Abstract
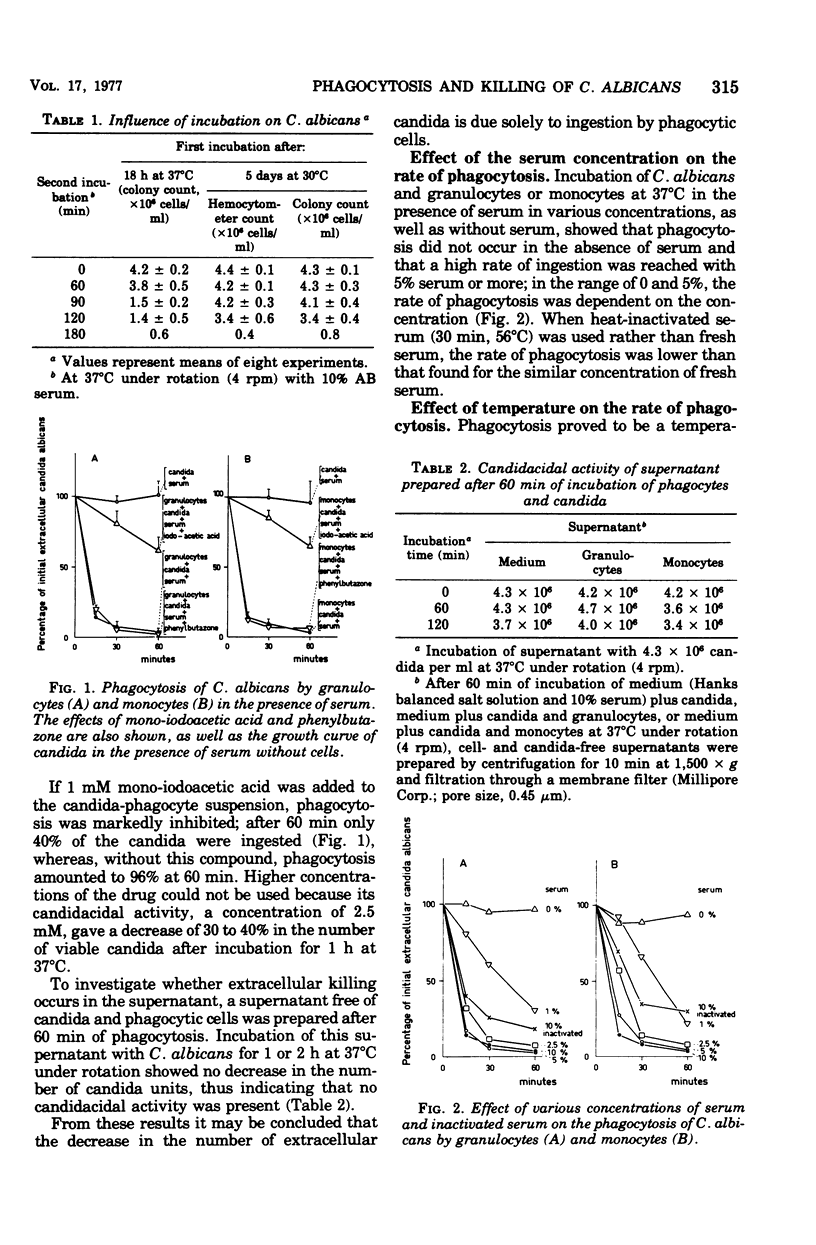
The study of the phagocytosis and intracellular killing of Candida albicans by granulocytes and monocytes has been hampered by the budding and pseudomycelium formation of this yeast during a relatively short incubation period at 37°C and by the similar density of candida cells and phagocytes, which makes differential centrifugation impossible. In the present study, C. albicans was used after 5 days of preculture at 30°C, after which the number of candida cells remained constant during incubation at 37°C for 90 min. On this basis, phagocytosis and intracellular killing were limited to a period of 60 min. Phagocytosis of C. albicans by granulocytes and monocytes was measured with a hemocytometer, the number of extracellular candida being a measure of the ingestion of these microorganisms. After 60 min, 96% of the candida cells were ingested by normal human granulocytes and monocytes. This process was dependent on the opsonin concentration and temperature and was inhibited by mono-iodoacetic acid. Heat-inactivated serum was less active than fresh serum, reflecting the role of complement factors with respect to opsonization. Intracellular killing was measured by a microbiological assay. After 60 min of incubation of phagocytes together with C. albicans and serum, human granulocytes and monocytes killed 58 and 50% of the ingested candida, respectively. This process was inhibited by phenylbutazone. Phagocytes from patients with chronic granulomatous disease showed impaired intracellular killing.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chilgren R. A., Hong R., Quie P. G. Human serum interactions with Candida albicans. J Immunol. 1968 Jul;101(1):128–132. [PubMed] [Google Scholar]
- Kernbaum S. Effects of hyperosmolality on candidacidal activity of human neutrophil polymorphonuclear leukocytes and on clumping of Candida albicans by human serum. Biomedicine. 1976 May;25(3):109–114. [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Measurement of candidacidal activity of specific leukocyte types in mixed cell populations I. Normal, myeloperoxidase-deficient, and chronic granulomatous disease neutrophils. Infect Immun. 1970 Jul;2(1):42–47. doi: 10.1128/iai.2.1.42-47.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid L., Brune K. Assessment of phagocytic and antimicrobial activity of human granulocytes. Infect Immun. 1974 Nov;10(5):1120–1126. doi: 10.1128/iai.10.5.1120-1126.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg C. O. Influence of therapeutic concentrations of phenylbutazone on granulocyte function. Acta Pathol Microbiol Scand B. 1975 Apr;83(2):100–102. doi: 10.1111/j.1699-0463.1975.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Boler J., Valdimarsson H. A chromium release assay for phagocytic killing of Candida albicans. J Immunol Methods. 1976;13(3-4):227–233. doi: 10.1016/0022-1759(76)90069-7. [DOI] [PubMed] [Google Scholar]


