Abstract
Over last 20 years, extracellular matrices have been shown to be useful in promoting tissue regeneration. Recently, they have been used and have had success in achieving neurogenesis. Recent developments in extracellular matrix design have allowed their successful in vivo incorporation to engender an environment favorable for neural regeneration in animal models. Promising treatments under investigation include manipulation of the intrinsic extracellular matrix and incorporation of engineered naometer-sized scaffolds through which inhibition of molecules serving as barriers to neuroregeneration and delivery of neurotrophic factors and/or cells for successful tissue regeneration can be achieved. Further understanding of the changes incurred within the extracellular matrix following central nervous system injury will undoubtedly help design a clinically efficacious extracellular matrix scaffold that can mitigate or reverse neural degeneration in the clinical setting.
Keywords: extracellular matrix, neurogenesis, scaffolds, neural stem cells
Introduction
For many years, management of neurological injury secondary to trauma or stroke has focused on mediating the cellular changes that cause secondary damage (Greve et al., 2009). Advances in the understanding of the role of extracellular matrix (ECM) in influencing neurogenesis, however, have presented novel strategies for tissue regeneration (Hallbergson et al., 2003). In this review, we discuss the functions of the ECM and summarize the basic science advances and several novel treatments under investigation that highlight the potential for intrinsically altering the ECM and the use of artificial ECM scaffolds to achieve neural regeneration.
The repair of a damaged axon involves a complex series of cellular changes to coordinate the formation of a growth cone which can successfully follow a sea of inhibitory and permissive signals to reunite with its prior synapses and properly regenerate (Szpara et al., 2007). In the peripheral nervous system (PNS), these changes are called Wallerian degeneration. Here denervated Schwann cells differentiate and proliferate, producing neurotropic molecules, cell receptors, and basement membrane elements which allows the growth cone to reach its original target. In the central nervous system (CNS), in which oligodendrocytes myelinate multiple neurons, injury causes more widespread demyelination. Injury to the CNS causes a common pathway of destruction through secondary injuries like hemorrhage, edema, ischemia, and inflammation (Fitch et al., 1997). In spinal cord injuries, these secondary injuries lead to necrosis and apoptosis in 1–2 segments above the original site of injury, with reactive astrocytes forming a glial scar (Faulkner et al., 2004). Wallerian degeneration like that seen in the PNS is furthermore impeded by the inhibiting properties of slowly cleared myelin degradation products (McKerracher et al., 1994) and the absence of an organized basal lamina. The glial scar, itself, contains inhibitory molecules (Fawcett et al., 1997) which, at first, limit areas of damage by reestablishing the blood-brain barrier and containing inflammation and released neurotransmitters (Faulkner et al., 2004); however, overtime, the ECM deposited within the glial scar contains inhibitory molecules like chondroitin sulfate proteoglycans (CSPGs) which prevent further neurogenesis (McKeon et al., 1991).
The ECM
In recent years, the role of the ECM in regulating the survival, differentiation, growth, and migration of cells in the nervous system and its impact on neurogenesis have been increasingly characterized. The ECM, which constitutes 10–20% of the brain's volume, is composed of a network of proteins and glycans which are produced intracellularly and get secreted into the extracellular space (Bignami et al., 1993). In addition to its important structural, anchoring, and organizing functions, the ECM also contains signals which regulate growth, differentiation, synapse remodeling, and migration (Bandtlow et al., 2000; Dityatev et al., 2003). The network is organized roughly into three compartments (Figure 1): the basement membrane (basal lamina) that surrounds cerebral vessels, the perineuronal nets (PNNs) surrounding cell bodies and dendrites, and a diffusely distributed interstitial matrix lying between CNS parenchyma (Lau et al., 2013). The basement membrane is composed of collagen, fibronectin, perlecan, dystroglycan and laminin-nidogen (entactin) complexes. It surrounds the pial surface of the CNS and separates the endothelial cells from parenchyma, thus forming the blood-brain barrier. During cerebral ischemia, increased expression of matrix metalloproteinases 9 and 2 results in the breakdown of the basement membrane, which may facilitate hemorrhage, edema, and secondary injury (Wang et al., 2007). PNNs are primarily composed of a dense matrix of hyaluronan (HA), chondroitin sulfate proteoglycans (CSPGs), tenascin R and link proteins, and serve as crucial regulators of synaptic plasticity (Kwok et al., 2011). The negatively charged glycosaminoglycans (GAG) regulate the diffusion of important cations, such as sodium, potassium, and calcium, which supports rapid neuronal firing (Hartig et al., 1999).
Figure 1.
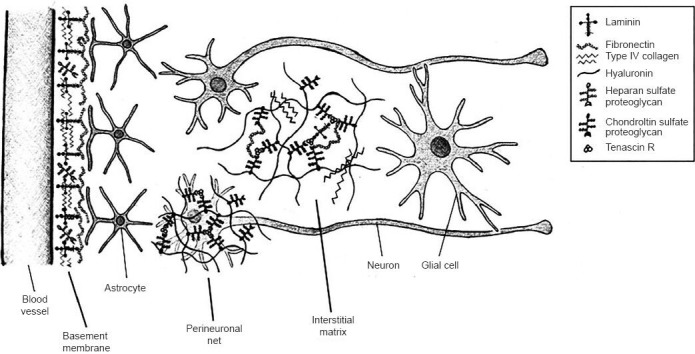
Microscopic anatomy of the extracellular matrix within the central nervous system (CNS).
Adapted from: Lau et al. (2013). The three major compartments of the extracellular matrix in the CNS are the basement membrane, perineuronal net, and neuronal interstitial matrix. The basement membrane is found surrounding cerebral blood vessels, the perineuronal net is a dense matrix immediately surrounding neuronal cell bodies and dendrites, and the neuronal interstitial matrix occupies the space between neurons and glial cells.
ECM modifications following injury
CNS injury causes the proliferation of astrocytes, fibroblasts, and oligodendrocyte precursors which form a glial scar (Hatten et al., 1991; Chen et al., 2002). Within this glial scar, upregulated proteoglycans like CSPGs and changes in sulfation patterns within the ECM itself result in the creation of a barrier to regeneration (Massey et al., 2008). Multiple studies using the bacterial enzyme ChaseABC to digest chondroitin sulfate chains in CSPGs have demonstrated reversal of these inhibitory functions (McKeon et al., 1995). Promising in vitro work led to successful in vivo injection of ChaseABC into rats with spinal cord contusion injuries, leading to successful CSPG degradation (Lemons et al., 1999). Multiple other models have since demonstrated post-injury removal of chondroitin sulfates, promoting axon regeneration (Moon et al., 2001), and even functional recovery in rats with lesioned dorsal columns (Bradbury et al., 2002) and cats with spinal cord hemisection (Tester et al., 2008). Alternative strategies besides injection to deliver ChaseABC to modify the PNN in injured areas have included transgenic astrocytes with a GFAP promoter (Cafferty et al., 2008), neutralization with antibodies (Tan et al., 2006), and siRNA knocking down CS GAG elongation enzymes (Laabs et al., 2007).
Artificial scaffolds
Besides manipulating the intrinsic extracellular matrix to engender an environment favoring neural regeneration, an alternative strategy involves the use of artificially created, nanometer-sized scaffolds to overcome the body's natural barriers to repair. The use of a tissue-engineered scaffold offers a way to the poor regenerative capacity of the CNS to reconstruct formed cavities and reconnect neuronal processes. Thus, the artificial scaffold functions to enhance the communication between cells, allowing for improvement in proliferation, migration, and differentiation (Figure 2). Studies have demonstrated efficacy of bare scaffolds within the CNS (Huang et al., 2012), but regeneration and functional recovery in lesioned rat models have been enhanced with the addition of other substrates such as vascular endothelial growth factor (Zhang et al., 2007) or hyaluronic acid with laminin (Hou et al., 2005).
Figure 2.
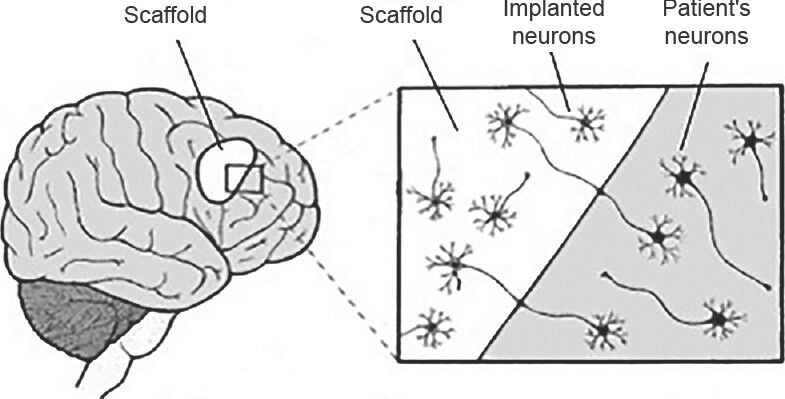
Implantation of extracellular matrix scaffold into damaged brain tissue.
Adapted from: Orive G et al. (2009). Placing a scaffold into an area of brain damage provides structural support and enhancement of communication between cells, in turn, allowing for improved proliferation, migration, and differentiation, especially in the setting of implantation of exogenous neurons or neural stem cells.
Increasingly, stem cells have been incorporated into these engineered scaffolds to support three-dimensional cell organization. Neural stem cells (NSCs) are the multipotent, self-renewing progenitors of neurons, astrocytes, and oligodendrocytes. Three distinct populations exist in the subventricular zone, external germinal layer of the cerebellum, and subgranular layer of the dentate gyrus (Doetsch et al., 1999). Cells can be harvested directly from these regions in adult or embryonic tissues, or by inducing embryonic stem cell (ESCs) differentiation, which may have less fate restrictions but greater tumorigenic potential (Nishikawa et al., 2007). In addition, cells taken from other areas, such as marrow stromal cells (MSCs), olfactory ensheathing cells, or Schwann cells, can give rise to cells for autotransplantation in a minimally-invasive and easily accessible process (Hayase et al., 2009). Transplantation can aid neuroregeneration not only by differentiating to replace the damaged or degenerative cell types (Zhu et al., 2009), but also by secreting various neurotropic factors like nerve growth facture and brain-derived neurotrophic factor (BDNF) (Lu et al., 2003), and by inhibiting T cell activation, thus preventing further injury (Bacigaluppi et al., 2009). Schwann cells promote CNS regeneration by naturally expressing numerous surface adhesion molecules and growth factors, and by producing the ECM components laminin and fibronectin (Volpato et al., 2013).
The use of biomaterials and polymers has been shown to be an essential strategy to maximize cell transplantation efficacy. Indeed, the success of cell transplantation and eventual neurogenesis is a complex process that relies upon: a) the delivery of the stem cells into a framework where cells can interact, b) the appropriate signaling molecules within the microenvironment, and c) an adequate blood supply. The ideal cell scaffold is physiochemically similar to the surrounding tissues, non-toxic, poorly immunogenic, biodegradable, durable, cheap, conformable to various structures, and highly porous to allow for a high density of cell seeding (Madigan et al., 2009). These scaffolds appear in various forms, including hydrogels, sponges, membranes, and channel containing tubules, and can be from naturally occurring or synthetic components.
Natural scaffolds made of proteins and carbohydrates are generally highly biocompatible and easily available. As one of the major components of ECM, collagen has been well studied. In combination with neurotropic factors like BDNF and neurotrophin-3 (NT-3), axonal regeneration and partial functional recovery after dorsal spinal cord transection have been noted (East et al., 2010; Han et al., 2010). Addition of NSCs also demonstrated improved remyelination and recovery in rat lesional models (Hatami et al., 2009), and combination delivery with ChaseABC also showed improved bladder function (Fouad et al., 2009). Fibrin, a natural component of blood clots, can also be used as an injected or biodegradable scaffold to promote migration of neural support cells and promote implanted NSC survival (Johnson et al., 2010). Other naturally occurring scaffolds include hyaluronic acid, which has lymphocyte and astrocyte inhibition properties and has been shown to lead to diminished glial scar formation (Wei et al., 2010), or alginate, agarose, and chitin sponges or gels, which have proved a versatile delivery vehicle for various cells and growth factors (Kataoka et al., 2004; Stokols et al., 2006; Bozkurtet al., 2010; Barminko et al., 2011).
Artificial scaffolds, designed to mimic endogenous ECM, formed from type 1 collagen and glycosaminoglycans (CGs), have already been successfully used to facilitate regeneration of skin, bone, and peripheral nerves (Yannas et al., 2010). Synthetic scaffolds are an attractive alternative to animal-derived biomaterials due to their minimal risk of pathogen transmission (Kyle et al., 2009), similar structure to naturally occurring ECM (Zhang et al., 2005), ease of liquid delivery to in vivo targets, and lack of immune response (Rudra et al., 2010).
Polymeric scaffolds designed for the CNS rely upon the self-assembling properties of engineered peptides, whereas a liquid monomer can be injected to spontaneously form highly ordered architectures (Bonzani et al., 2006). The list of synthetic compounds is rapidly expanding, and includes compounds like poly-E-caprolactone, poly-lactic acid, poly-lactic-co-glycolic acid, poly-B-hydroxybutirate, polyethylene glycol, and poly (2-hydroxyethyl mathacrylate) (Kim et al., 2014). These can be utilized as sponges, sheets, channels, films, or hydrogels. Hydrogels, from either natural or synthetic compounds are easily implanted by simple injection and conform easily to surrounding tissue (Phillips et al., 2004). Guidance channels, derived from synthetic biomaterials, may prove superior at axonal regeneration, but must be surgically implanted, given their structural stiffness (Wong et al., 2008). In addition to NSCs, MSCs, Schwann cells, and epithelial stem cells are transplanted either alone or together for improved efficacy (Oh et al., 2011). Likewise, growth factors most commonly used in combination include NT-3, FGF-2, GDNF, and BDNF (Zurita et al., 2010).
Conclusion
Given the heterogenous nature of injury to the CNS through ischemia, trauma, or degeneration to diverse anatomic locations and cell populations, it is not surprising that therapeutic strategies have also become increasingly complex. Manipulating the extracellular matrix allows for the delivery of targeted scaffolds containing tailored combinations of cells and neurotrophic factors, many of which have demonstrated marked efficacy in animal models. Although the application of these findings has yet to significantly alter the way clinicians view or treat these conditions, there is promise for significant therapeutic advances in our lifetime.
Footnotes
Conflicts of interest: None declared.
References
- 1.Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, Brambilla E, West MJ, Comi G, Martino G, Hermann DM. Delayed post-ischaemicneuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–2251. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- 2.Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev. 2000;80:1267–1290. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- 3.Barminko J, Kim JH, Otsuka S, Gray A, Schloss R, Grumet M, Yarmush ML. Encapsulated mesenchymal stromal cells for in vivo transplantation. Biotechnol Bioeng. 2011;108:2747–2758. doi: 10.1002/bit.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bignami A, Hosley M, Dahl D. Hyaluronic acid and hyaluronic acid-binding proteins in brain extracellular matrix. Anat Embryol (Berl) 1993;188:419–433. doi: 10.1007/BF00190136. [DOI] [PubMed] [Google Scholar]
- 5.Bonzani IC, George JH, Stevens MM. Novel materials for bone and cartilage regeneration. Curr Opin Chem Biol. 2006;10:568–575. doi: 10.1016/j.cbpa.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Bozkurt G, Mothe AJ, Zahir T, Kim H, Shoichet MS, Tator CH. Chitosan channels containing spinal cord-derived stem/progenitor cells for repair of subacute spinal cord injury in the rat. Neurosurgery. 2010;67:1733–1744. doi: 10.1227/NEU.0b013e3181f9af35. [DOI] [PubMed] [Google Scholar]
- 7.Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 8.Cafferty WB, Bradbury EJ, Lidierth M, Jones M, Duffy PJ, Pezet S, Mc-Mahon SB. Chondroitinase ABC-mediated plasticity of spinal sensory function. J Neurosci. 2008;28:11998–12009. doi: 10.1523/JNEUROSCI.3877-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen ZJ, Negra M, Levine A, Ughrin Y, Levine JM. Oligodendrocyte precursor cells: reactive cells that inhibit axon growth and regeneration. J Neurocytol. 2002;31:481–495. doi: 10.1023/a:1025791614468. [DOI] [PubMed] [Google Scholar]
- 10.Dityatev A, Schachner M. Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci. 2003;4:456–468. doi: 10.1038/nrn1115. [DOI] [PubMed] [Google Scholar]
- 11.Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 12.East E, de Oliveira DB, Golding JP, Phillips JB. Alignment of astrocytes increases neuronal growth in three-dimensional collagen gels and is maintained following plastic compression to form a spinal cord repair conduit. Tissue Eng Part A. 2010;16:3173–3184. doi: 10.1089/ten.tea.2010.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 15.Fitch MT, Silver J. Activated macrophages and the blood-brain barrier: inflammation after CNS injury leads to increases in putative inhibitory molecules. Exp Neurol. 1997;148:587–603. doi: 10.1006/exnr.1997.6701. [DOI] [PubMed] [Google Scholar]
- 16.Fouad K, Pearse DD, Tetzlaff W, Vavrek R. Transplantation and repair: combined cell implantation and chondroitinase delivery prevents deterioration of bladder function in rats with complete spinal cord injury. Spinal Cord. 2009;47:727–732. doi: 10.1038/sc.2009.10. [DOI] [PubMed] [Google Scholar]
- 17.Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. Mt Sinai J Med. 2009;76:97–104. doi: 10.1002/msj.20104. [DOI] [PubMed] [Google Scholar]
- 18.Hallbergson AF, Gnatenco C, Peterson DA. Neurogenesis and brain injury: managing a renewable resource for repair. J Clin Invest. 2003;112:1128–1133. doi: 10.1172/JCI20098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han Q, Jin W, Xiao Z, Ni H, Wang J, Kong J, Wu J, Liang W, Chen L, Zhao Y, Chen B, Dai J. The promotion of neural regeneration in an extreme rat spinal cord injury model using a collagen scaffold containing a collagen binding neuroprotective protein and an EGFR neutralizing antibody. Biomaterials. 2010;31:9212–9220. doi: 10.1016/j.biomaterials.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 20.Härtig W, Derouiche A, Welt K, Brauer K, Grosche J, Mäder M, Reichenbach A, Brückner G. Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res. 1999;842:15–29. doi: 10.1016/s0006-8993(99)01784-9. [DOI] [PubMed] [Google Scholar]
- 21.Hatami M, Mehrjardi NZ, Kiani S, Hemmesi K, Azizi H, Shahverdi A, Baharvand H. Human embryonic stem cell-derived neural precursor transplants in collagen scaffolds promote recovery in injured rat spinal cord. Cytotherapy. 2009;11:618–630. doi: 10.1080/14653240903005802. [DOI] [PubMed] [Google Scholar]
- 22.Hatten ME, Liem RK, Shelanski ML, Mason CA. Astroglia in CNS injury. Glia. 1991;4:233–243. doi: 10.1002/glia.440040215. [DOI] [PubMed] [Google Scholar]
- 23.Hayase M, Kitada M, Wakao S, Itokazu Y, Nozaki K, Hashimoto N, Takagi Y, Dezawa M. Committed neural progenitor cells derived from genetically modified bone marrow stromal cells ameliorate deficits in a rat model of stroke. J Cereb Blood Flow Metab. 2009;29:1409–1420. doi: 10.1038/jcbfm.2009.62. [DOI] [PubMed] [Google Scholar]
- 24.Hou S, Xu Q, Tian W, Cui F, Cai Q, Ma J, Lee IS. The repair of brain lesion by implantation of hyaluronic acid hydrogels modified with laminin. J Neurosci Methods. 2005;148:60–70. doi: 10.1016/j.jneumeth.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Huang KF, Hsu WC, Chiu WT, Wang JY. Functional improvement and neurogenesis after collagen-GAG matrix implantation into surgical brain trauma. Biomaterials. 2012;33:2067–2075. doi: 10.1016/j.biomaterials.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 26.Johnson PJ, Tatara A, McCreedy DA, Shiu A, Sakiyama-Elbert SE. Tissue-engineered fibrin scaffolds containing neural progenitors enhance functional recovery in a subacute model of SCI. Soft Matter. 2010;6:5127–5137. doi: 10.1039/c0sm00173b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kataoka K, Suzuki Y, Kitada M, Hashimoto T, Chou H, Bai H, Ohta M, Wu S, Suzuki K, Ide C. Alginate enhances elongation of early regenerating axons in spinal cord of young rats. Tissue Eng. 2004;10:493–504. doi: 10.1089/107632704323061852. [DOI] [PubMed] [Google Scholar]
- 28.Kim M, Park SR, Choi BH. Biomaterial scaffolds used for the regeneration of spinal cord injury (SCI) Histol Histopathol [Epub ahead of print] 2014 doi: 10.14670/HH-29.1395. [DOI] [PubMed] [Google Scholar]
- 29.Kwok JC, Dick G, Wang D, Fawcett JW. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol. 2011;71:1073–1089. doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- 30.Kyle S, Aggeli A, Ingham E, McPherson MJ. Production of self-assembling biomaterials for tissue engineering. Trends Biotechnol. 2009;27:423–433. doi: 10.1016/j.tibtech.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laabs TL, Wang H, Katagiri Y, McCann T, Fawcett JW, Geller HM. Inhibiting glycosaminoglycan chain polymerization decreases the inhibitory activity of astrocyte-derived chondroitin sulfate proteoglycans. J Neurosci. 2007;27:14494–14501. doi: 10.1523/JNEUROSCI.2807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau LW, Cua R, Keough MB, Haylock-Jacobs S, Yong VW. Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat Rev Neurosci. 2013;14:722–729. doi: 10.1038/nrn3550. [DOI] [PubMed] [Google Scholar]
- 33.Lemons ML, Howland DR, Anderson DK. Chondroitin sulfate proteoglycan immunoreactivity increases following spinal cord injury and transplantation. Exp Neurol. 1999;160:51–65. doi: 10.1006/exnr.1999.7184. [DOI] [PubMed] [Google Scholar]
- 34.Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 35.Madigan NN, McMahon S, O’Brien T, Yaszemski MJ, Windebank AJ. Current tissue engineering and novel therapeutic approaches to axonal regeneration following spinal cord injury using polymer scaffolds. Respir Physiol Neurobiol. 2009;169:183–199. doi: 10.1016/j.resp.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massey JM, Amps J, Viapiano MS, Matthews RT, Wagoner MR, Whitaker CM, Alilain W, Yonkof AL, Khalyfa A, Cooper NG, Silver J, Onifer SM. Increased chondroitin sulfate proteoglycan expression in denervated brainstem targets following spinal cord injury creates a barrier to axonal regeneration overcome by chondroitinase ABC and neurotrophin-3. Exp Neurol. 2008;209:426–445. doi: 10.1016/j.expneurol.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKeon RJ, Höke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol. 1995;136:32–43. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- 39.McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 40.Moon LD, Asher RA, Rhodes KE, Fawcett JW. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci. 2001;4:465–466. doi: 10.1038/87415. [DOI] [PubMed] [Google Scholar]
- 41.Nishikawa S, Jakt LM, Era T. Embryonic stem-cell culture as a tool for developmental cell biology. Nat Rev Mol Cell Biol. 2007;8:502–507. doi: 10.1038/nrm2189. [DOI] [PubMed] [Google Scholar]
- 42.Oh JS, Kim KN, An SS, Pennant WA, Kim HJ, Gwak SJ, Yoon DH, Lim MH, Choi BH, Ha Y. Cotransplantation of mouse neural stem cells (mNSCs) with adipose tissue-derived mesenchymal stem cells improves mNSC survival in a rat spinal cord injury model. Cell Transplant. 2011;20:837–849. doi: 10.3727/096368910X539083. [DOI] [PubMed] [Google Scholar]
- 43.Phillips JB, King VR, Ward Z, Porter RA, Priestley JV, Brown RA. Fluid shear in viscous fibronectin gels allows aggregation of fibrous materials for CNS tissue engineering. Biomaterials. 2004;25:2769–2779. doi: 10.1016/j.biomaterials.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 44.Rudra JS, Tian YF, Jung JP, Collier JH. A self-assembling peptide acting as an immune adjuvant. Proc Natl Acad Sci U S A. 2010;107:622–627. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stokols S, Sakamoto J, Breckon C, Holt T, Weiss J, Tuszynski MH. Templated agarose scaffolds support linear axonal regeneration. Tissue Eng. 2006;12:2777–2787. doi: 10.1089/ten.2006.12.2777. [DOI] [PubMed] [Google Scholar]
- 46.Szpara ML, Vranizan K, Tai YC, Goodman CS, Speed TP, Ngai J. Analysis of gene expression during neurite outgrowth and regeneration. BMC Neurosci. 2007;8:100. doi: 10.1186/1471-2202-8-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan AM, Colletti M, Rorai AT, Skene JH, Levine JM. Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J Neurosci. 2006;26:4729–4739. doi: 10.1523/JNEUROSCI.3900-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tester NJ, Howland DR. Chondroitinase ABC improves basic and skilled locomotion in spinal cord injured cats. Exp Neurol. 2008;209:483–496. doi: 10.1016/j.expneurol.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volpato FZ, Führmann T, Migliaresi C, Hutmacher DW, Dalton PD. Using extracellular matrix for regenerative medicine in the spinal cord. Biomaterials. 2013;34:4945–4955. doi: 10.1016/j.biomaterials.2013.03.057. [DOI] [PubMed] [Google Scholar]
- 50.Wang CX, Shuaib A. Critical role of microvasculature basal lamina in ischemic brain injury. Prog Neurobiol. 2007;83:140–148. doi: 10.1016/j.pneurobio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 51.Wei YT, He Y, Xu CL, Wang Y, Liu BF, Wang XM, Sun XD, Cui FZ, Xu QY. Hyaluronic acid hydrogel modified with nogo-66 receptor antibody and poly-L-lysine to promote axon regrowth after spinal cord injury. J Biomed Mater Res B Appl Biomater. 2010;95:110–117. doi: 10.1002/jbm.b.31689. [DOI] [PubMed] [Google Scholar]
- 52.Wong DY, Leveque JC, Brumblay H, Krebsbach PH, Hollister SJ, Lamarca F. Macro-architectures in spinal cord scaffold implants influence regeneration. J Neurotrauma. 2008;25:1027–1037. doi: 10.1089/neu.2007.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yannas IV, Tzeranis DS, Harley BA, So P. Biologically active collagen-based scaffolds: advances in processing and characterization. Philos Trans A Math Phys Eng Sci. 2010;368:2123–2139. doi: 10.1098/rsta.2010.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H, Hayashi T, Tsuru K, Deguchi K, Nagahara M, Hayakawa S, Nagai M, Kamiya T, Osaka A, Abe K. Vascular endothelial growth factor promotes brain tissue regeneration with a novel biomaterial polydimethylsiloxane-tetraethoxysilane. Brain Res. 2007;1132:29–35. doi: 10.1016/j.brainres.2006.09.117. [DOI] [PubMed] [Google Scholar]
- 55.Zhang S, Gelain F, Zhao X. Designer self-assembling peptide nanofiber scaffolds for 3D tissue cell cultures. Semin Cancer Biol. 2005;15:413–420. doi: 10.1016/j.semcancer.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Zhu Qf, Ma J, Yu Ll, Yuan Cg. Grafted neural stem cells migrate to substantianigra and improve behavior in Parkinsonian rats. Neurosci Lett. 2009;462:213–218. doi: 10.1016/j.neulet.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 57.Zurita M, Otero L, Aguayo C, Bonilla C, Ferreira E, Parajón A, Vaquero J. Cell therapy for spinal cord repair: optimization of biologic scaffolds for survival and neural differentiation of human bone marrow stromal cells. Cytotherapy. 2010;12:522–537. doi: 10.3109/14653241003615164. [DOI] [PubMed] [Google Scholar]


