Abstract
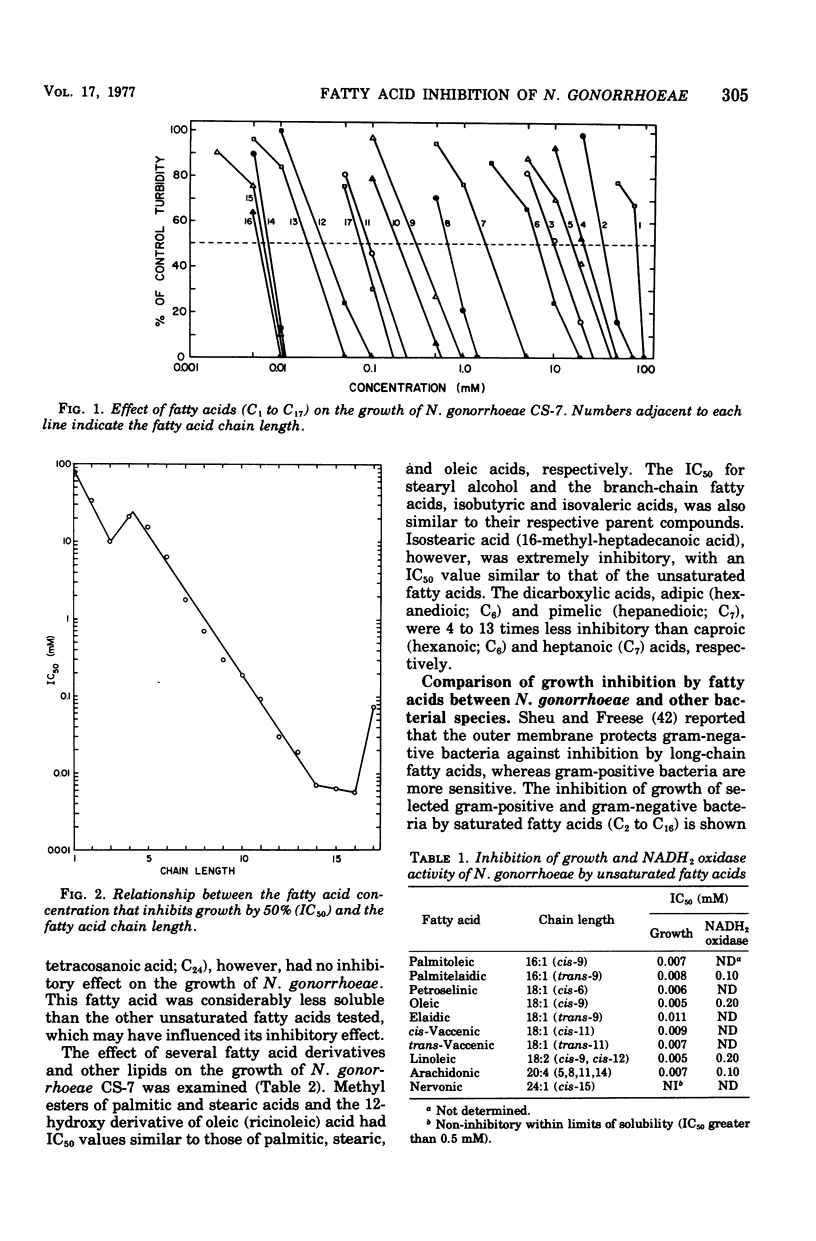
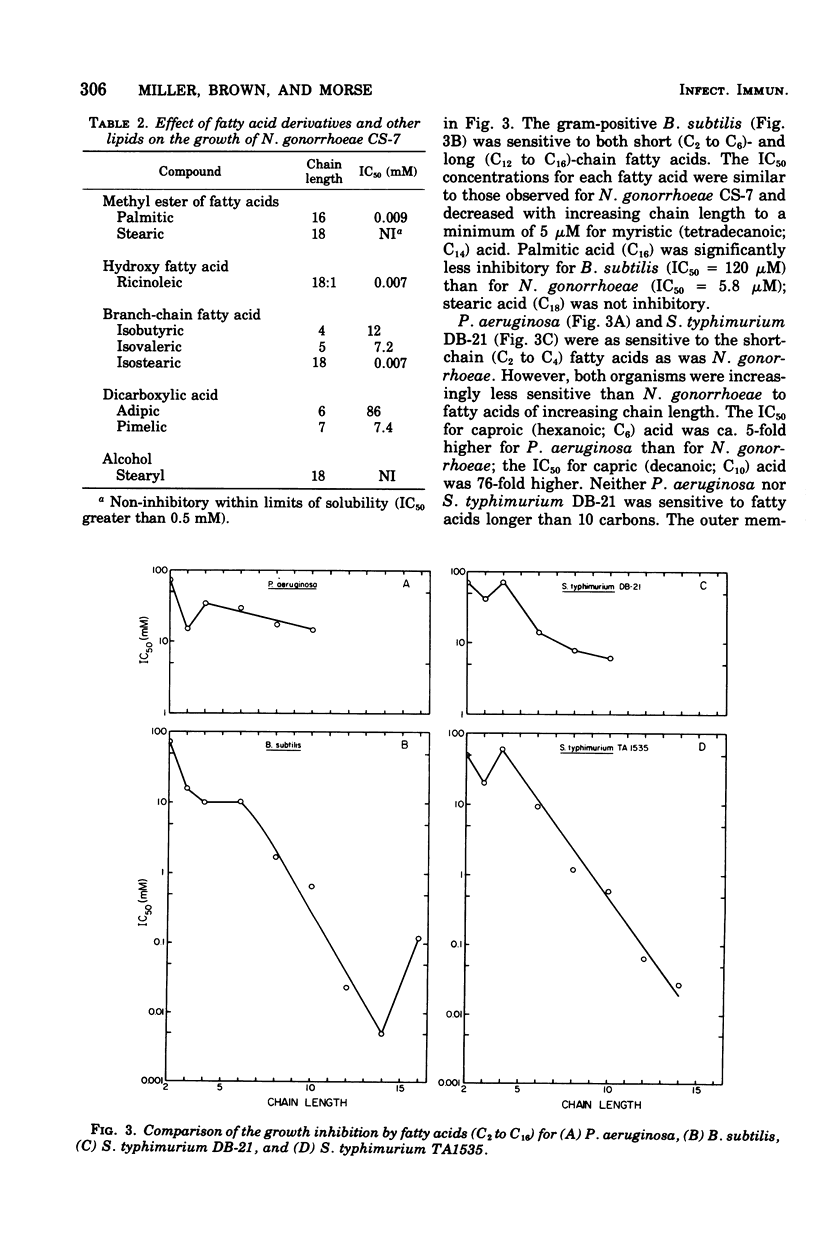
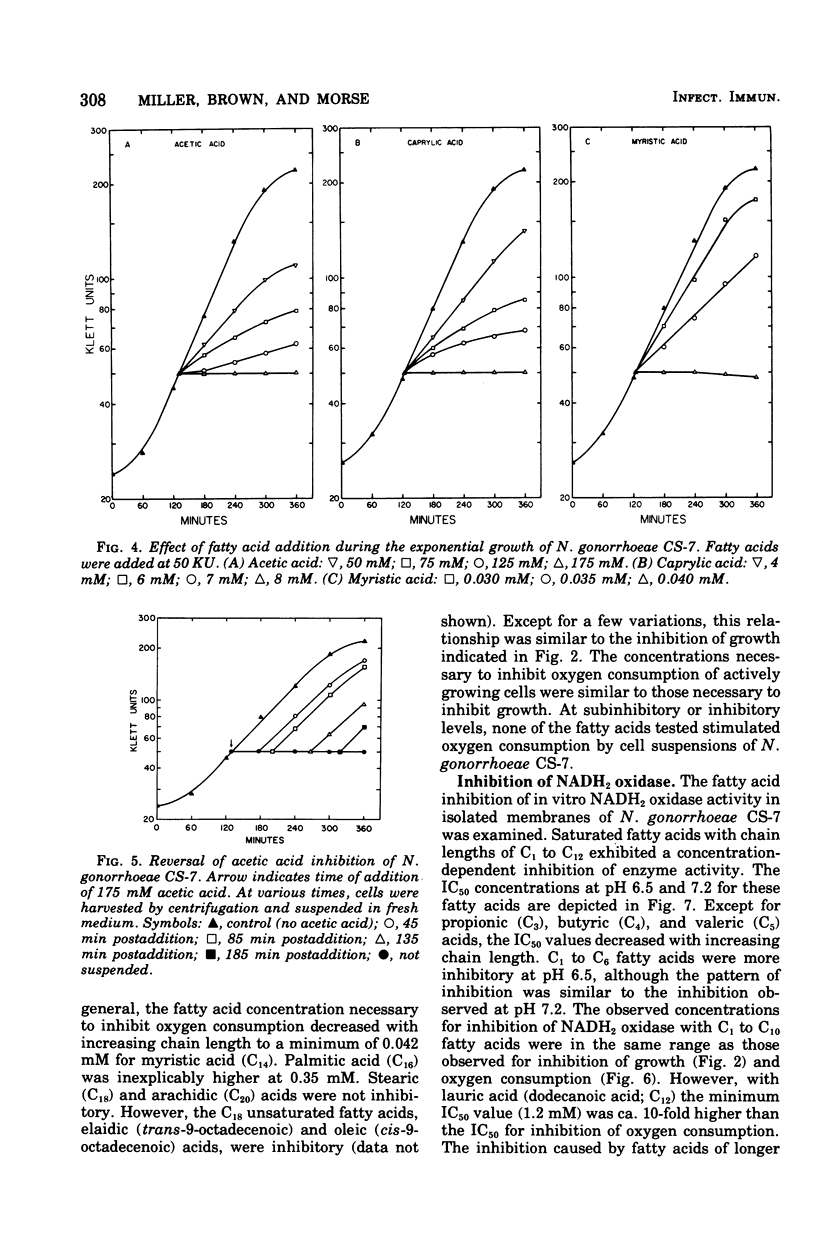
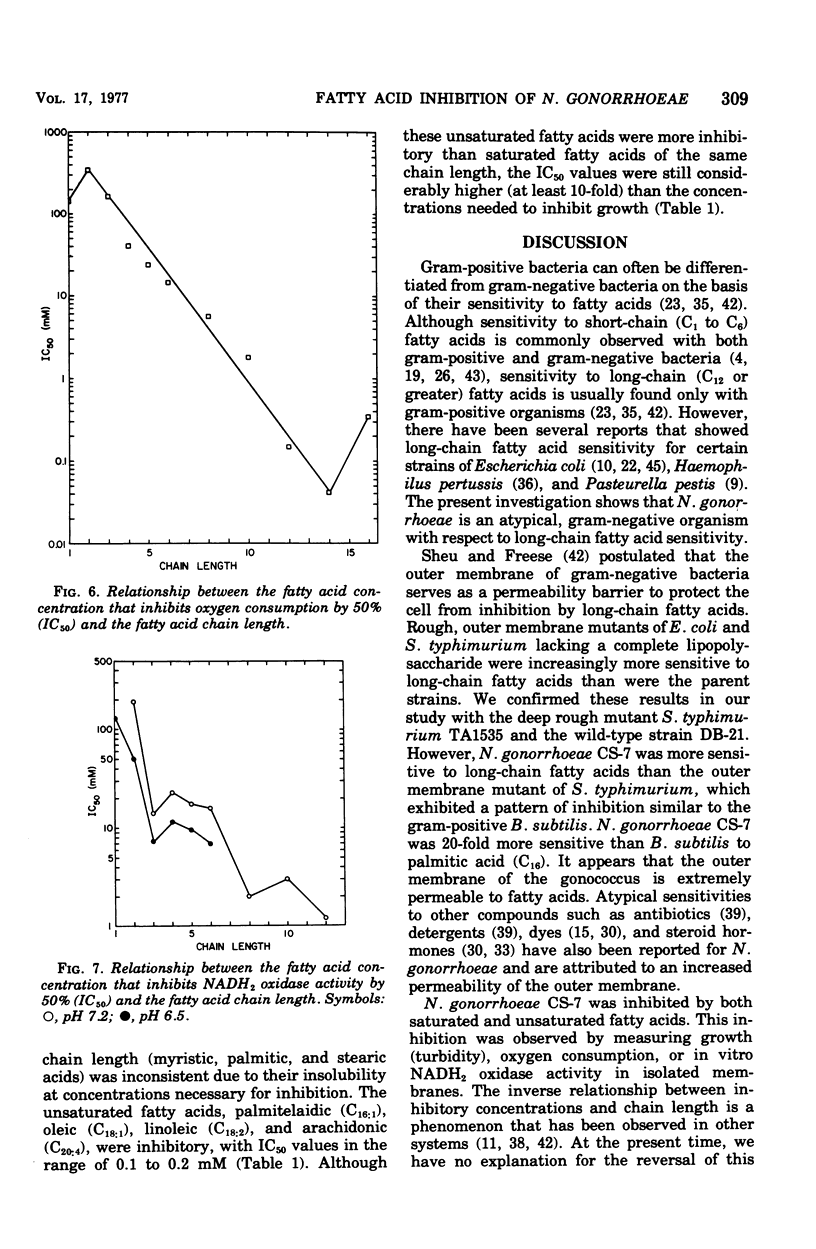
Fatty acids of various chain lengths (C1 to C24) were examined for their effects on growth, oxygen consumption, and in vitro reduced nicotinamide adenine dinucleotide oxidase activity of Neisseria gonorrhoeae CS-7. The growth inhibition caused by saturated fatty acids increased with increasing chain length to a maximum with palmitic acid (C16). Stearic acid (C18) and longer saturated fatty acids showed little inhibition of growth. However, unsaturated fatty acids of chain length C16 to C20 were inhibitory. Similar inhibition was observed with Bacillus subtilis and a deep rough mutant of Salmonella typhimurium. Wildtype S. typhimurium and Pseudomonas aeruginosa were more resistant to medium-chain (C7 to C10) fatty acids and completely resistant to long-chain (C12 to C18) fatty acids. Thus, sensitivity of N. gonorrhoeae to long-chain fatty acids appears to be related to the permeability of the outer membrane. Growth inhibition by short-chain (C1 to C6) fatty acids was pH dependent; inhibition of growth increased with decreasing pH. Saturated fatty acids inhibited oxygen consumption by log-phase cells of N. gonorrhoeae. This inhibition increased with increasing chain length to a maximum observed with myristic acid (C14). Whereas stearic acid (C18) had little effect upon oxygen consumption, unsaturated C18 fatty acids were inhibitory. An in vitro inhibition of reduced nicotinamide adenine dinucleotide oxidase activity by saturated (C1 to C12) and unsaturated (C16 to C20) fatty acids was also observed. Although the inhibitory concentrations were generally higher than those required to inhibit growth or oxygen consumption, an inhibition of electron transport may be partially responsible for the observed growth inhibition.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORST P., LOOS J. A., CHRIST E. J., SLATER E. C. Uncoupling activity of long-chain fatty acids. Biochim Biophys Acta. 1962 Aug 27;62:509–518. doi: 10.1016/0006-3002(62)90232-9. [DOI] [PubMed] [Google Scholar]
- Borst-Pauwels G. W., Jager S. Inhibition of phosphate and arsenate uptake in yeast by monoiodoacetate, fluoride, 2,4-dinitrophenol and acetate. Biochim Biophys Acta. 1969 Apr 8;172(3):399–406. doi: 10.1016/0005-2728(69)90136-4. [DOI] [PubMed] [Google Scholar]
- Brooks J. B., Kellogg D. S., Thacker L., Turner E. M. Analysis by gas chromatography of fatty acids found in whole cultural extracts of Neisseria species. Can J Microbiol. 1971 Apr;17(4):531–543. doi: 10.1139/m71-088. [DOI] [PubMed] [Google Scholar]
- CAMIEN M. N., DUNN M. S. Potassium acetate inhibition of Lactobacillus casei and its reversal by lithium, sodium and fatty acids. Proc Soc Exp Biol Med. 1957 Aug-Sep;95(4):697–700. doi: 10.3181/00379727-95-23334. [DOI] [PubMed] [Google Scholar]
- Cruess W. V., Irish J. H. Further Observations on the Relation of pH Value to Toxicity of Preservatives to Microörganisms. J Bacteriol. 1932 Feb;23(2):163–166. doi: 10.1128/jb.23.2.163-166.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS S. W., BALL E. G. The action of phospholipases on succinate oxidase and cytochrome oxidase. J Biol Chem. 1954 Aug;209(2):619–633. [PubMed] [Google Scholar]
- Eisler D. M., Von Metz E. K. Anti-Pasteurella pestis factor. 3. Effects of fatty acids on Pasteurella pestis. J Bacteriol. 1968 May;95(5):1767–1773. doi: 10.1128/jb.95.5.1767-1773.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay J. P., Farias R. N. The inhibitory action of fatty acids on the growth of Escherichia coli. J Gen Microbiol. 1975 Dec;91(2):233–240. doi: 10.1099/00221287-91-2-233. [DOI] [PubMed] [Google Scholar]
- Ferdinandus J., Clark J. B. Selective inhibition of bacterial enzymes by free fatty acids. J Bacteriol. 1969 Jun;98(3):1109–1113. doi: 10.1128/jb.98.3.1109-1113.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Sheu C. W., Galliers E. Function of lipophilic acids as antimicrobial food additives. Nature. 1973 Feb 2;241(5388):321–325. doi: 10.1038/241321a0. [DOI] [PubMed] [Google Scholar]
- Guymon L. F., Sparling P. F. Altered crystal violet permeability and lytic behavior in antibiotic-resistant and -sensitive mutants of Neisseria gonorrhoeae. J Bacteriol. 1975 Nov;124(2):757–763. doi: 10.1128/jb.124.2.757-763.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDESTY B. A., MITCHELL H. K. The interaction of fatty acids with mammalian cytochrome c. Arch Biochem Biophys. 1963 Jan;100:1–8. doi: 10.1016/0003-9861(63)90025-0. [DOI] [PubMed] [Google Scholar]
- HARDWICK W. A., GUIRARD B., FOSTER J. W. Antisporulation factors in complex organic media. II. Saturated fatty acids as antisporulation factors. J Bacteriol. 1951 Feb;61(2):145–151. doi: 10.1128/jb.61.2.145-151.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULSMANN W. C., ELLIOTT W. B., SLATER E. C. The nature and mechanism of action of uncoupling agents present in mitochrome preparations. Biochim Biophys Acta. 1960 Apr 8;39:267–276. doi: 10.1016/0006-3002(60)90163-3. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Hentges D. J. Influence of pH on the inhibitory activity of formic and acetic acids for Shigella. J Bacteriol. 1967 Jun;93(6):2029–2030. doi: 10.1128/jb.93.6.2029-2030.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A., Arima K. Inhibitory effect of sucrose ester of lauric acid on the growth of Escherichia coli. Biochem Biophys Res Commun. 1971 Feb 19;42(4):596–601. doi: 10.1016/0006-291x(71)90529-8. [DOI] [PubMed] [Google Scholar]
- Kodicek E. The effect of unsaturated fatty acids on Lactobacillus helveticus and other Gram-positive micro-organisms. Biochem J. 1945;39(1):78–85. doi: 10.1042/bj0390078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus S. J., Geller R. C., Perkins G. H., Rhoden D. L. Interference by Neisseria gonorrhoeae growth by other bacterial species. J Clin Microbiol. 1976 Sep;4(3):288–295. doi: 10.1128/jcm.4.3.288-295.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARDY H. A., PRESSMAN B. C. Effect of surface active agents on the latent ATPase of mitochondria. Biochim Biophys Acta. 1956 Sep;21(3):458–466. doi: 10.1016/0006-3002(56)90182-2. [DOI] [PubMed] [Google Scholar]
- LASER H. The isolation of a haemolytic substance from animal tissues and its biological properties. J Physiol. 1949 Dec;110(3-4):338–355. doi: 10.1113/jphysiol.1949.sp004443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levison M. E. Effect of colon flora and short-chain fatty acids on growth in vitro of Pseudomonas aeruginsoa and Enterobacteriaceae. Infect Immun. 1973 Jul;8(1):30–35. doi: 10.1128/iai.8.1.30-35.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley H. L., Jr, Mueller J. H. On the Isolation from Agar of an Inhibitor for Neisseria gonorrhoeae. J Bacteriol. 1946 Oct;52(4):453–460. doi: 10.1128/jb.52.4.453-460.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael R. P., Bonsall R. W., Warner P. Human vaginal secretions: volatile fatty acid content. Science. 1974 Dec 27;186(4170):1217–1219. doi: 10.1126/science.186.4170.1217. [DOI] [PubMed] [Google Scholar]
- Miller R. D., Morse S. A. Binding of progesterone to Neisseria gonorrhoeae and other gram-negative bacteria. Infect Immun. 1977 Apr;16(1):115–123. doi: 10.1128/iai.16.1.115-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974 Apr;145(4):1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Fitzgerald T. J. Effect of progesterone on Neisseria gonorrhoeae. Infect Immun. 1974 Dec;10(6):1370–1377. doi: 10.1128/iai.10.6.1370-1377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Stein S., Hines J. Glucose metabolism in Neisseria gonorrhoeae. J Bacteriol. 1974 Nov;120(2):702–714. doi: 10.1128/jb.120.2.702-714.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEMAN C. Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol Rev. 1954 Jun;18(2):147–163. doi: 10.1128/br.18.2.147-163.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMSON F. E., KATZ A. M., HARRIS D. L. Effects of acetate and other short-chain fatty acids on yeast metabolism. Arch Biochem Biophys. 1955 Feb;54(2):406–423. doi: 10.1016/0003-9861(55)90054-0. [DOI] [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Sparling P. F., Blackman E., Lewis E. Loss of low-level antibiotic resistance in Neisseria gonorrhoeae due to env mutations. J Bacteriol. 1975 Nov;124(2):750–756. doi: 10.1128/jb.124.2.750-756.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senff L. M., Wegener W. S., Brooks G. F., Finnerty W. R., Makula R. A. Phospholipid composition and phospholipase A activity of Neisseria gonorrhoeae. J Bacteriol. 1976 Aug;127(2):874–880. doi: 10.1128/jb.127.2.874-880.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu C. W., Freese E. Effects of fatty acids on growth and envelope proteins of Bacillus subtilis. J Bacteriol. 1972 Aug;111(2):516–524. doi: 10.1128/jb.111.2.516-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu C. W., Freese E. Lipopolysaccharide layer protection of gram-negative bacteria against inhibition by long-chain fatty acids. J Bacteriol. 1973 Sep;115(3):869–875. doi: 10.1128/jb.115.3.869-875.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu C. W., Konings W. N., Freese E. Effects of acetate and other short-chain fatty acids on sugar and amino acid uptake of Bacillus subtilis. J Bacteriol. 1972 Aug;111(2):525–530. doi: 10.1128/jb.111.2.525-530.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater E. C. The action of inhibitors on the system of enzymes which catalyse the aerobic oxidation of succinate. Biochem J. 1949;45(1):8–13. doi: 10.1042/bj0450008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERESI J. D., LUCK J. M. The combination of organic anions with serum albumin. VIII. Fatty acid salts. J Biol Chem. 1952 Feb;194(2):823–834. [PubMed] [Google Scholar]
- WOJTCZAK L., WOJTCZAK A. B. Uncoupling of oxidative phosphorylation and inhibition of ATP-Pi exchange by a substance from insect mitochondria. Biochim Biophys Acta. 1960 Apr 8;39:277–286. doi: 10.1016/0006-3002(60)90164-5. [DOI] [PubMed] [Google Scholar]
- Walstad D. L., Reitz R. C., Sparling P. F. Growth inhibition among strains of Neisseria gonorrhoeae due to production of inhibitory free fatty acids and lysophosphatidylethanolamine: absence of bacteriocins. Infect Immun. 1974 Sep;10(3):481–488. doi: 10.1128/iai.10.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbach E. C., Garbus J. Mechanism of action of reagents that uncouple oxidative phosphorylation. Nature. 1969 Mar 15;221(5185):1016–1018. doi: 10.1038/2211016a0. [DOI] [PubMed] [Google Scholar]


