Abstract
Previous studies have shown that vagus nerve stimulation can improve the prognosis of traumatic brain injury. The aim of this study was to elucidate the mechanism of the neuroprotective effects of vagus nerve stimulation in rabbits with brain explosive injury. Rabbits with brain explosive injury received continuous stimulation (10 V, 5 Hz, 5 ms, 20 minutes) of the right cervical vagus nerve. Tumor necrosis factor-α, interleukin-1β and interleukin-10 concentrations were detected in serum and brain tissues, and water content in brain tissues was measured. Results showed that vagus nerve stimulation could reduce the degree of brain edema, decrease tumor necrosis factor-α and interleukin-1β concentrations, and increase interleukin-10 concentration after brain explosive injury in rabbits. These data suggest that vagus nerve stimulation may exert neuroprotective effects against explosive injury via regulating the expression of tumor necrosis factor-α, interleukin-1β and interleukin-10 in the serum and brain tissue.
Keywords: nerve regeneration, brain injury, vagus nerve stimulation, tumor necrosis factor-α, interleukin-1β, interleukin-10, brain tissue pathology, protection, explosive injury, mechanisms, hydrocephalus, neural regeneration
Introduction
Traumatic brain injury is termed the “silent epidemic disease” and has a major public health impact worldwide (Hayward, 2008). Patients with traumatic brain injury often exhibit low blood pressure, hypoxemia, edema, inflammatory cytokine damage, and oxygen free radical injury in addition to the primary damage caused by the direct mechanical force, especially in the traumatic acute stage (Maas et al., 2007). Furthermore, increased levels of inflammatory factors such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-10 can lead to blood-brain barrier damage, nerve interstitial cells edema, neuronal necrosis and apoptosis, and chronic degeneration of the central nervous system (Lu et al., 2000, 2001, 2009). Such changes result in a secondary pattern of brain injury and increase the morbidity and mortality of traumatic brain injury patients. Since Borovikova et al. (2000) proposed the “cholinergic anti-inflammatory pathway” in 2000, there has been increasing interest on the role of cholinergic nerves and their transmitters and receptors. At the same time, vagus nerve stimulation technology in the field of functional neurosurgery is relatively mature, and is important for neuroprotection in diseases such as epilepsy, Parkinson's disease, and Alzheimer's disease (Ben-Menachem, 2002; Sjogren et al., 2002; Merrill et al., 2006; Englot et al., 2012). However, at present, the role of vagus nerve stimulation in the treatment of traumatic brain injury remains unclear (Kumaria and Tolias, 2012). Thus, in the present study, we examined the neuroprotective effects of vagus nerve stimulation on rabbits with brain explosive injury.
Materials and Methods
Experimental instruments and animals
A total of 28 healthy adult male New Zealand rabbits weighing 2.0–2.5 kg were provided by the Shanghai Songjiang Songlian Experimental Animal Farm (Shanghai, China; animal license No. SCXK (Hu) 2012-0011). The ‘Destructor’ is a commercial lightning firecracker, with specifications of 28 × 8 mm2 (Liling Huabu Export Firecrackers Factory (Xiang) YH AXZZ (2011) 001069) and charge quantity of 50 ± 5 mg black powder. Sweepings were obtained from after-product of metal removal by a planer, approximately 2–3 mm in diameter, weighing 3.21 ± 1.09 mg. Experiments were approved by the Animal Ethnic Committee, Affiliated Dongnan Hospital of Xiamen University, China.
Grouping and establishment of rabbit models of traumatic brain injury
Twenty-eight male rabbits were randomly assigned to blank control group (n = 4), sham surgery group (n = 6), explosive injury group (n = 10), and vagus nerve stimulation group (n = 8). A total of 24 rabbits in the sham surgery, explosive injury, and vagus nerve stimulation groups were intramuscularly anesthetized with Xylazine Sailaqin (4 mg/kg; Huamu Animal and Health Care Products Company, Jilin Province, China) and ketamine hydrochloride (10 mg/kg; Pharmacy Department, the 175 Hospital of PLA, China) at a 1:1 ratio. After shaving and sterilizing, the rabbits were fixed on a dissecting table in the prone position. A 4 cm straight incision was made 1.5 cm lateral to and 4.5 cm posterior to the midpoint of the median sagittal line and inner eye canthus line. A hole was drilled with a hand drill and enlarged with a rongeur forceps. Thus, a 1.5 cm-diameter round bone window was made. The dura mater was cut open to expose the right parietal cortex with no hemostasis.
Explosive injury group and vagus nerve stimulation group
After craniotomy, the lightning firecracker was wrapped with a fine iron wire at 2 mm from its end. The forelimbs of the rabbits were loosely fixed, and the midpoint of the firecracker was placed 0.5 cm away from the rabbits’ brain tissues. Five pieces of metal debris were placed on the right parietal cortex. At 15 minutes after the surgery, the rabbits were conscious, and could raise their heads and crawl. The firecrackers were lit outdoors to induce a rabbit model of explosive injury. Next, without hemostasis, the bone window was blocked with bone wax, and a whole-layer suture was performed. The rabbits were fixed on the dissecting table in the supine position at 1 hour after injury. An operation to dissociate the right vagus nerve in the neck was then performed. In vagus nerve stimulation group, a bipolar platinum hook electrode (pole spacing of 2 mm) was implanted on the neural stem segment of the right vagus. The vagus was then continuously stimulated at 10 V, 5 Hz, and 5 ms for 20 minutes while the electrode was connected to the continuous nerve stimulator (YLS-9A Physiological, pharmacological electronic stimulator; Beijing Zhong-Shi-Di-Chuang Technology Company, China).
Sham surgery group
The rabbits with opening of the right top skull were fixed on the dissecting table in the supine position at 1 hour after injury. An operation on neck was performed to dissociate the right vagus nerve, but animals received no stimulation.
Blank control group
Rabbits did not receive any treatment. Data were collected at corresponding time points, and brain tissues were obtained.
After regaining consciousness, all rabbits were returned to their cages. At 5 hours after injury, cranial CT scans (2 mm thick; the 175th Hospital of Chinese PLA, Trauma Neurosurgery Center of Nanjing Military Region, Fujian Province, China) were performed in all groups. At 6 hours, blood was collected from the central artery of the ear. At 24 hours, the rabbits were sacrificed and their brain tissues collected.
Detection of TNF-α, IL-1β, and IL-10 concentrations in serum and brain tissues in right parietal lobe
At 6 hours after injury (Wang et al., 2013), 2 mL of blood was collected from the central artery of the ear of each rabbit in each group. The blood was placed in a pro-coagulation tube for 4 hours at room temperature, and then centrifuged at 3,500 r/min for 20 minutes. Approximately 1 mL of supernatant was stored in an Eppendorf tube at −20°C. At 24 hours, rabbits from each group were sacrificed by air embolism. Approximately 0.1 g of contused brain tissues were rapidly homogenized with 3 mL of physiological saline at a low temperature, and centrifuged at 3,500 r/min for 5 minutes. Approximately 1 mL of supernatant was stored in an Eppendorf tube at −20°C. TNF-α, IL-1β, and IL-10 concentrations were detected in strict accordance with the instructions of enzyme linked immunosorbent assay (ELISA) kit (Shanghai Yili Biotechnology, Shanghai, China). The primary antibody was a rat anti-rabbit monoclonal antibody, and the second antibody was an HRP-conjugated sheep anti-rabbit antibody.
Preparation of pathological sections of brain tissues and determination of brain water content
At 24 hours after injury (Wang et al., 2013), rabbits from each group were sacrificed by air embolism. Brain tissues surrounding the contused foci within a radius of 1 cm (0.5 cm × 0.5 cm × 0.5 cm) were fixed in 4% formaldehyde for hematoxylin-eosin staining. A portion of 0.2–0.25 g of brain tissue was weighed with an analytical balance (with accuracy up to 0.001 g) after blood and water was absorbed with absorbent paper. After drying at 110°C for 10 hours in an oven, brain tissues were weighed. Brain water content was calculated by the following formula: water content = (wet weight – dry weight)/wet weight × 100%.
Statistical analysis
Data are expressed as the mean ± SD. All data were processed using SPSS 13.0 software (SPSS, Chicago, IL, USA) and analyzed with one-way analysis of variance. Intergroup mean value was compared by paired comparison of the least significant difference test. A value of P < 0.05 was considered statistically significant.
Results
General conditions
Of 18 rabbits in the explosive injury and vagus nerve stimulation groups, 1 died immediately after injury, while 17 presented transient apnea, startle, twitch, and falling after explosive injury. After resuscitation, spontaneous breathing and heart rate were stable. The survival time was longer than 24 hours, with a survival rate of 94.4%.
Cranial CT manifestations following brain explosive injury
Cranial CT scans were performed at 5 hours after model induction. Results revealed that obvious contusion (17/17, 100%), intracerebral hematoma (12/17, 70.6%), subarachnoid hemorrhage (10/17, 58.8%), contused brain tissue protruding into the bone window (12/17, 70.6%), intracranial pneumatocele (5/17, 29.4%), and residual iron (7/17, 41.2%) in the brain tissue of rabbits in the explosive injury and vagus nerve stimulation groups (Figure 1).
Figure 1.
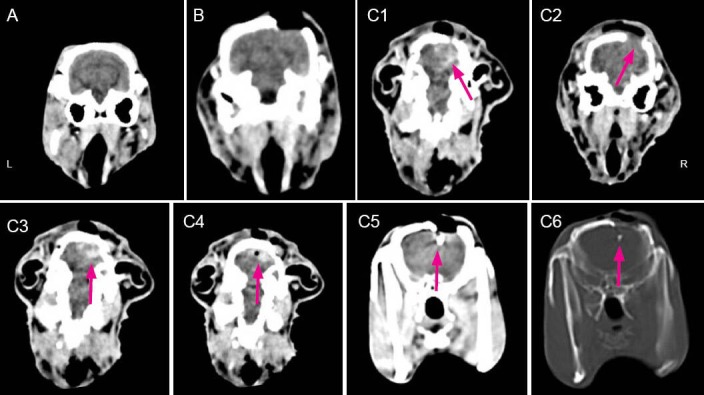
CT images of rabbit skull at 5 hours after brain explosive injury.
(A) Blank control group: normal performance. (B) Sham surgery group: normal performance. (C) Explosive injury group: C1, subarachnoid hemorrhage (arrow); C2, brain tissue protrusion (arrow); C3, intracerebral hematoma (arrow); C4, intracranial pneumatocele (arrow); C5, residual iron (soft tissue window) (arrow), and C6 residual iron (bone window) (arrow). L: Left; R: right.
Effect of vagus nerve stimulation on pathological manifestations of brain tissues following brain explosive injury
In the explosive injury and vagus nerve stimulation groups, light microscope analysis of hematoxylin-eosin staining demonstrated neuronal swelling, karyolysis, nerve cell necrosis, unclear nuclear structure, vacuolization, loose stroma, edema, microcapsule, and vasodilatation, with disappearance of Nissl bodies. The number of glial cells was obviously increased. Erythrocytes were adherent, and vascular engorgement, abundant erythrocyte extravasation, and inflammatory cell infiltration were visible in the contused site. By contrast, the brain tissue edema conditions were improved in the vagus nerve stimulation group compared with the explosive injury group, although they were still worse than the sham surgery group (Figure 2).
Figure 2.
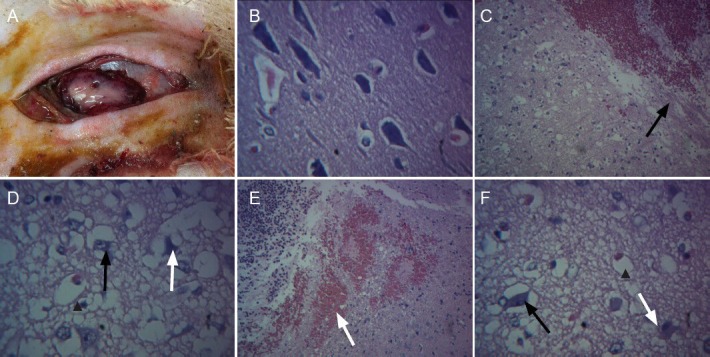
Effect of vagus nerve stimulation on pathological manifestations of brain tissues following brain explosive injury.
(A) Gross appearance of rabbit brain in the explosive injury group. (B) Pathological sections of brain tissues in the sham surgery group (hematoxylin-eosin staining, × 400). (C) Explosive injury group: loose partial region or stroma, edema, increased glial cell size, clear cytoplasm (cellular edema); interstitial hemorrhage and fibrinoid necrosis (upper right), scattered glial cells (black arrow), repaired brain tissues (hematoxylin-eosin staining, × 100). (D) Explosive injury group: some neuronal dissolution and necrosis, unclear nuclear structure, disappearance of Nissl bodies (white arrow); some neuronal swelling (black arrow), loose stroma, edema, microcapsule, vasodilatation, adherent erythrocytes (arrowhead) (hematoxylin-eosin staining, × 400). (E) Explosive injury group: interstitial hemorrhage (white arrow), abundant inflammatory cell infiltration (black arrow) (hematoxylin-eosin staining, × 100). (F) Vagus nerve stimulation group: partial neuronal dissolution and necrosis, unclear nuclear structure, disappearance of Nissl bodies (white arrow); some neuronal swelling (black arrow), loose stroma, slight edema, slight vasodilatation, and adherent erythrocytes (arrowhead) (hematoxylin-eosin staining, × 400).
Effect of vagus nerve stimulation on brain water content following brain explosive injury
Significant differences in brain water content were detected among blank control, sham surgery, explosive injury, and vagus nerve stimulation groups at 24 hours after injury (P < 0.01). The brain water content was significantly greater in the explosive injury group than that in the blank control and sham surgery groups (P < 0.01). In the vagus nerve stimulation group, brain water content was significantly higher than that in the blank control and sham surgery groups (P < 0.05), but lower than that in the explosive injury group (P = 0.06). These data suggest that vagus nerve stimulation reduced the degree of brain tissue edema after explosive injury (Figure 3).
Figure 3.
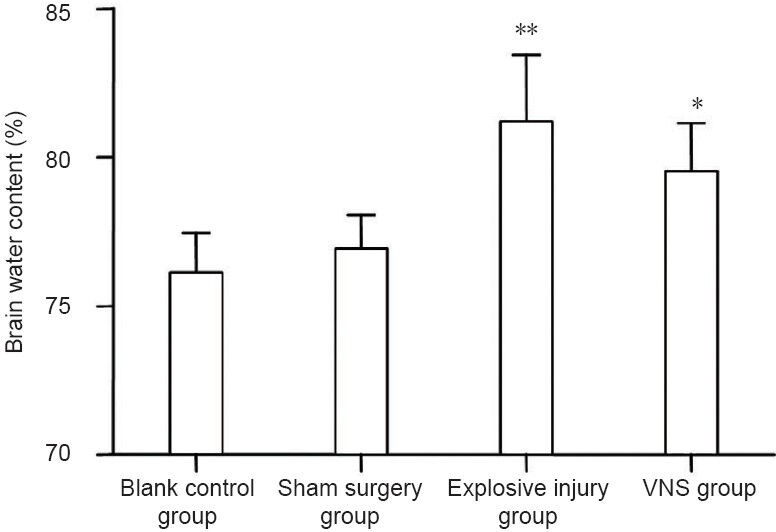
Effect of vagus nerve stimulation (VNS) on brain water content following brain explosive injury.
Brain water content was significantly higher in the vagus nerve stimulation group (n = 8) than that in the blank control (n = 4) and sham surgery (n = 6) groups, but lower than that in the explosive injury group (n = 9). *P < 0.05, **P < 0.01, vs. blank control group and sham surgery group. Data are expressed as the mean ± SD in all rabbits for each group (one-way analysis of variance and the least significant difference test).
Effect of vagus nerve stimulation on pro-inflammatory cytokines TNF-α and IL-1β levels in serum and brain tissues of rabbits with explosive injury
Significant differences in TNF-α and IL-1β levels were detectable between the blank control group, sham surgery group, explosive injury group, and vagus nerve stimulation group (serum: FTNF-α = 31.189, PTNF-β < 0.01; FIL-1α = 71.189, PIL-1β < 0.01; brain tissues: FTNF-α = 28.349, PTNF-α< 0.01; FIL-1β = 31.802, PIL-1β < 0.01). TNF-α and IL-1β levels in serum and brain tissues were significantly higher in the vagus nerve stimulation group than those in the blank control and the sham surgery (P < 0.01) groups, but significantly lower than those in the explosive injury group (P < 0.01). These data suggest that vagus nerve stimulation reduced the whole body inflammatory response after explosive injury (Figure 4).
Figure 4.
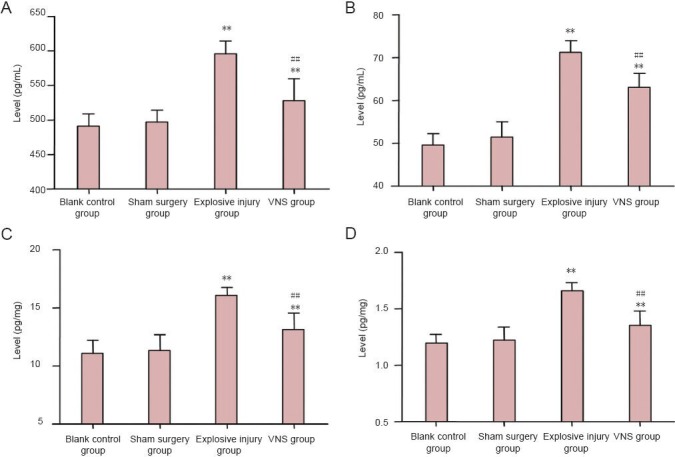
Effect of vagus nerve stimulation (VNS) on levels of the pro-inflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-1β in serum and brain tissues of rabbits with explosive injury.
(A) Serum TNF-α; (B) serum IL-1β; (C) brain tissue TNF-α; (D) brain tissue IL-1β. TNF-α and IL-1β levels in serum and brain tissues were significantly higher in the vagus nerve stimulation group (n = 8) than those in the blank control group (n = 4) and the sham surgery group (n = 6) (P < 0.01), but significantly lower than those in the explosive injury group (n = 9). **P < 0.01, vs. blank control group and sham surgery group; ##P < 0.01, vs. explosive injury group. Data are expressed as the mean ± SD in all rabbits for each group (one-way analysis of variance and the least significant difference test).
Effect of vagus nerve stimulation on anti-inflammatory cytokine IL-10 level in serum and brain tissues of rabbits with explosive injury
Significant differences in IL-10 levels were observed among the blank control, sham surgery, explosive injury, and vagus nerve stimulation groups (serum: FIL-10 = 25.986, PIL-10 < 0.01; brain tissues: FIL-10 = 25.318, PIL-10 < 0.01). IL-10 levels in the serum and brain tissues were significantly higher in the explosive injury group than that in the blank control and sham surgery (P < 0.05) groups, but significantly lower than that in the vagus nerve stimulation group (P < 0.01). These data suggest that vagus nerve stimulation promoted and maintained the production of the anti-inflammatory cytokine IL-10 after explosive injury (Figure 5).
Figure 5.
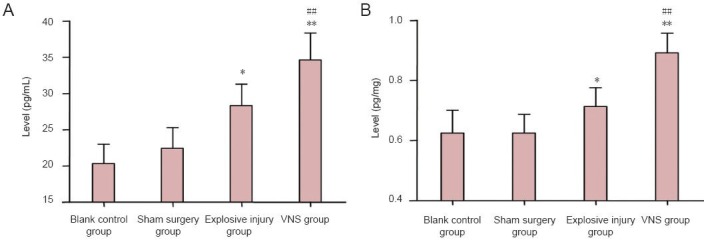
Effect of vagus nerve stimulation (VNS) on anti-inflammatory cytokine interleukin (IL)-10 levels in serum and brain tissues of rabbits with explosive injury.
(A) Serum IL-10; (B) brain tissue IL-10. IL-10 levels in serum and brain tissues were significantly higher in the explosive injury group (n = 9) than that in the blank control (n = 4) and sham surgery (n = 6) groups, but significantly lower than that in the vagus nerve stimulation group (n = 8). *P < 0.05, **P < 0.01, vs. blank control group and sham surgery group; ##P < 0.01, vs. explosive injury group. Data are expressed as the mean ± SD in all rabbits for each group (one-way analysis of variance and the least significant difference test).
Discussion
Despite modern advances in recovery of brain function after brain injury, traumatic brain injury continues to have a bad prognosis (Shi et al., 2013). Recent studies suggest that the pathological development of traumatic brain injury is not an instant and irreversible event, and that there is potential for saving and restoring brain function (Arundine et al., 2004; Kumaria and Tolias, 2008). However, current drug treatments have reached a bottleneck stage, and new treatment methods are required (Kumaria and Tolias, 2012). In the present study, we innovatively applied neural control technology in traumatic brain injury using vagus nerve stimulation technology as a neuroprotective strategy.
Hematoxylin-eosin staining results revealed that contused brain tissues after explosive injury exhibited neuronal swelling, karyolysis, necrosis, unclear nuclear structure, disappearance of Nissl bodies, increased number of glial cells, vacuolization, loose stroma, edema, microcapsule, vasodilatation, adherent erythrocytes, vascular engorgement, abundant erythrocyte extravasation, and inflammatory cell infiltration, consistent with a previously published study (Kilbourne et al., 2009). These manifestations indicated the occurrence of neural cell necrosis, glial cell proliferation, blood-brain barrier damage, cerebral edema, and inflammatory reaction. Levels of TNF-α, IL-1β, and IL-10 were increased in the serum (6 hours) and brain tissues (24 hours) of rabbits with explosive injury. However, vagus nerve stimulation effectively reduced levels of TNF-α and IL-1β, but increased levels of IL-10. Brain water content was consistent with TNF-α and IL-1β levels in brain tissues of rabbits of each group at 24 hours after injury. Pathological sections of brain tissues also revealed neuronal necrosis, hemorrhage, edema, inflammatory cell infiltration, and glial cell proliferation after injury, while the degree of cerebral edema was reduced by vagus nerve stimulation. These data suggest that TNF-α and IL-1β may participate in the pathophysiological processes of secondary brain injury and edema formation. The inflammatory reaction plays a key role in promoting nerve cell degeneration and necrosis. TNF-α and IL-1β are pro-inflammatory mediators that induce inflammation and regulate immunity, and can also promote a cascade of inflammatory reaction amplification, resulting in secondary brain tissue lesions, secondary edema, and brain cell necrosis. Our data show that vagus nerve stimulation can effectively reduce TNF-α and IL-1β levels, but increase IL-10 levels after traumatic brain injury. This is likely due to the large number of afferent fibers in the vagus nerve that project into the nucleus tractus solitarius, which in turn has a wide range of projections that contact with the forebrain and brainstem. Thus, vagus nerve stimulation can reduce the extent of the damage of nerve cells after traumatic brain injury through these widespread cholinergic anti-inflammatory pathways (Clough et al., 2007; Neese et al., 2007). Interestingly, vagus nerve stimulation was also reported to reduce blood-brain barrier damage by weakening the blood-brain barrier vessel permeability and limit AQP-4 water channel upregulation (Lopez et al., 2012).
Craniocerebral explosive injury is a type of open craniocerebral injury, and is frequently associated with presence of injuries to the scalp, skull, cerebral dura mater, and brain tissue. As such, hair, bone chips, metal, rubble, and gunpowder can be observed at the injury site. The incidence of intracranial infection was also reported to be high following craniocerebral gunshot wounds (Jimenez et al., 2013). Furthermore, intracranial brain contusion, intracranial hemorrhage, brain tissue swelling, and foreign body retention can be seen in skull CT. After traumatic brain injury in the presence of contusion, laceration, combined ischemia and hypoxia, a large number of inflammatory cytokines are activated and released, which results in nerve cell degeneration, necrosis, blood-brain barrier damage and edema, and accelerated secondary brain injury. This secondary brain injury contributes to the secretion of inflammatory factors and produces a vicious cycle of cell death. Without effective intervention, irreversible secondary pathological alterations can develop resulting in aggravated neurological function (Ziebell and Morganti-Kossmann, 2010; Wang et al., 2011; Stella, 2012). Therefore, therapeutic measures that target the inflammatory reaction and cerebral edema after traumatic brain injury are useful for reducing secondary brain damage, thus protecting neurological function and diminishing the sequelae of traumatic brain injury. In the present study, we used stimulation of the right vagus nerve to decrease brain edema and inflammation on the brain and serum in our rabbit brain explosion injury model. We found that vagus nerve stimulation reduced the degree of edema in rabbits with explosive injury, delayed edema process, significantly decreased TNF-α and IL-1β levels, but increased IL-10 levels, in serum and brain tissues. Because of a limitation of experimental funds and conditions, we were only able to analyze a limited number of factors such as brain tissue hematoxylin-eosin staining, inflammatory factors, and cephaledema. Future studies are required to provide more direct indicators of the neuroprotective effects of vagus nerve stimulation.
In conclusion, brain explosive injury was associated with inflammatory cytokine release through various pathophysiological mechanisms, blood-brain barrier injury, aggravated edema, and increased secondary brain injury. Vagus nerve stimulation exerts effectively protective effects on the brain via anti-inflammatory pathways and reduction of cephaledema. Future in-depth basic and clinical studies are required to determine optimal therapeutic strategy for use of vagus nerve stimulation.
Footnotes
Conflicts of interest: None declared.
Copyedited by Dean J, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
References
- 1.Arundine M, Aarts M, Lau A, Tymianski M. Vulnerability of central neurons to secondary insults after in vitro mechanical stretch. J Neurosci. 2004;24:8106–8123. doi: 10.1523/JNEUROSCI.1362-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 2002;1:477–482. doi: 10.1016/s1474-4422(02)00220-x. [DOI] [PubMed] [Google Scholar]
- 3.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 4.Clough RW, Neese SL, Sherill LK, Tan AA, Duke A, Roosevelt RW, Browning RA, Smith DC. Cortical edema in moderate fluid percussion brain injury is attenuated by vagus nerve stimulation. Neuroscience. 2007;147:286–293. doi: 10.1016/j.neuroscience.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 5.Englot DJ, Rolston JD, Wang DD, Hassnain KH, Gordon CM, Chang EF. Efficacy of vagus nerve stimulation in posttraumatic versus nontraumatic epilepsy. J Neurosurg. 2012;117:970–977. doi: 10.3171/2012.8.JNS122. [DOI] [PubMed] [Google Scholar]
- 6.Hayward P. Traumatic brain injury: the signature of modern conflicts. Lancet Neurol. 2008;7:200–201. doi: 10.1016/S1474-4422(08)70032-2. [DOI] [PubMed] [Google Scholar]
- 7.Jimenez CM, Polo J, Espana JA. Risk factors for intracranial infection secondary to penetrating craniocerebral gunshot wounds in civilian practice. World Neurosurg. 2013;79:749–755. doi: 10.1016/j.wneu.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Kilbourne M, Kuehn R, Tosun C, Caridi J, Keledjian K, Bochicchio G, Scalea T, Gerzanich V, Simard JM. Novel model of frontal impact closed head injury in the rat. J Neurotrauma. 2009;26:2233–2243. doi: 10.1089/neu.2009.0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumaria A, Tolias CM. In vitro models of neurotrauma. Br J Neurosurg. 2008;22:200–206. doi: 10.1080/02688690701772413. [DOI] [PubMed] [Google Scholar]
- 10.Kumaria A, Tolias CM. Is there a role for vagus nerve stimulation therapy as a treatment of traumatic brain injury? Br J Neurosurg. 2012;26:316–320. doi: 10.3109/02688697.2012.663517. [DOI] [PubMed] [Google Scholar]
- 11.Lopez NE, Krzyzaniak MJ, Costantini TW, Putnam J, Hageny AM, Eliceiri B, Coimbra R, Bansal V. Vagal nerve stimulation decreases blood-brain barrier disruption after traumatic brain injury. J Trauma Acute Care Surg. 2012;72:1562–1566. doi: 10.1097/TA.0b013e3182569875. [DOI] [PubMed] [Google Scholar]
- 12.Lu J, Goh SJ, Tng PY, Deng YY, Ling EA, Moochhala S. Systemic inflammatory response following acute traumatic brain injury. Front Biosci (Landmark Ed) 2009;14:3795–3813. doi: 10.2741/3489. [DOI] [PubMed] [Google Scholar]
- 13.Lu J, Moochhala S, Kaur C, Ling E. Changes in apoptosis-related protein (p53, Bax, Bcl-2 and Fos) expression with DNA fragmentation in the central nervous system in rats after closed head injury. Neurosci Lett. 2000;290:89–92. doi: 10.1016/s0304-3940(00)01307-0. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Moochhala S, Kaur C, Ling EA. Cellular inflammatory response associated with breakdown of the blood-brain barrier after closed head injury in rats. J Neurotrauma. 2001;18:399–408. doi: 10.1089/089771501750170976. [DOI] [PubMed] [Google Scholar]
- 15.Maas AI, Marmarou A, Murray GD, Teasdale SG, Steyerberg EW. Prognosis and clinical trial design in traumatic brain injury: the IMPACT study. J Neurotrauma. 2007;24:232–238. doi: 10.1089/neu.2006.0024. [DOI] [PubMed] [Google Scholar]
- 16.Merrill CA, Jonsson MA, Minthon L, Ejnell H, Blennow K, Karlsson M, Nordlund A, Rolstad S, Warkentin S, Ben-Menachem E, Sjogren MJ. Vagus nerve stimulation in patients with Alzheimer's disease: additional follow-up results of a pilot study through 1 year. J Clin Psychiatry. 2006;67:1171–1178. doi: 10.4088/jcp.v67n0801. [DOI] [PubMed] [Google Scholar]
- 17.Neese SL, Sherill LK, Tan AA, Roosevelt RW, Browning RA, Smith DC, Duke A, Clough RW. Vagus nerve stimulation may protect GABAergic neurons following traumatic brain injury in rats: an immunocytochemical study. Brain Res. 2007;1128:157–163. doi: 10.1016/j.brainres.2006.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi C, Flanagan SR, Samadani U. Vagus nerve stimulation to augment recovery from severe traumatic brain injury impeding consciousness: a prospective pilot clinical trial. Neurol Res. 2013;35:263–276. doi: 10.1179/1743132813Y.0000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sjogren MJ, Hellstrom PT, Jonsson MA, Runnerstam M, Silander HC, Ben-Menachem E. Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer's disease: a pilot study. J Clin Psychiatry. 2002;63:972–980. doi: 10.4088/jcp.v63n1103. [DOI] [PubMed] [Google Scholar]
- 20.Stella N. Neuroscience. Inflammation to rebuild a brain. Science. 2012;338:1303–1304. doi: 10.1126/science.1232331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang GH, Jiang ZL, Li YC, Li X, Shi H, Gao YQ, Vosler PS, Chen J. Free-radical scavenger edaravone treatment confers neuroprotection against traumatic brain injury in rats. J Neurotrauma. 2011;28:2123–2134. doi: 10.1089/neu.2011.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang XL, Sun GZ, Li SC, Zhao ZM. Effect of nuclear factor-KB on inflammatory cytokines expression after fluid percussion brain injury in rats. Zhonghua Shiyan Waike Zazhi. 2013;30:1484–1486. [Google Scholar]
- 23.Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


