Abstract
Edaravone has been shown to reduce ischemia/reperfusion-induced peripheral nerve injury. However, the therapeutic effect of edaravone on peripheral nerve injury caused by mechanical factors is unknown. In the present study, we established a peripheral nerve injury model by crushing the sciatic nerve using hemostatic forceps, and then administered edaravone 3 mg/kg intraperitoneally. The sciatic functional index and superoxide dismutase activity of the sciatic nerve were increased, and the malondialdehyde level was decreased in animals in the edaravone group compared with those in the model group. Bcl-2 expression was increased, but Bax expression was decreased in anterior horn cells of the L4-6 spinal cord segments. These results indicated that edaravone has a neuroprotective effect following peripheral nerve injury caused by mechanical factors through alleviating free radical damage to cells and inhibiting lipid peroxidation, as well as regulating apoptosis-related protein expression.
Keywords: nerve regeneration, peripheral nerve injury, mechanical injury, sciatic nerve injury, edaravone, lipid peroxidation, free radical damage, malondialdehyde, superoxide dismutase, Bcl-2, Bax, NSFC grant, neural regeneration
Introduction
Peripheral nerve injury is usually followed by Wallerian degeneration as well as neuronal apoptosis (Wang et al., 2012b). Thus, even if we could repair the injured peripheral nerve, various factors, especially those responsible for neuronal apoptosis, could prevent the satisfactory recovery of the injured peripheral nerve. So, how to prevent neuronal death at an early stage so as to promote the recovery of injured nerves is a hot issue at present.
One of the most important factors for apoptosis is free radicals, especially oxygen radicals. More recently, it has been shown that reactive oxygen species generated in injured peripheral nerves could increase lipid peroxidation, which alters enzymes and structural proteins, directing the cell toward an apoptotic pathway (Casares et al., 2012). Edaravone is a potent free radical scavenger of hydroxyl radicals (Watanabe et al., 2008; Lapchak, 2010; Kikuta et al., 2013). Edaravone is currently widely used in neurology departments for recovery from cerebral infarctions in humans (Ikeda et al., 2013). It was reported that edaravone can attenuate peripheral nerve injury induced by ischemia/reperfusion (Iida et al., 2009). Furthermore, an analgesic effects of edaravone on neuropathic pain in rats was demonstrated (Mao et al., 2009). Hence, little is known about the therapeutic role of edaravone in peripheral nerve injury caused by mechanical factors. The objective of the present study was to study the effect of edaravone following peripheral nerve injury caused by mechanical factors by making a rat model of sciatic nerve injury.
Materials and Methods
Animals
Thirty-six clean healthy adult Sprague-Dawley rats, weighing 185 ± 10 g, irrespective of gender, were provided by the Animal Experimental Center of Dalian Medical University, China (license No. SCXK (Liao) 2012-0002). During the trial period, standard laboratory conditions of Dalian Medical University were provided in a 12-hour light/dark cycle, at 20–22°C. The feeding and management conditions were the same for all rats. The rats were given as much food and water as they could consume and food and water were delivered by the same breeder. Until execution, all operative incisions on the rats healed well. Animal experiments were approved by the Animal Ethics Committee of Dalian Medical University, China. A total of 36 rats were assigned equally and randomly to a sham surgery group, a model group and an edaravone group.
Establishment of the injured sciatic nerve model
Chloral hydrate (10%) was administered intraperitoneally for anesthesia at a dose of 350 mg/kg. After shaving and preparing the operation area at the right hip of a rat with 5% iodine, the rat was placed in the prone position. The sciatic nerve was exposed by opening the fascial plane between the gluteal and femoral musculature via a longitudinal incision. Following the dissection of the gluteus maximus, the right sciatic nerve was exposed. Hemostatic forceps were used to crush the mid-point of right sciatic nerve three times, each time for 10 seconds, with an interval of 10 seconds. Similar methods have been previously reported (Gao et al., 2008; Cheng et al., 2013; Suslu et al., 2013). Then, we marked the injured area of the sciatic nerve with a micro-suture. Acute blunt sciatic nerve injury was induced by the sciatic nerve crush technique, which allowed us to perform a standard direct trauma in each rat, and which also resulted in a lesion similar to those seen in patients with peripheral nerve injury. Paralysis of the right shank and right foot of rats indicated successful modeling. All surgeries were performed by the same investigator using the same forceps at the same position of the sciatic nerve (10 mm distal to the sciatic notch). In the sham surgery group, after the sciatic nerves were exposed, we only gave the sciatic nerve a simulative traction.
Drug administration
In the edaravone group, after making the injured sciatic nerve model, we administered rats edaravone (chemical name: 3-methyl-1-phenyl-2-pyrazolin-5-one, chemical formula: C10H10N2O, Jiangsu Simcere Pharmaceutical Co., Ltd., license No. H20031342) at a dose of 3 mg/kg per day, intraperitoneally, for 2 weeks. The required amount of edaravone was diluted with normal saline to a total volume of 1 mL. In the model group, we administered rats intraperitoneally 1 mL of saline per day for the same period.
General assessment
At 2 weeks after modeling, we performed a general assessment. The general assessment included: fur loss and palsy of the right lower extremity, gait and limb activity of rats, areflexia of claw-spreading and muscular atrophy.
Measurement of the sciatic functional index
At 1 and 2 weeks after modeling, the following method was used to test sciatic function in the rats. This method describes an index based on measurements of the footprints of walking rats, and provides a reliable and easily quantifiable method for evaluating the functional condition of the sciatic nerve (Koka and Hadlock, 2001). For this test, the rats, whose plantar hind feet were dyed with blue ink, were trained to walk over a white sheet of paper covering the bottom of a 50-cm-long, 10-cm-wide box. The rat footprints were used to determine the following measurements: distance from the heel to the third toe [print length (PL)], distance from the first to the fifth toe [toe spread (TS)], and distance from the second to the fourth toe [intermediary toe spread (ITS)]. These three measurements were obtained from both the experimental (E) and normal (N) sides of the animal. Bilateral footprints were clearly measured and recorded once a week, and the parameters were put into the sciatic functional index formula as follows: sciatic functional index = −38.3(EPL –NPL)/NPL + 109.5(ETS – NTS)/NTS + 13.3(EITS – NITS)/NITS − 8.8. The result obtained was considered a functional index of the sciatic nerve, where the normal value is 0. When the sciatic nerve was totally denervated, the value of the sciatic functional index is −100.
Specimen collection
At 2 weeks after modeling, the sciatic nerve was exposed again in the same way as mentioned above. Specimens of the sciatic nerve tissue at the point 1 cm away from the injured site were obtained. The sciatic nerve tissue was stored at −70°C after being washed with brine ice. The heart was exposed and then a catheter was inserted into the left ventricle, through which normal saline was constantly injected until the liquid flowing out from the auricula dextra was clear. The rats were transcardially fixed with 4% paraformaldehyde for 24 hours. The L4–6 spinal cord was obtained and fixed in 10% paraformaldehyde buffer solution for 24 hours, dehydrated in an ethanol series, and embedded in paraffin. After that, 5-mm thick sections including spinal cord were collected for immunohistochemical staining.
Measurement of superoxide dismutase activity and malondialdehyde level in rat sciatic nerve by spectrophotometry
Preparation of 10% sciatic nerve tissue homogenate: superoxide dismutase test kit was purchased from Nanjing KeyGEN Biotech. Co., Ltd., China. Superoxide dismutase activity was measured using the xanthine oxidase test (Su et al., 2003; Liu and Lei, 2013; Zhou and Zheng, 2013). Superoxide dismutase activity in tissue homogenate was calculated as follows: superoxide dismutase activity (U/mg) = [(absorbance of the control tube − absorbance of the test tube)/absorbance of the control tube]/50% × (total volume of the reaction liquid/the volume of the sample)/protein content of the tissue (mg/mL).
Preparation of 10% sciatic nerve tissue homogenate: An malondialdehyde test kit was purchased from Nanjing KeyGEN Biotech. Co., Ltd. Using thibabituric acid as the substrate, the malondialdehyde level was measured. The product of the condensation reaction between malondialdehyde and thibabituric acid has a maximum absorption at 532 nm by spectrophotometer (Shanghai Jingke Industrial Co., Ltd., China) (Rahmat et al., 2004; Grotto et al., 2007; Candan and Tuzmen, 2008).
Immunohistochemistry of apoptotic factors Bcl-2 and Bax expression in L4–6 spinal anterior horn cells of rats
The paraffin sections of rat L4–6 spinal anterior horn were dewaxed with xylene, dehydrated in an ethanol series, and incubated with 3% hydrogen peroxide solution for 30 minutes at room temperature to block endogenous peroxidase activity. After cleaning the sections with distilled water, a microwave was used for tissue antigen retrieval. The buffer solution was cooled and the section was taken out. The sections were cleaned with distilled water, and put into PBS for 5 minutes. After PBS was wiped off, the appropriate amount of sheep serum was added into the section at room temperature for 30 minutes. After removal of the serum, the sections were incubated with rabbit anti-rat Bcl-2 monoclonal antibody (1:100; Nanjing KeyGEN Biotech. Co., Ltd.) and rabbit anti-rat Bax monoclonal antibody (1:100; Nanjing KeyGEN Biotech. Co., Ltd.) at 4°C overnight. The sections were placed at room temperature for 30 minutes, and rinsed with PBS (pH 7.4) three times, each for 5 minutes. After removal of PBS, the sections were incubated with goat anti-rabbit IgG (1:100; Nanjing KeyGEN Biotech. Co., Ltd.) at 37°C for an hour and a half, and rinsed three times with PBS (pH 7.4), each for 5 minutes. After removal of PBS, the sections were visualized with 3,3′-diaminobenzidine (Beijing Zhongbin Biotech. Co., Ltd.), and observed under a microscope to visualize development of color. Following rinsing with distilled water and running water successively, the sections were counterstained with hematoxylin, permeabilized with xylene, mounted with resin, and treated with a decreasing ethanol series. Particles that were specifically stained on a clear background under a light microscope (Carton optical microscope, Ningbo, Zhejiang Province, China) represented Bcl-2 and Bax labelling, and the cell nucleus was dyed blue. Light microscopy images were collected using an Image Acquisition System (Neusoft, Dalian, Liaoning Province, China). Three 200-fold fields were randomly selected in each section; the amount of positive cells per hundred cells was counted, and the average value was calculated using the Image Acquisition System.
Statistical analysis
Data were presented as the mean ± SD and were analyzed using SPSS 16.0 (SPSS, Chicago, IL, USA). Statistical significance was determined by one-way analysis of variance followed by Tukey's post hoc test. A value of P < 0.05 was considered statistically significant.
Results
Edaravone improved the behavior of rats following mechanical peripheral nerve injury
The general assessment of rats in the sham surgery group at 2 weeks after modeling was almost the same as before surgery: the muscle strength of the right foot and toes and reflex of the claw-spreading, as well as the appearance of fur and muscle on the right lower extremity were normal. Two weeks after modeling, complete palsy of the right foot and toes, areflexia of the claw-spreading, as well as fur loss and neuralgic amyotrophy on the right lower extremity were seen in rats in the model group. The symptoms mentioned above improved in the rats in the edaravone group.
The sciatic functional index of animals in the sham surgery group after 14 days was almost the same as that before surgery, which was nearly normal. After 2 weeks of edaravone administration, the sciatic functional index in rats with sciatic nerve injury increased (P < 0.05), and their footprints became clearer (Figure 1), revealing that edaravone improves the right lower extremity function of rats whose right sciatic nerve is mechanically injured.
Figure 1.
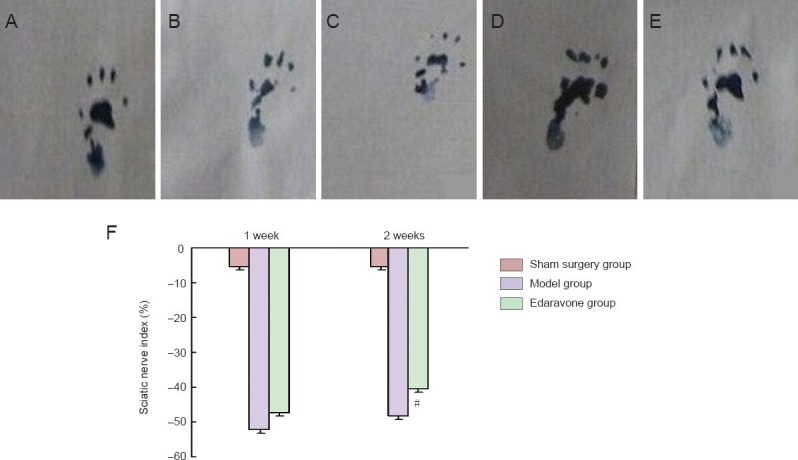
Effect of edaravone on the sciatic functional index of rats with mechanical peripheral nerve injury.
(A–E) The right footprint of normal rats, rats in the model group at 1 and 2 weeks after modeling, and rats in the edaravone group at 1 and 2 weeks after modeling. (F) Sciatic functional index in the model, edaravone and sham surgery groups. The result obtained was considered a functional index of the sciatic nerve, whose normal value is 0. When the sciatic nerve is totally denervated, the value of sciatic functional index is –100. Data were presented as the mean ± SD, and there were 12 rats in each group. #P < 0.05, vs. model group. Statistical significance was determined by one-way analysis of variance followed by Tukey's post hoc test.
Edaravone increased superoxide dismutase activity and malondialdehyde level in the sciatic nerves of rats with mechanical peripheral nerve injury
Spectrophotometry results showed that, after 2 weeks of edaravone administration, the superoxide dismutase activity was increased (P < 0.05), but the malondialdehyde level was decreased in rats with sciatic nerve injury (P < 0.05; Figure 2).
Figure 2.
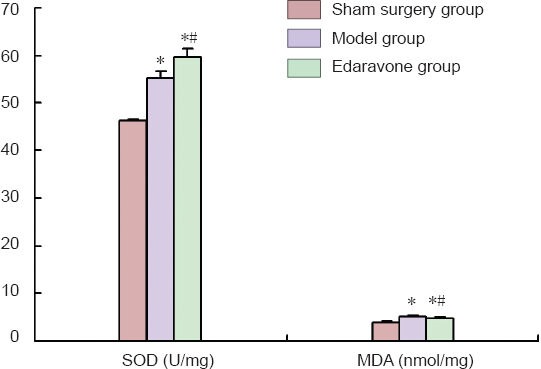
Effect of edaravone on superoxide dismutase (SOD) activity and malondialdehyde (MDA) level in the sciatic nerves of rats with mechanical peripheral nerve injury.
Data were presented as the mean ± SD, and there were 12 rats in each group. *P < 0.05, vs. sham surgery group; #P < 0.05, vs. model group. Statistical significance was determined by one-way analysis of variance followed by Tukey's post hoc test.
Effect of edaravone on expression of the apoptotic factors Bcl-2 and Bax in the L4–6 spinal anterior horn cells of rats with mechanical peripheral nerve injury
Immunohistochemical staining revealed that, after 2 weeks of edaravone administration, Bcl-2 expression was increased (P < 0.05), but Bax expression was decreased in L4–6 spinal anterior horn cells in rats with sciatic nerve injury (P < 0.05; Figure 3).
Figure 3.
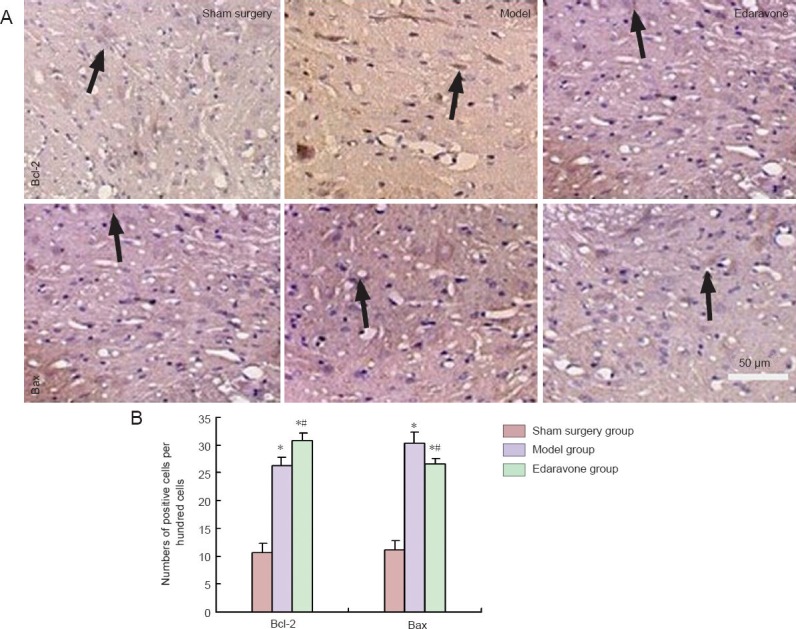
Effect of edaravone on expression of the apoptotic factors Bcl-2 and Bax in the L4–6 spinal anterior horn cells of rats with mechanical peripheral nerve injury (immunohistochemistry staining).
(A) Expression of Bcl-2 and Bax in anterior horn cells of L4–6 spinal cord segments (light microscope, × 200). Arrows indicate positive cells. (B) Amounts of Bcl-2- and Bax-positive cells among anterior horn cells of L4–6 spinal cord segments. Data were presented as the mean ± SD, and there were 12 rats in each group. *P < 0.05, vs. sham surgery group; #P < 0.05, vs. model group. Statistical significance was determined by one-way analy-sis of variance followed by Tukey's post hoc test.
Discussion
Edaravone is used to treat many diseases, such as acute cerebral infarction and ischemia/reperfusion-induced nerve injury, through scavenging free radicals. Hence, we deduced that edaravone could also be applied to the treatment of mechanical peripheral nerve injury. In the present study, we evaluated the effect of edaravone on a peripheral nerve injury caused by mechanical factors in a rat sciatic nerve injured by hemostatic forceps. This model was chosen because it was verified that these forceps could be used to establish mechanical peripheral nerve injury models (Xu and Li, 2008; Bauder and Ferguson, 2012; Wang et al., 2012a). This study confirmed that edaravone improved the general condition, lowered malondialdehyde levels and Bcl-2 expression, and enhanced superoxide dismutase activity and Bax expression after 2 weeks of administration, indicating that edaravone can promote recovery from mechanical peripheral nerve injury at an early stage.
Complete palsy of the right foot and toes, areflexia of the claw-spreading, as well as fur loss and muscular atrophy on the right lower extremity indicate the loss of any neurotrophic effect owing to denervation of sciatic nerves after injury (Wang et al., 2004). In particular, muscular atrophy, which is mainly caused by motor nerve injury (Zhao et al., 2012), can be observed quite obviously. After two weeks of edaravone treatment, the symptoms mentioned above had improved. Several studies have provided evidence that edaravone exhibits a neuroprotective effect by enhancing the expression of brain-derived neurotrophic factors (Lee et al., 2010; Wang et al., 2013). Moreover, edaravone can increase the expression of nerve growth factor in human astrocytes subjected to hypoxia/reoxygenation (Yoshida et al., 2010). Hence, based on the above result, it is possible that edaravone has a neurotrophic effect on sciatic nerve injured by mechanical factors. The sciatic functional index is a direct behavioral indicator for sciatic nerve functional recovery and has long been the standard method for assessing motor recovery in rat sciatic nerve models (Kaplan et al., 2014; Tamaddonfard et al., 2014). In the second week after injury, the sciatic functional index of the edaravone group rats was better than that of the model group, indicating an effect of edaravone on functional recovery following sciatic nerve injury.
Superoxide dismutase is an antioxidant enzyme that can reduce products of lipid peroxidation such as malondialdehyde (Ghyasi et al., 2012; Di Cesare Mannelli et al., 2013; Gao et al., 2013). Lipid peroxidation products can induce upregulation of reactive oxygen species formation (Ambrozova et al., 2011). Under physiological conditions, superoxide dismutase is effectively able to dismutate superoxide anion free radical into hydrogen peroxide and dioxygen; as a result, the toxic effect of the superoxide anion free radical on cells is eliminated. Superoxide dismutase is often used to evaluate the free radical scavenging ability of medicines as well as to attenuate oxidative damage such as radiation-induced lung injury in mice (Pan et al., 2012; Xu et al., 2012). Malondialdehyde, a degraded product of lipid peroxidation, can also produce cytotoxicity (Yang et al., 2014). Compared with other sources of lipid peroxidation, malondialdehyde has a longer life span. Hence, changes in malondialdehyde levels and superoxide dismutase activity are indicators of the degree of lipid peroxidation and, therefore, reflect the severity of tissue damage (Ye et al., 2011; Turkoglu et al., 2012). Edaravone has potent radical scavenging activity (Kamogawa and Sueishi, 2014). Edaravone can eliminate hydrogen oxide radicals that trigger lipid peroxidation and subsequently inhibit the initiation and progression of lipid peroxidation induced by hydrogen peroxide radicals (Gutteridge, 1984). Our data revealed that edaravone significantly lowered malondialdehyde levels and enhanced superoxide dismutase activity in sciatic nerve tissue. This finding suggests that edaravone could promote recovery following mechanical sciatic nerve injury in rats, at the early stage, by scavenging free radicals and inhibiting lipid peroxidation.
Bax and Bcl-2 are two important members of the Bcl-2 gene family involved in the regulation of cellular apoptosis at an early stage (Gillies and Kuwana, 2014; Mignard et al., 2014). In vivo manipulation of their expression has identified Bcl-2 protein as an anti-apoptosis factor for the early stage of apoptosis (Hockenbery et al., 1990; Niture and Jaiswal, 2012), while the Bax protein is an pro-apoptosis factor (Yang and Korsmeyer, 1996). Experimental evidence suggests that Bcl-2 protein must be in equilibrium with Bax protein in most cells. Drugs stabilizing this equilibrium can keep cells alive (Korbakis and Scorilas, 2012). On the contrary, drugs shifting the equilibrium toward free Bax can induce apoptosis. Studies using nerve cells (Zhao et al., 2013) and animal models (Shimoda et al., 2012) have shown that edaravone can prevent apoptosis, findings that have already been applied to the treatment of many diseases such as Parkinson's disease and cardiovascular disease (Kikuchi et al., 2013; Li et al., 2013). The results of our study suggested that edaravone exerts a neuroprotective effect against behavioral functional loss after sciatic nerve injury at an early stage, which is partly mediated through inhibition of Bcl-2/Bax apoptotic pathways by suppressing Bax protein overexpression and increasing the level of Bcl-2 protein. What is more, oxidative stress can accelerate the apoptotic process (Zhao and Wang, 2012; Deramaudt et al., 2013; Walluscheck et al., 2013; Zhou et al., 2013). Hence, edaravone slows down the apoptotic process via its anti-oxidative mechanism (Chen et al., 2012).
Based on the above results, we draw the conclusion that edaravone promotes regeneration of peripheral nerve injury caused by mechanical factors at an early age through alleviating free radical damage to cells, then inhibiting lipid peroxidation and celluar apoptotic effects.
This study had certain limitations. First, the study was not sufficiently powered to conclusively analyze the optimal edaravone dose or the optimal length of edaravone use. More studies are needed to identify the best dose, therapeutic period, safety and efficacy of edaravone for its clinical use in treating mechanical peripheral nerve injury. Second, we did not dissect out the mechanism of action of edaravone in improving function following peripheral nerve injury. Although edaravone is far from being used in patients with mechanical peripheral nerve injury, the present study provides basic theoretical foundation for it.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 30901515; Dalian Municipal Science and Technology Project Foundation in China, No. 2009J22DW029.
Conflicts of interest: None declared.
Copyedited by McGowan D, Robens J, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Ambrozova G, Pekarova M, Lojek A. The effect of lipid peroxidation products on reactive oxygen species formation and nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 macrophages. Toxicol In Vitro. 2011;25:145–152. doi: 10.1016/j.tiv.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Bauder AR, Ferguson TA. Reproducible mouse sciatic nerve crush and subsequent assessment of regeneration by whole mount muscle analysis. J Vis Exp. 2012 doi: 10.3791/3606. doi:10.3791/3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Candan N, Tuzmen N. Very rapid quantification of malondialdehyde (MDA) in rat brain exposed to lead, aluminium and phenolic antioxidants by high-performance liquid chromatography-fluorescence detection. Neurotoxicology. 2008;29:708–713. doi: 10.1016/j.neuro.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Casares C, Ramírez-Camacho R, Trinidad A, Roldán A, Jorge E, García-Berrocal JR. Reactive oxygen species in apoptosis induced by cisplatin: review of physiopathological mechanisms in animal models. Eur Arch Otorhinolaryngol. 2012;269:2455–2459. doi: 10.1007/s00405-012-2029-0. [DOI] [PubMed] [Google Scholar]
- 5.Chen SD, Yin JH, Hwang CS, Tang CM, Yang DI. Anti-apoptotic and anti-oxidative mechanisms of minocycline against sphingomyelinase/ceramide neurotoxicity: implication in Alzheimer's disease and cerebral ischemia. Free Radical Res. 2012;46:940–950. doi: 10.3109/10715762.2012.674640. [DOI] [PubMed] [Google Scholar]
- 6.Cheng X, Gan L, Zhao J, Chen M, Liu Y, Wang Y. Changes in Ataxin-10 expression after sciatic nerve crush in adult rats. Neurochem Res. 2013;38:1013–1021. doi: 10.1007/s11064-013-1011-6. [DOI] [PubMed] [Google Scholar]
- 7.Deramaudt TB, Dill C, Bonay M. Regulation of oxidative stress by Nrf2 in the pathophysiology of infectious diseases. Med Maladies Infect. 2013;43:100–107. doi: 10.1016/j.medmal.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Di Cesare Mannelli L, Bani D, Bencini A, Brandi ML, Calosi L, Cantore M, Carossino AM, Ghelardini C, Valtancoli B, Failli Therapeutic effects of the superoxide dismutase mimetic compound MnIIMe2DO2A on experimental articular pain in rats. Mediators Inflamm 2013. 2013:905360. doi: 10.1155/2013/905360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao S, Fei M, Cheng C, Yu X, Chen M, Shi S, Qin J, Guo Z, Shen A. Spatiotemporal expression of PSD-95 and nNOS after rat sciatic nerve injury. Neurochem Res. 2008;33:1090–1100. doi: 10.1007/s11064-007-9555-y. [DOI] [PubMed] [Google Scholar]
- 10.Gao YY, Xie QM, Ma JY, Zhang XB, Zhu JM, Shu DM, Sun BL, Jin L, Bi YZ. Supplementation of xanthophylls increased antioxidant capacity and decreased lipid peroxidation in hens and chicks. Br J Nutr. 2013;109:977–983. doi: 10.1017/S0007114512002784. [DOI] [PubMed] [Google Scholar]
- 11.Ghyasi R, Sepehri G, Mohammadi M, Badalzadeh R, Ghyasi A. Effect of mebudipine on oxidative stress and lipid peroxidation in myocardial ischemic-reperfusion injury in male rat. J Res Med Sci. 2012;17:1150–1155. [PMC free article] [PubMed] [Google Scholar]
- 12.Gillies LA, Kuwana T. Apoptosis regulation at the mitochondrial outer membrane. J Cell Biochem. 2014;115:632–640. doi: 10.1002/jcb.24709. [DOI] [PubMed] [Google Scholar]
- 13.Grotto D, Santa Maria LD, Boeira S, Valentini J, Charão MF, Moro AM, Nascimento PC, Pomblum VJ, Garcia SC. Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography-visible detection. J Pharmaceut Biomed. 2007;43:619–624. doi: 10.1016/j.jpba.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Gutteridge JMC. Lipid peroxidation initiated by superoxide-dependent hydroxyl radicals using complexed iron and hydrogen peroxide. FEBS Lett. 1984;172:245–249. doi: 10.1016/0014-5793(84)81134-5. [DOI] [PubMed] [Google Scholar]
- 15.Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 16.Iida H, Nagasaka T, Shindo K, Shiozawa Z. Effect of the free radical scavenger edaravone on peripheral nerve ischemia-reperfusion injury. Muscle Nerve. 2009;40:582–588. doi: 10.1002/mus.21388. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda S, Harada K, Ohwatashi A, Kamikawa Y. Effects of edaravone, a free radical scavenger, on photochemically induced cerebral infarction in a rat hemiplegic model. ScientificWorldJournal 2013. 2013:175280. doi: 10.1155/2013/175280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamogawa E, Sueishi Y. A multiple free-radical scavenging (MULTIS) study on the antioxidant capacity of a neuroprotective drug, edaravone as compared with uric acid, glutathione, and trolox. Bioorg Med Chem Lett. 2014;24:1376–1379. doi: 10.1016/j.bmcl.2014.01.045. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan T, Kafa IM, Cansev M, Bekar A, Karli N, Taskapilioglu MO, Kanar F. Investigation of the dose-dependency of citicoline effects on nerve regeneration and functional recovery in a rat model of sciatic nerve injury. Turk Neurosurg. 2014;24:54–62. doi: 10.5137/1019-5149.JTN.8451-13.0. [DOI] [PubMed] [Google Scholar]
- 20.Kikuchi K, Tancharoen S, Takeshige N, Yoshitomi M, Morioka M, Murai Y, Tanaka E. The efficacy of edaravone (radicut), a free radical scavenger, for cardiovascular disease. Int J Mol Sci. 2013;14:13909–13930. doi: 10.3390/ijms140713909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kikuta M, Shiba T, Yoneyama M, Kawada K, Yamaguchi T, Hinoi E, Yoneda Y, Ogita K. In vivo and in vitro treatment with edaravone promotes proliferation of neural progenitor cells generated following neuronal loss in the mouse dentate gyrus. J Pharmacol Sci. 2013;121:74–83. doi: 10.1254/jphs.12162fp. [DOI] [PubMed] [Google Scholar]
- 22.Koka R, Hadlock TA. Quantification of functional recovery following rat sciatic nerve transection. Exp Neurol. 2001;168:192–195. doi: 10.1006/exnr.2000.7600. [DOI] [PubMed] [Google Scholar]
- 23.Korbakis D, Scorilas A. Quantitative expression analysis of the apoptosis-related genes BCL2, BAX and BCL2L12 in gastric adenocarcinoma cells following treatment with the anticancer drugs cisplatin etoposide and taxol. Tumour Biol. 2012;33:865–875. doi: 10.1007/s13277-011-0313-z. [DOI] [PubMed] [Google Scholar]
- 24.Lapchak PA. A critical assessment of edaravone acute ischemic stroke efficacy trials: is edaravone an effective neuroprotective therapy? Expert Opin Pharmacother. 2010;11:1753–1763. doi: 10.1517/14656566.2010.493558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee BJ, Egi Y, van Leyen K, Lo EH, Arai K. Edaravone, a free radical scavenger, protects components of the neurovascular unit against oxidative stress in vitro. Brain Res. 2010;1307:22–27. doi: 10.1016/j.brainres.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li B, Yu D, Xu Z. Edaravone prevents neurotoxicity of mutant L166P DJ-1 in Parkinson's disease. J Mol Neurosci. 2013;51:539–549. doi: 10.1007/s12031-013-0022-8. [DOI] [PubMed] [Google Scholar]
- 27.Liu CZ, Lei B. Effect of acupuncture intervention on learning-memory ability and cerebral superoxide dismutase activity and malonaldehyde concentration in chronic fatigue syndrome rats. Zhen Ci Yan Jiu. 2013;38:478–481. [PubMed] [Google Scholar]
- 28.Mao YF, Yan N, Xu H, Sun JH, Xiong YC, Deng XM. Edaravone, a free radical scavenger, is effective on neuropathic pain in rats. Brain Res. 2009;1248:68–75. doi: 10.1016/j.brainres.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 29.Mignard V, Lalier L, Paris F, Vallette FM. Bioactive lipids and the control of Bax pro-apoptotic activity. Cell Death Dis. 2014;5:e1266. doi: 10.1038/cddis.2014.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niture SK, Jaiswal AK. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J Biol Chem. 2012;287:9873–9886. doi: 10.1074/jbc.M111.312694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan J, Su Y, Hou X, He H, Liu S, Wu J, Rao P. Protective effect of recombinant protein SOD-TAT on radiation-induced lung injury in mice. Life Sci. 2012;91:89–93. doi: 10.1016/j.lfs.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Rahmat A, Kumar V, Fong LM, Endrini S, Sani HA. Determination of total antioxidant activity in three types of local vegetables shoots and the cytotoxic effect of their ethanolic extracts against different cancer cell lines. Asia Pac J Clin Nutr. 2004;13:308–311. [PubMed] [Google Scholar]
- 33.Shimoda M, Iwasaki Y, Okada T, Kubota K. Edaravone inhibits apoptosis caused by ischemia/reperfusion injury in a porcine hepatectomy model. World J Gastroenterol. 2012;18:3520–3526. doi: 10.3748/wjg.v18.i27.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su Y, Xia YF, Yang HL, He JH, Wu QL, Zheng Q, Hou JH. Changes of superoxide dismutase (SOD) and metallothionien (MT) before, during, and after radiotherapy for nasopharyngeal carcinoma and their significance. Ai Zheng. 2003;22:629–633. [PubMed] [Google Scholar]
- 35.Suslu H, Altun M, Erdivanli B, Turan Suslu H. Comparison of the effects of local and systemic dexamethasone on the rat traumatic sciatic nerve model. Turk Neurosurg. 2013;23:623–629. doi: 10.5137/1019-5149.JTN.7761-13.0. [DOI] [PubMed] [Google Scholar]
- 36.Tamaddonfard E, Farshid AA, Maroufi S, Kazemi-Shojaei S, Erfanparast A, Asri-Rezaei S, Taati M, Dabbaghi M, Escort M. Effects of safranal, a constituent of saffron, and vitamin E on nerve functions and histopathology following crush injury of sciatic nerve in rats. Phytomedicine. 2014;21:717–723. doi: 10.1016/j.phymed.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 37.Turkoglu E, Serbes G, Dolgun H, Oztuna S, Bagdatoglu OT, Yilmaz N, Bagdatoglu C, Sekerci Z. Effects of α-MSH on ischemia/reperfusion injury in the rat sciatic nerve. Surg Neurol Int. 2012;3:74. doi: 10.4103/2152-7806.98501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walluscheck D, Poehlmann A, Hartig R, Lendeckel U, Schönfeld P, Hotz-Wagenblatt A, Reissig K, Bajbouj K, Roessner A, Schneider-Stock R. ATF2 knockdown reinforces oxidative stress-induced apoptosis in TE7 cancer cells. J Cell Mol Med. 2013;17:976–988. doi: 10.1111/jcmm.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang G, Su J, Li L, Feng J, Shi L, He W, Liu Y. Edaravone alleviates hypoxia-acidosis/reoxygenation-induced neuronal injury by activating ERK1/2. Neurosci Lett. 2013;543:72–77. doi: 10.1016/j.neulet.2013.02.067. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Luo Y, Song W. Study on early motor neurotrophism of denervated red and white muscles. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2004;18:250–253. [PubMed] [Google Scholar]
- 41.Wang XH, Wan L, Li XY, Meng YQ, Zhu NX, Yang M, Feng BH, Zhang WC, Zhu SG, Li ST. A standardized method to create peripheral nerve injury in dogs using an automatic non-serrated forceps. Neural Regen Res. 2012a;7:2516–2521. doi: 10.3969/j.issn.1673-5374.2012.32.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Tang X, Yu B, Gu Y, Yuan Y, Yao D, Ding F, Gu X. Gene Network Revealed Involvements of Birc2, Birc3 and Tnfrsf1a in Anti-Apoptosis of Injured Peripheral Nerves. PLoS One. 2012b;7:e43436. doi: 10.1371/journal.pone.0043436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe T, Tahara M, Todo S. The novel antioxidant edaravone: from bench to bedside. Cardiovasc Ther. 2008;26:101–114. doi: 10.1111/j.1527-3466.2008.00041.x. [DOI] [PubMed] [Google Scholar]
- 44.Xu J, Li XS. An experimental study of nerve bypass graft. Chin J Traumatol. 2008;11:175–178. doi: 10.1016/s1008-1275(08)60037-1. [DOI] [PubMed] [Google Scholar]
- 45.Xu Q, Zhang B, Li X-m, Gao X. Traditional Chinese medicine formula Qing Huo Yi Hao as superoxide anion scavenger in high glucose-treated endothelial cells. Acta Pharmacol Sin. 2012;33:496–502. doi: 10.1038/aps.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang E, Korsmeyer S. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- 47.Yang TC, Chen YJ, Chang SF, Chen CH, Chang PY, Lu SC. Malondialdehyde mediates oxidized LDL-induced coronary toxicity through the Akt-FGF2 pathway via DNA methylation. J Biomed Sci. 2014;21:11. doi: 10.1186/1423-0127-21-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye N, Liu S, Lin Y, Rao P. Protective effects of intraperitoneal injection of TAT-SOD against focal cerebral ischemia/reperfusion injury in rats. Life Sci. 2011;89:868–874. doi: 10.1016/j.lfs.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida H, Metoki N, Ishikawa A, Imaizumi T, Matsumiya T, Tanji K, Ota K, Ohyama C, Satoh K. Edaravone improves the expression of nerve growth factor in human astrocytes subjected to hypoxia/reoxygenation. Neurosci Res. 2010;66:284–289. doi: 10.1016/j.neures.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Zhao F, Wang Q. The protective effect of peroxiredoxin II on oxidative stress induced apoptosis in pancreatic β-cells. Cell Biosci. 2012;2:22. doi: 10.1186/2045-3701-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao L, Lv GM, Jiang SY, Yan ZQ, Sun JM, Wang L, Jiang DL. Morphological differences in skeletal muscle atrophy of rats with motor nerve and/or sensory nerve injury. Neural Regen Res. 2012;7:2507–2515. doi: 10.3969/j.issn.1673-5374.2012.32.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao ZY, Luan P, Huang SX, Xiao SH, Zhao J, Zhang B, Gu BB, Pi RB, Liu J. Edaravone protects HT22 neurons from H 2 O 2 -induced apoptosis by inhibiting the MAPK signaling pathway. CNS Neurosci Ther. 2013;19:163–169. doi: 10.1111/cns.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou S, Zheng C. Preparation of microspheres of superoxide dismutase and their activities. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2013;42:666–670. doi: 10.3785/j.issn.1008-9292.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 54.Zhou S, Liu R, Yuan K, Yi T, Zhao X, Huang C, Wei Y. Proteomics analysis of tumor microenvironment: Implications of metabolic and oxidative stresses in tumorigenesis. Mass Spectrom Rev. 2013;32:267–311. doi: 10.1002/mas.21362. [DOI] [PubMed] [Google Scholar]


