangzhou, Guangdong Province, China) were seeded in T25 culture flasks. Sufficient rabbit bone marrow mesenchymal stem cell complete medium (Cyagen, Santa Clara, CA, USA) was added to the culture flask, which was placed in a 5% CO2 moisturizing incubator at 37°C. When the cells reached 80–90% confluency, the medium was discarded. Bone marrow mesenchymal stem cells were subcultured by digestion in 100 mL PBS containing 0.25 g trypsin and 0.04 g ethylenediamine tetraacetic acid. Bone marrow mesenchymal stem cells were viewed under a light microscope (Olympus, Tokyo, Japan). Single cell suspensions (1 × 105/mL) were prepared for further use. Bone marrow mesenchymal stem cells from passages 2, 4 and 6 were measured for 7 consecutive days, as described previously (Kuo et al., 2009). In brief, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT; 5 mg/mL, 10 μL/well) and dimethyl sulfoxide working solution (150 μL/well) were added to the culture plate and mixed. Optical density values were measured at 490 nm using a microplate reader (TECAN, Berlin, Germany). A growth curve was drawn and cell viability was calculated. Passage 4 bone marrow mesenchymal stem cells were incubated with specific mouse anti-rabbit CD34 (1:3,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and CD45 (1:3,000; Santa Cruz Biotechnology) monoclonal antibodies. Cell surface antigens were identified using a flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) (Meixner et al., 2000; Kuo et al., 2009).
Tracking migration of bone marrow mesenchymal stem cells using CM-DiI dye
CM-DiI dye (4 μg/mL; Molecular Probes, Eugene, OR, USA) was added to a single-cell suspension from a primary culture of bone marrow mesenchymal stem cells. CM-DiI-labeled bone marrow mesenchymal stem cells were measured using flow cytometry.
Detection of protein phosphatase 2A and MAP1B expression in bone marrow mesenchymal stem cells using immunohistochemistry
A single-cell suspension from primary bone marrow mesenchymal stem cells was cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. Samples were incubated with mouse anti-rat protein phosphatase 2A (1:2,000) and MAP1B (1:3,000) monoclonal antibodies (Abcam, Cambridge, MA, USA) at room temperature for 2 hours, and then washed with PBS three times for 5 minutes each. The samples were then incubated with rabbit anti-mouse horseradish peroxidase-labeled secondary antibody (1:4,000; Abcam) for 50 minutes, and then washed three times as before. All samples were observed under a light microscope (Olympus).
Experimental groups and intervention
Bone marrow mesenchymal stem cells were randomly assigned to an agonist group, an inhibitor group and a control group. Bone marrow mesenchymal stem cells in the inhibitor and agonist groups were treated with the protein phosphatase 2A inhibitor okadaic acid (100 μmol/mL; Sigma, St. Louis, MO, USA) and the protein phosphatase 2A agonist N-acetyl-D-erythro-sphingosine (50 μmol/mL; Sigma) respectively, for 24 hours. Growth curves of bone marrow mesenchymal stem cells in each group were measured using the MTT assay.
Establishment of spinal cord contusion models
A total of 96 healthy 6-month-old specific-pathogen-free New Zealand rabbits weighing 2.3–2.5 kg, irrespective of gender, were provided by the Experimental Animal Center, Jilin University, China (animal license No. SCXK (Ji) 2008-0004). All rabbits were housed in a positive-pressure airtight room. The protocols were approved by the Animal Ethics Committee, Jilin University, China. Rabbits were equally and randomly divided into an inhibitor group, an agonist group, a bone marrow mesenchymal stem cell group and a model group. Spinal cord ischemic injury models were established in each group as described previously (Urdzíková et al., 2006). In brief, rabbits were anesthetized with sodium pentobarbital (40 mg/kg) through the ear vein. Bilateral L3–5 lumbar arteries were bluntly isolated, and occluded with a vascular clamp for 25 minutes. After removal of the vascular clamp, the incision was sutured. Motor function was evaluated using the modified Tarlov scale after spinal cord injury. Rabbits scoring less than 3 were considered successful models (Urdzíková et al., 2006).
Bone marrow mesenchymal stem cell transplantation
Seven days after model establishment, rabbits in the inhibitor, agonist, bone marrow mesenchymal stem cell and model groups were injected through the ear vein with 1 mL bone marrow mesenchymal stem cells treated with okadaic acid for 24 hours (1 × 107/mL), 1 mL bone marrow mesenchymal stem cells treated with N-acetyl-D-erythro-sphingosine for 24 hours (1 × 107/mL), 1 mL untreated bone marrow mesenchymal stem cells (1 × 107/mL) or 1 mL physiological saline, respectively.
Bone marrow mesenchymal stem cell migration observed by laser scanning confocal microscope
Eight rabbits were selected randomly from each group at 2, 4 or 7 days after transplantation, and sacrificed by anesthesia with sodium pentobarbital (40 mg/kg) through the ear vein. L3–5 spinal cord tissue was obtained and freeze-sectioned. The sections were observed under a laser scanning confocal microscope (Nikon, Tokyo, Japan) at excitation and emission wavelengths of 553 and 570 nm, respectively.
Western blot assay for MAP1B phosphorylation
MAP1B and P1-MAP1B expression levels were quantified in each group by western blot assay at 1, 3 and 5 days after intervention. The phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 (Cell Signaling Technology, Danvers, MA, USA) or the extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitor U0126 (Sigma) were added to the bone marrow mesenchymal stem cell suspension. Protein was extracted by lysing the cells with 0.1 mmol/L NaCl, 10 mmol/L NaF, 1 mmol/L Na3VO4, 5 mmol/L ethylenediamine tetraacetic acid, 1 mmol/L okadaic acid, and protease inhibitors (2 mmol/L phenylmethyl sulfonylfluoride, 10 mg/mL aprotinin, 10 mg/mL leupeptin, and 10 mg/mL pepstatin), and protein was extracted. The control group did not receive any treatment. The gel was prepared and protein samples were added to 4 × loading buffer at a ratio of 3:1. The mixture was placed in a water bath at 100°C for 5 minutes, 60 μg protein/well. Pre-stained protein marker was added, and the proteins were separated by electrophoresis (80 V in the stacking gel and 160 V in the separating gel). Protein concentrations were determined by Bradford assay (Bio-Rad, Hercules, CA, USA). Equal amounts of protein were separated by sodiumdodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane at 4°C. The membrane was blocked and incubated at room temperature for 2 hours with mouse anti-rat MAP1B monoclonal antibody (1:2,000), mouse anti-rat P1-MAP1B monoclonal antibody (1:2,000; Abcam), and mouse anti-rat β-actin monoclonal antibody (1:3,000; Abcam). The membrane was washed three times, for 5 minutes each, and incubated with rabbit anti-mouse horseradish peroxidase-labeled secondary antibody (1:4,000) at room temperature for 50 minutes, and then washed again. The samples were visualized with enhanced chemiluminescence before photographing. Optical density values were calculated using Quantity One software (Bio-Rad, Hercules, CA, USA).
Statistical analysis
The data were expressed as the mean ± SD, and one-way analysis of variance or independent samples t-tests were performed using SPSS 17.0 software (SPSS, Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Bone marrow mesenchymal stem cell culture and identification
MTT assay revealed that bone marrow mesenchymal stem cells entered the latent phase at 1–2 days, the logarithmic phase at 3–5 days, and the platform phase at 5 days, forming an “S”-shaped growth curve (Figure 1A). In accordance with the growth curve, passage 4 bone marrow mesenchymal stem cells with good viability were selected for further experiments. Passage 4 bone marrow mesenchymal stem cells were plump and spindle-shaped, and a few were polygonal at 4 days of culture (Figure 1B). Flow cytometry results revealed that bone marrow mesenchymal stem cells were negative for the hematopoietic stem cell markers CD34 and CD45 (Figure 1C).
Figure 1.
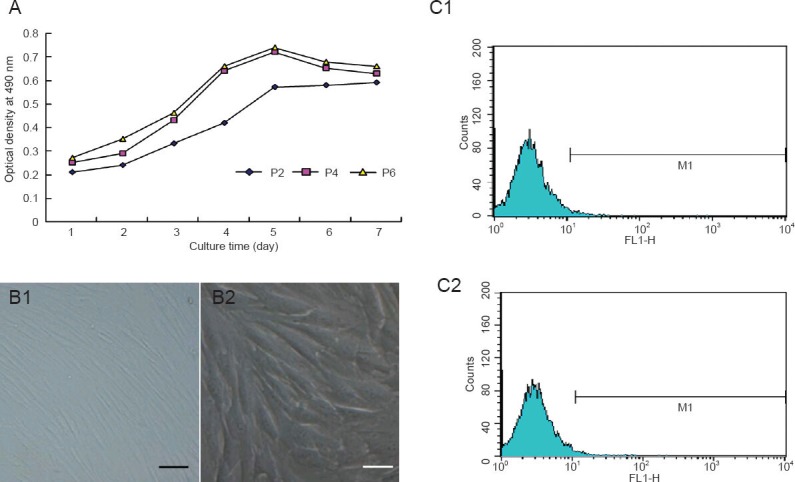
Growth, morphology and surface antigens of subcultured bone marrow mesenchymal stem cells (BMSCs).
(A) Growth curves of BMSCs from passages (P) 2, 4 and 6; (B) morphology of passage 4 BMSCs (inverted microscope). Scale bars: B1: 10 μm; B2: 5 μm); (C) flow cytometry shows surface antigens of rabbit BMSCs. CD34 (C1) and CD45 (C2): Negative reaction.
Effects of tracker CM-DiI, pretreatment of protein phosphatase 2A inhibitor and agonist on bone marrow mesenchymal stem cells
The cells appeared bright red after fluorescent labeling (Figure 2A). Using flow cytometry, the labeling rate of passage 4 bone marrow mesenchymal stem cells reached 99.63% (Figure 2B). Immunohistochemistry showed that MAP1B and protein phosphatase 2A were expressed in bone marrow mesenchymal stem cells (Figure 2C). Western blot assay revealed that neither the inhibitor for protein phosphatase 2A, okadaic acid, nor the protein phosphatase 2A agonist N-acetyl- D-erythro-sphingosine, affected MAP1B expression in bone marrow mesenchymal stem cells (P > 0.05); however, both significantly altered the expression of P1-MAP1B, especially at 1 and 3 days after intervention (P < 0.05; Figure 2D). The MTT assay showed that okadaic acid and N-acetyl-D-erythro-sphingosine suppressed bone marrow mesenchymal stem cell proliferation (P < 0.05 at 7 days; Figure 2E).
Figure 2.
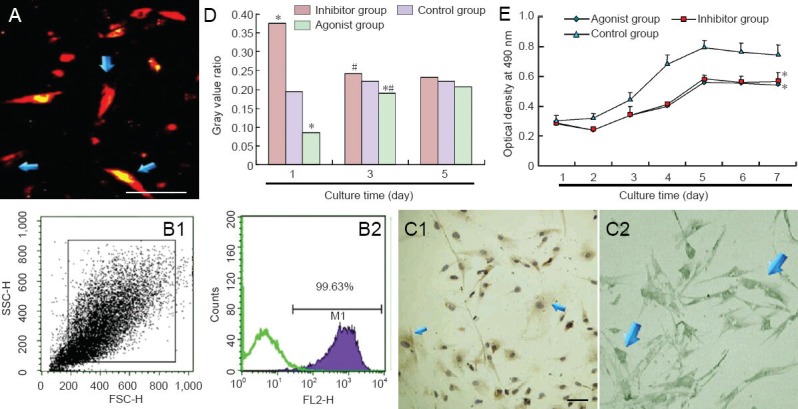
Fluorescent labeling and pretreatment in bone marrow mesenchymal stem cells (BMSCs).
(A) CM-DiI-labeled BMSCs are red and spindle shaped. Scale bar: 50 μm. (B) Flow cytometry: Labeling rate of CM-DiI reached 99.63%. (C) Im-munohistochemical staining of protein phosphatase 2A (PP2A) (C1) and microtubule-associated protein 1B (MAP1B) (C2) expression in BMSC cytoplasm. Scale bar: 100 μm. (D) Effects of okadaic acid and N-acetyl-D-erythro-sphingosine on P1-MAP1B content in BMSCs (western blot assay). (E) Effects of okadaic acid and N-acetyl-D-erythro-sphingosine on BMSC viability. Data were expressed as the mean ± SD. Experiment was conducted in triplicate. *P < 0.05, vs. control group; #P < 0.05, vs. previous time point (one-way analysis of variance and independent samples t-test).
Protein phosphatase 2A affected targeted migration of bone marrow mesenchymal stem cells during treatment of ischemic spinal cord injury
In the bone marrow mesenchymal stem cell group, few bone marrow mesenchymal stem cells were detected in the injured site at 2 days after transplantation, but the number gradually increased at 4 and 7 days. Protein phosphatase 2A inhibitor and agonist both delayed bone marrow mesenchymal stem cell migration. Bone marrow mesenchymal stem cells were detected in the injured site at 4 days after transplantation, increasing by 7 days. Moreover, there were fewer bone marrow mesenchymal stem cells in the agonist and inhibitor groups than in the bone marrow mesenchymal stem cell group. No red fluorescence was detectable in the injured site of rabbits injected with physiological saline (model group) (Figure 3).
Figure 3.
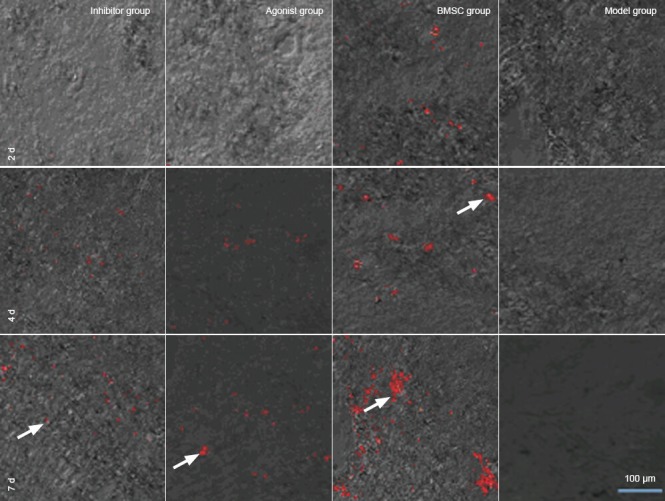
Protein phosphatase 2A (PP2A) affected the targeted migration of bone marrow mesenchymal stem cells (BMSCs) during treatment of ischemic spinal cord injury (confocal laser scanning microscope).
PP2A inhibitor and agonist delayed BMSC migration. There were fewer BMSCs in the inhibitor and agonist groups than in the BMSC group. Arrows show transplanted BMSCs. Red: CM-DiI-labeled cells. Scale bar: 100 μm. d: Days.
PI3K and ERK1/2 regulated MAP1B phosphorylation levels in bone marrow mesenchymal stem cells
PI3K inhibitor LY294002 and ERK1/2 inhibitor U0126 did not affect MAP1B contents in bone marrow mesenchymal stem cells (P > 0.05), but had a considerable effect on MAP1B phosphorylation levels. Western blot assay showed that P1-MAP1B protein content was significantly greater after suppression of PI3K, but significantly lower after ERK1/2 suppression (P < 0.01; Figure 4).
Figure 4.
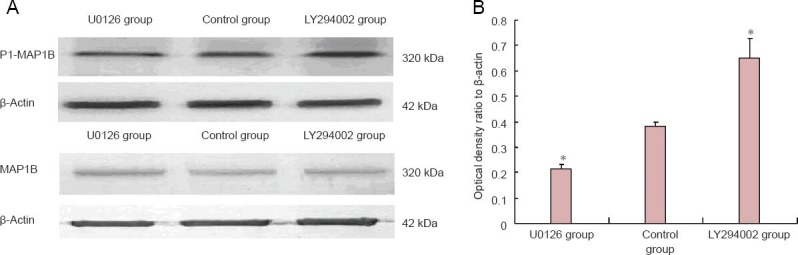
PI3K and ERK1/2 regulate MAP1B phosphorylation levels in bone marrow mesenchymal stem cells (BMSCs).
(A) Immunoblot results of MAP1B and P1-MAP1B expression levels in BMSCs. (B) Quantitative expression of P1-MAP1B in BMSCs. Data were expressed as the mean ± SD. Experiment was performed in triplicate. *P < 0.05, vs. control group (one-way analysis of variance and independent samples t-test). ERK1/2: Extracellular signal-regulated kinase 1/2; PI3K: phosphatidylinositol 3-kinase; MAPIB: microtubule-associated protein 1B; U0126: ERK1/2 inhibitor; LY294002: PI3K inhibitor.
Discussion
Bone marrow mesenchymal stem cell viability was good after resuscitation. Most cells were plump and spindle-shaped after proliferation. There were some unusually-shaped cells but they did not affect the proliferation of bone marrow mesenchymal stem cells. During culture, cells adhered to the wall. The growth curve of cultured cells was “S”-shaped. Flow cytometry showed that cultured cells did not express the hematopoietic lineage markers CD34 and CD45, confirming that bone marrow mesenchymal stem cells had successfully been obtained. CM-DiI was used to label bone marrow mesenchymal stem cells for tracking because of its staining for over 3 weeks (Fan et al., 2008). The labeling rate of living cells as detected by flow cytometry was 99.63%.
Meixner et al. (2000) believed that MAP1B played an important role in the development of the central nervous system in animals, and Gong et al. (2000) found that protein phosphatase 2A mediated MAP1B dephosphorylation. In the present study, we found that protein phosphatase 2A and MAP1B are distributed extensively in the cytoplasm of rabbit bone marrow mesenchymal stem cells, so we sought to regulate P1-MAP1B levels by modulating protein phosphatase 2A activity. Takai et al. (1987) confirmed that okadaic acid is a specific protein phosphatase inhibitor that selectively inhibits protein phosphatase 1 and, in particular, protein phosphatase 2A, whereas N-acetyl-D-erythro-sphingosine elevates protein phosphatase 2A activity (Dobrowsky et al., 1993). P1-MAP1B expression was noticeably greater in cells treated with the inhibitor, because okadaic acid reduced protein phosphatase 2A activity. Thus, MAP1B dephosphorylation was inhibited, and P1-MAP1B expression was ultimately increased. Conversely, P1-MAP1B expression was significantly lower in bone marrow mesenchymal stem cells of the agonist group. Together, these results suggest that there is a strong relationship between protein phosphatase 2A and MAP1B dephosphorylation. The results from the present study show that pretreatment of okadaic acid and N-acetyl-D-erythro-sphingosine affect cell viability, but the cells exhibit a logarithmic growth phase, which can be used in subsequent research.
Several previous studies have shown that early focal ischemia, necrosis, and toxic substances (such as oxygen free radicals and resolvase) in the spinal cord are not conducive to bone marrow mesenchymal stem cell survival and migration (Park et al., 2011; Ren et al., 2013; Torres-Espín et al., 2014). Seven days after injury, unfavorable factors subsided and the concentrations of various inflammatory factors and chemotactic factors in the spinal cord were at a level that promoted bone marrow mesenchymal stem cell migration. Stem cell transplantation 5–7 days after injury contributes to the recovery of neurological function (Park et al., 2011; Ren et al., 2013; Torres-Espín et al., 2014). We therefore transplanted bone marrow mesenchymal stem cells 7 days after model establishment. Pal et al. (2010) confirmed that the therapeutic effect of bone marrow mesenchymal stem cells was ideal when the number of transplanted cells was 5 × 106/kg, whereas Li et al. (2011) demonstrated that mesenchymal stem cell transplantation (5 × 107) for treating femoral head necrosis did not induce immune rejection. Therefore, we selected 1 × 107 per animal as the dose of bone marrow mesenchymal stem cells to inject. The growth and viability of CM-DiI-labeled bone marrow mesenchymal stem cells were slightly low, but the number of surviving cells nevertheless met the criteria for transplantation. Bone marrow mesenchymal stem cell migration was notably impaired whether P1-MAP1B expression had been elevated or diminished. Because MAP1B content was not greatly changed, we hypothesized that our intervention caused the changes in MAP1B/P1-MAP1B ratio, which further affected bone marrow mesenchymal stem cell migration. MAP1B and P1-MAP1B can induce cytoskeletal rearrangement and changes in cell polarity by the regulation of actin and microtubule function, and maintain specific microtubule dynamics in cells (Kawauchi et al., 2003; Utreras et al., 2008). Considering that MAP1B is associated with neuronal migration and that alterations in protein phosphatase 2A activity result in MAP1B phosphorylation changes, this may affect actin and microtubule functions, disrupt microtubule dynamics, cause cytoskeletal reorganization and polarity changes and damage to cell motility, ultimately resulting in the disruption of targeted bone marrow mesenchymal stem cell migration.
Protein phosphatase 2A regulates the expression of intracellular ERK1/2 (Tsuchiya et al., 2014), microtubule-associated protein (MAP)-tau (Guadagna et al., 2012) and MAP2 (Gong et al., 2000) and induces their dephosphorylation. ERK1/2 can directly or indirectly regulate downstream MAP1B phosphorylation (Goold and Gordon-Weeks, 2005; Ramos-Miguel and García-Sevilla, 2012). Protein phosphatase 2A induces ERK1/2 dephosphorylation and deactivation, indirectly suppressing MAP1B phosphorylation. MAP1B, tau and MAP2 are all microtubule binding proteins, but their functions are quite different. Knockout of MAP1B with MAP2 or tau causes serious damage to neuronal migration and the development of the nervous system, but knockout of MAP2 or tau alone does not have the same outcome (Takei et al., 2000; Zhang et al., 2014b), suggesting that MAP1B has a crucial role in the regulation of cytoskeletal reorganization during neuronal movement. Therefore, MAP1B is the main link between protein phosphatase 2A activity and bone marrow mesenchymal stem cell migration. In the present study, P1-MAP1B protein expression was significantly greater after suppression of PI3K, but lower after ERK1/2 was suppressed. P1-MAP1B affects neuronal migration in vivo, which is mainly regulated by the neuronal migration-related signaling molecule Reelin (González-Billault et al., 2005). Reelin activates glycogen synthase kinase 3β and cyclin dependent kinase 5, and co-induces P1-MAP1B production. It also activates PI3K and ERK1/2 signaling pathways (Simó et al., 2007), and PI3K simultaneously regulates ERK1/2 phosphorylation (Jossin and Goffinet, 2007). Zhou et al. (2005) found that PI3K indirectly suppresses P1-MAP1B production by inhibiting glycogen synthase kinase 3β phosphorylation. Consistent with our findings, a previous study found that ERK1/2 activation increased P1-MAP1B expression (Dashiell et al., 2002). PI3K in bone marrow mesenchymal stem cells regulates and induces cytoskeletal reorganization, triggers downstream signal transduction pathways, and contributes to the targeted migration of bone marrow mesenchymal stem cells (Kim et al., 2008; Monypenny et al., 2009). ERK1/2 mediates cytoskeletal reorganization and polarity changes, participates in bone marrow mesenchymal stem cell migration (Fu et al., 2009; Joiner et al., 2012; Cao et al., 2013; Jeon et al., 2013; Kwon et al., 2013; Melo et al., 2013; Hou et al., 2014; Wu et al., 2014). Our study demonstrates that PI3K and ERK1/2 in bone marrow mesenchymal stem cells affect MAP1B phosphorylation in neurons, as well as affecting targeted bone marrow mesenchymal stem cell migration. Protein phosphatase 2A directly induces P1-MAP1B dephosphorylation, regulates the dephosphorylation of signaling molecules upstream of MAP1B, and thus indirectly negatively regulates MAP1B phosphorylation levels. Our findings suggest that signal transduction molecules in bone marrow mesenchymal stem cells, such as protein phosphatase 2A, PI3K and ERK1/2, can affect bone marrow mesenchymal stem cell migration by regulating type 1 MAP1B phosphorylation. Moreover, these signaling molecules form cross-links with others at different levels in the pathway, composing a signaling network by which to regulate MAP1B phosphorylation and bone marrow mesenchymal stem cell migration (Gu et al., 2010; Mohammad-Gharibani et al., 2012; Wang et al., 2013).
In summary, the dynamic equilibrium of P1-MAP1B and MAP1B is important in the regulation of the targeted migration of bone marrow mesenchymal stem cells.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81350013, 81250016; the Youth Science Project of National Natural Science Foundation of China, No. 81301289; the Youth Scientific Research Project of Jilin Provincial Science and Technology Development Plan, No. 20130522032JH, 20130522039JH.
Conflicts of interest: None declared.
Copyedited by Slone-Murphy J, Haase R, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Ahn SY, Chang YS, Sung DK, Sung SI, Yoo HS, Lee JH, Oh WI, Park WS. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke. 2013;44:497–504. doi: 10.1161/STROKEAHA.112.679092. [DOI] [PubMed] [Google Scholar]
- 2.Bottai D, Madaschi L, Di Giulio AM, Gorio A. Viability-dependent promoting action of adult neural precursors in spinal cord injury. Mol Med. 2008;14:634–644. doi: 10.2119/2008-00077.Bottai. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Y, Xia DS, Qi SR, Du J, Ma P, Wang SL, Fan ZP. Epiregulin can promote proliferation of stem cells from the dental apical papilla via MEK/Erk and JNK signalling pathways. Cell Prolif. 2013;46:447–456. doi: 10.1111/cpr.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caplan AI, Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthopaed Res. 2011;29:1795–1803. doi: 10.1002/jor.21462. [DOI] [PubMed] [Google Scholar]
- 5.Dashiell SM, Tanner SL, Pant HC, Quarles RH. Myelin-associated glycoprotein modulates expression and phosphorylation of neuronal cytoskeletal elements and their associated kinases. J Neurochem. 2002;81:1263–1272. doi: 10.1046/j.1471-4159.2002.00927.x. [DOI] [PubMed] [Google Scholar]
- 6.Do H, Park HJ, Sohn EH, Kim BO, Um S, Kwak JH, Moon EY, Rhee DK, Pyo S. Ethanol induces cell cycle arrest and triggers apoptosis via Sp1-dependent p75NTR expression in human neuroblastoma cells. Cell Biol Toxicol. 2013;29:365–380. doi: 10.1007/s10565-013-9260-3. [DOI] [PubMed] [Google Scholar]
- 7.Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268:15523–15530. [PubMed] [Google Scholar]
- 8.Fan L, Du F, Cheng BC, Peng H, Liu SQ. Migration and distribution of bone marrow stromal cells in injured spinal cord with different transplantation techniques. Chin J Traumatol. 2008;11:94–97. doi: 10.1016/s1008-1275(08)60020-6. [DOI] [PubMed] [Google Scholar]
- 9.Fehlings MG, Vawda R. Cellular treatments for spinal cord injury: the time is right for clinical trials. Neurotherapeutics. 2011;8:704–720. doi: 10.1007/s13311-011-0076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu X, Han B, Cai S, Lei Y, Sun T, Sheng Z. Migration of bone marrow-derived mesenchymal stem cells induced by tumor necrosis factor-α and its possible role in wound healing. Wound Repair Regen. 2009;17:185–191. doi: 10.1111/j.1524-475X.2009.00454.x. [DOI] [PubMed] [Google Scholar]
- 11.Gong CX, Wegiel J, Lidsky T, Zuck L, Avila J, Wisniewski HM, Grundke-Iqbal I, Iqbal K. Regulation of phosphorylation of neuronal microtubule-associated proteins MAP1b and MAP2 by protein phosphatase-2A and -2B in rat brain. Brain Res. 2000;853:299–309. doi: 10.1016/s0006-8993(99)02294-5. [DOI] [PubMed] [Google Scholar]
- 12.González-Billault C, Del Río JA, Ureña JM, Jiménez-Mateos EM, Barallobre MJ, Pascual M, Pujadas L, Simó S, Torre AL, Gavin R, Wandosell F, Soriano E, Ávila J. A role of MAP1B in Reelin-dependent Neuronal Migration. Cereb Cortex. 2005;15:1134–1145. doi: 10.1093/cercor/bhh213. [DOI] [PubMed] [Google Scholar]
- 13.Goold RG, Gordon-Weeks PR. The MAP kinase pathway is upstream of the activation of GSK3β that enables it to phosphorylate MAP1B and contributes to the stimulation of axon growth. Mol Cell Neurosci. 2005;28:524–534. doi: 10.1016/j.mcn.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Gu Y, Wang J, Ding F, Hu N, Wang Y, Gu X. Neurotrophic actions of bone marrow stromal cells on primary culture of dorsal root ganglion tissues and neurons. J Mol Neurosci. 2010;40:332–341. doi: 10.1007/s12031-009-9304-6. [DOI] [PubMed] [Google Scholar]
- 15.Guadagna S, Esiri MM, Williams RJ, Francis PT. Tau phosphorylation in human brain: relationship to behavioral disturbance in dementia. Neurobiol Aging. 2012;33:2798–2806. doi: 10.1016/j.neurobiolaging.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Hou M, Cui J, Liu J, Liu F, Jiang R, Liu K, Wang Y, Yin L, Liu W, Yu B. Angiopoietin-like 4 confers resistance to hypoxia/serum deprivation-induced apoptosis through PI3K/Akt and ERK1/2 signaling pathways in mesenchymal stem cells. PLoS One. 2014;9:e85808. doi: 10.1371/journal.pone.0085808. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Hu SL, Luo HS, Li JT, Xia YZ, Li L, Zhang LJ, Meng H, Cui GY, Chen Z, Wu N, Lin JK, Zhu G, Feng H. Functional recovery in acute traumatic spinal cord injury after transplantation of human umbilical cord mesenchymal stem cells. Crit Care Med. 2010;38:2181–2189. doi: 10.1097/CCM.0b013e3181f17c0e. [DOI] [PubMed] [Google Scholar]
- 18.Jeon BJ, Yang Y, Kyung Shim S, Yang HM, Cho D, Ik Bang S. Thymosin beta-4 promotes mesenchymal stem cell proliferation via an interleukin-8-dependent mechanism. Exp Cell Res. 2013;319:2526–2534. doi: 10.1016/j.yexcr.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Joiner DM, Tayim RJ, Kadado A, Goldstein SA. Bone marrow stromal cells from aged male rats have delayed mineralization and reduced response to mechanical stimulation through nitric oxide and ERK1/2 signaling during osteogenic differentiation. Biogerontology. 2012;13:467–478. doi: 10.1007/s10522-012-9391-6. [DOI] [PubMed] [Google Scholar]
- 20.Jossin Y, Goffinet AM. Reelin signals through phosphatidylinositol 3-kinase and Akt to control cortical development and through mTor to regulate dendritic growth. Mol Cell Biol. 2007;27:7113–7124. doi: 10.1128/MCB.00928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawauchi T, Chihama K, Nabeshima Y, Hoshino M. The in vivo roles of STEF/Tiam1, Rac1 and JNK in cortical neuronal migration. 2003;22:4190–4201. doi: 10.1093/emboj/cdg413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim EK, Tucker DF, Yun SJ, Do KH, Kim MS, Kim JH, Kim CD, Birnbaum MJ, Bae SS. Linker region of Akt1/protein kinase Bα mediates platelet-derived growth factor-induced translocation and cell migration. Cell Signal. 2008;20:2030–2037. doi: 10.1016/j.cellsig.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Kuo TY, Hong CJ, Hsueh YP. Bcl11A/CTIP1 regulates expression of DCC and MAP1b in control of axon branching and dendrite outgrowth. Mol Cell Neurosci. 2009;42:195–207. doi: 10.1016/j.mcn.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Kwon HS, Johnson TV, Tomarev SI. Myocilin stimulates osteogenic differentiation of mesenchymal stem cells through mitogen-activated protein kinase signaling. J Biol Chem. 2013;288:16882–16894. doi: 10.1074/jbc.M112.422972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Wen Y, Luo Y, Lan X, Wang D, Sun Z, Hu L. Transplantation of bone marrow mesenchymal stem cells into spinal cord injury: a comparison of delivery different times. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;24:180–184. [PubMed] [Google Scholar]
- 27.Li ZH, Liao W, Cui XL, Zhao Q, Liu M, Chen YH, Liu TS, Liu NL, Wang F, Yi Y, Shao NS. Intravenous transplantation of allogeneic bone marrow mesenchymal stem cells and its directional migration to the necrotic femoral head. Int J Med Sci. 2011;8:74–83. doi: 10.7150/ijms.8.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meixner A, Haverkamp S, Wässle H, Führer S, Thalhammer J, Kropf N, Bittner RE, Lassmann H, Wiche G, Propst F. MAP1B is required for axon guidance and Is involved in the development of the central and peripheral nervous system. J Cell Biol. 2000;151:1169–1178. doi: 10.1083/jcb.151.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melo CS, Arantes Faria JA, Corrêa NC, de Andrade C, Carvalho JL, Goes AM, Rodrigues MA, Gomes DA. Cytoplasmic-targeted parvalbumin blocks the proliferation of multipotent mesenchymal stromal cells in prophase. Stem Cell Res Ther. 2013;4:92. doi: 10.1186/scrt291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammad-Gharibani P, Tiraihi T, Mesbah-Namin SA, Arabkheradmand J, Kazemi H. Induction of bone marrow stromal cells into GABAergic neuronal phenotype using creatine as inducer. Restor Neurol Neurosci. 2012;30:511–525. doi: 10.3233/RNN-2012-100155. [DOI] [PubMed] [Google Scholar]
- 31.Monypenny J, Zicha D, Higashida C, Oceguera-Yanez F, Narumiya S, Watanabe N. Cdc42 and Rac family GTPases regulate mode and speed but not direction of primary fibroblast migration during platelet-derived growth factor-dependent chemotaxis. Mol Cell Biol. 2009;29:2730–2747. doi: 10.1128/MCB.01285-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oudega M, Xu XM. Schwann cell transplantation for repair of the adult spinal cord. J Neurotrauma. 2006;23:453–467. doi: 10.1089/neu.2006.23.453. [DOI] [PubMed] [Google Scholar]
- 33.Pal R, Gopinath C, Rao NM, Banerjee P, Krishnamoorthy V, Venkataramana NK, Totey S. Functional recovery after transplantation of bone marrow-derived human mesenchymal stromal cells in a rat model of spinal cord injury. Cytotherapy. 2010;12:792–806. doi: 10.3109/14653249.2010.487899. [DOI] [PubMed] [Google Scholar]
- 34.Park SS, Byeon YE, Ryu HH, Kang BJ, Kim Y, Kim WH, Kang KS, Han HJ, Kweon OK. Comparison of canine umbilical cord blood- derived mesenchymal stem cell transplantation times: involvement of astrogliosis, inflammation, intracellular actin cytoskeleton pathways and neurotrophin-3. Cell Transplant. 2011;20:1867–1880. doi: 10.3727/096368911X566163. [DOI] [PubMed] [Google Scholar]
- 35.Ramos-Miguel A, García-Sevilla JA. Crosstalk between cdk5 and MEK-ERK signalling upon opioid receptor stimulation leads to upregulation of activator p25 and MEK1 inhibition in rat brain. Neuroscience. 2012;215:17–30. doi: 10.1016/j.neuroscience.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 36.Ren C, Liu X, Wan M, Geng D, Ge W, Li J, Zhang W. A comparative study on inducing non-homologous mesenchymal stem cells to differentiate into neural stem cells using non-homologous cerebrospinal fluid. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2013;30:1290–1297. [PubMed] [Google Scholar]
- 37.Simó S, Pujadas L, Segura MF, Torre AL, Del Río JA, Ureña JM, Comella JX, Soriano E. Reelin induces the detachment of postnatal subventricular zone cells and the expression of the Egr-1 through Erk1/2 activation. Cereb Cortex. 2007;17:294–303. doi: 10.1093/cercor/bhj147. [DOI] [PubMed] [Google Scholar]
- 38.Takai A, Bialojan C, Troschka M, Rüegg JC. Smooth muscle myosin phosphatase inhibition and force enhancement by black sponge toxin. FEBS Lett. 1987;217:81–84. doi: 10.1016/0014-5793(87)81247-4. [DOI] [PubMed] [Google Scholar]
- 39.Takei Y, Teng J, Harada A, Hirokawa N. Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J Cell Biol. 2000;150:989–1000. doi: 10.1083/jcb.150.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamama K, Kawasaki H, Wells A. Epidermal growth factor (EGF) treatment on multipotential stromal cells (MSCs). Possible enhancement of therapeutic potential of MSC. J Biomed Biotechnol 2010. 2010:795385. doi: 10.1155/2010/795385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, Fehlings MG, Kwon BK. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28:1611–1628. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torres-Espín A, Redondo-Castro E, Hernández J, Navarro X. Bone marrow mesenchymal stromal cells and olfactory ensheathing cells transplantation after spinal cord injury--a morphological and functional comparison in rats. Eur J Neurosci. 2014;39:1704–1717. doi: 10.1111/ejn.12542. [DOI] [PubMed] [Google Scholar]
- 43.Tsuchiya A, Kanno T, Shimizu T, Nakao S, Tanaka A, Tabata C, Nakano T, Nishizaki T. A novel PP2A enhancer induces caspase-independent apoptosis of MKN28 gastric cancer cells with high MEK activity. Cancer Lett. 2014;347:123–128. doi: 10.1016/j.canlet.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 44.Urdzíková L, Jendelová P, Glogarová K, Burian M, Hájek M, Syková E. Transplantation of bone marrow stem cells as well as mobilization by granulocyte-colony stimulating factor promotes recovery after spinal cord injury in rats. J Neurotrauma. 2006;23:1379–1391. doi: 10.1089/neu.2006.23.1379. [DOI] [PubMed] [Google Scholar]
- 45.Urdzíková L, Růži ka J, LaBagnara M, Kárová K, Kubinová Š, Jiráková K, Murali R, Syková E, Jhanwar-Uniyal M, Jendelová P. Human mesenchymal stem cells modulate inflammatory cytokines after spinal cord injury in rat. Int J Mol Sci. 2014;15:11275–11293. doi: 10.3390/ijms150711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Utreras E, Jiménez-Mateos EM, Contreras-Vallejos E, Tortosa E, Pérez M, Rojas S, Saragoni L, Maccioni RB, Avila J, González-Billault C. Microtubule-associated protein 1B interaction with tubulin tyrosine ligase contributes to the control of microtubule tyrosination. Dev Neurosci. 2008;30:200–210. doi: 10.1159/000109863. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Wang J, Bai D, Song J, Ye R, Zhao Z, Lei L, Hao J, Jiang C, Fang S, An S, Cheng Q, Li J. Cell proliferation is promoted by compressive stress during early stage of chondrogenic differentiation of rat BMSCs. J Cell Physiol. 2013;228:1935–1942. doi: 10.1002/jcp.24359. [DOI] [PubMed] [Google Scholar]
- 48.Wu J, Niu J, Li X, Li Y, Wang X, Lin J, Zhang F. Hypoxia induces autophagy of bone marrow-derived mesenchymal stem cells via activation of ERK1/2. Cell Physiol Biochem. 2014;33:1467–1474. doi: 10.1159/000358711. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Gong JF, Zhang W, Zhu WM, Li JS. Effects of transplanted bone marrow mesenchymal stem cells on the irradiated intestine of mice. J Biomed Sci. 2008;15:585–594. doi: 10.1007/s11373-008-9256-9. [DOI] [PubMed] [Google Scholar]
- 50.Zhang W, Shen ZY, Song HL, Yang Y, Wu BJ, Fu NN, Liu T. Protective effect of bone marrow mesenchymal stem cells in intestinal barrier permeability after heterotopic intestinal transplantation. World J Gastroenterol. 2014a;20:7442–7451. doi: 10.3748/wjg.v20.i23.7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Zhang H, Shao H, Xue Q, Yu B. ERK MAP kinase activation in spinal cord regulates phosphorylation of Cdk5 at serine 159 and contributes to peripheral inflammation induced pain/hypersensitivity. PLoS One. 2014b;9:e87788. doi: 10.1371/journal.pone.0087788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou FQ, Snider WD. Cell biology. GSK-3beta and microtubule assembly in axons. Science. 2005;308:211–214. doi: 10.1126/science.1110301. [DOI] [PubMed] [Google Scholar]


