Abstract
Flavonoids from the stems and leaves of Scutellaria baicalensis Georgi, an antioxidant, markedly improve memory impairments and neuronal injuries. In the present study, primary cortical neurons of rats were exposed to potassium cyanide to establish a model of in vitro neural cell apoptosis. Inhibition of apoptosis by flavonoids from the stems and leaves of Scutellaria baicalensis Georgi at concentrations of 18.98, 37.36, and 75.92 μg/mL was detected using this model. These flavonoids dramatically increased cell survival, inhibited cell apoptosis and excessive production of malondialdehyde, and increased the activities of superoxide dismutase, glutathione peroxidase, and Na+-K+-ATPase in primary cortical neurons exposed to potassium cyanide. The flavonoids from the stems and leaves of Scutellaria baicalensis Georgi were originally found to have a polyhydric structure and to protect against cerebral hypoxia in in vitro and in vivo models, including hypoxia induced by potassium cyanide or cerebral ischemia. The present study suggests that flavonoids from the stems and leaves of Scutellaria baicalensis Georgi exert neuroprotective effects via modulation of oxidative stress, such as malondialdehyde, superoxide dismutase, glutathione peroxidase and Na+-K+-ATPase disorders induced by potassium cyanide.
Keywords: nerve regeneration, brain injury, Scutellaria baicalensis Georgi, flavonoids, potassium cyanide, apoptosis, oxidative stress, Na+-K+-ATPase, neural regeneration
Introduction
Cerebral hypoxia is considered to be a hazardous factor in neurodegenerative diseases, accompanied by multiple neuropathological disturbances, including neuronal loss, energy exhaustion, neuronal inflammation, and other neuronal disorders (Chopra et al., 2011). In particular, oxidative stress and apoptosis propagate through distinctive and mutually exclusive signal transduction pathways and contribute to neuronal loss following hypoxic brain injury in neurodegenerative diseases (Blomgren et al., 2007). Thus, therapeutic interventions for hypoxic neuronal injuries should target the prevention of oxidative stress and apoptosis in a concerted way. In the laboratory, many in vitro or in vivo models have been used to study the neuro-pathophysiology of and provide drug screening for neurodegenerative diseases, including neuronal apoptosis.
Potassium cyanide is a respiratory inhibitor that induces especially strong inhibition of cytochrome oxidase. Cytochrome oxidase is a vital enzyme in the oxidative respiratory chain in mitochondria. There is evidence that electron transport in the respiratory chain will be cut off once cytochrome oxidase is inhibited, and consequently energy generation is blocked (Salkowski and Penney, 1994; Kunimoto et al., 1999). We know that neurons require a continuous energy supply from oxidative phosphorylation in the respiratory chain, and they are vulnerable to apoptosis induced by various kinds of hypoxia, including potassium cyanide insults (Mills et al., 1996). Potassium cyanide results in neuron detriments that are analogous to a series of neuronal morphology and metabolic disorders in which the brain was exposed to cerebral hypoxia (MacMillan, 1989). Thus, in the laboratory, potassium cyanide is commonly used as a hypoxic agent for reproducing states in patients who suffer from neurodegenerative diseases following hypoxia.
Flavonoids can be extracted from the aerial part of the stems and leaves of Scutellaria baicalensis Georgi, an antioxidant. These flavonoids have been found to ameliorate cognitive deficits and neuronal injuries in our previous studies. These functions on neurons are originally based on the polyhydric structure of flavonoids from the stems and leaves of Scutellaria baicalensis Georgi (Song et al., 2009; Liu et al., 2011; Shang et al., 2013). The present study, based on our previous studies, included an in vitro hypoxic model of rat primary cultured cortical cells to examine the effects of flavonoids from the stems and leaves of Scutellaria baicalensis Georgi on cell apoptosis and further explore the possible mechanism involved in oxidative stress and energy metabolites.
Materials and Methods
Animals
Pregnant Sprague-Dawley rats (16–18 days of gestation) were purchased from the Laboratory Animal Center of Hebei Medical University in China (certification No.10057). These rats were housed in a cage at 23 ± 1°C, kept in a 12-hour light-dark cycle, and were allowed free access to food and water. The protocols were approved by the Animals Ethics Committee of Chengde Medical College, China.
Drug
Flavonoid from the stems and leaves of Scutellaria baicalensis Georgi, a flavonoid compound, isolated from the aerial part of Scutellaria baicalensis Georgi were prepared by the Phytochemistry Laboratory, Institute of Traditional Chinese Medicine, Chengde Medical College, according to previously described procedures (Shang et al., 2005). The purity of flavonoids from the stems and leaves of Scutellaria baicalensis Georgi was about 61.88%, and scutellarein was identified to be the major ingredient by high performance liquid chromatography (Liu et al., 2011). Because some non-specific factors, such as pH, osmotic pressure, tannins, inorganic salts and other nonspecific substance in flavonoid from the stems and leaves of Scutellaria baicalensis Georgi may induce adverse effects on cells, rat serum was used to detect the properties of flavonoid from the stems and leaves of Scutellaria baicalensis Georgi against potassium cyanide-induced damage to primary cortical cells of rats. The preparation of rat serum containing flavonoids from the stems and leaves of Scutellaria baicalensis Georgi was based on a previous study (Shang et al., 2006).
Primary culture of cortical cells
The primary cortical cell culture in rats was carried out in accordance with a previous method (Xiao et al., 2000). Embryos were rapidly taken out from the womb of pregnant rats and the neocortices of embryos were dissected and put on a glass plate. The cortices were minced into 300–500 nm slices with scissors after the meninges and brain vessels were excised, and all slices were placed in a 50 mL conical flask containing 0.0625% trypsin by 20-time greater volume of minced sample. This triangular flask was placed on a magnetic mixer and stirred for 30 minutes at 37°C. The single cell suspension was centrifuged at 3,000 × g for 10 minutes, and the pellets of cells was re-suspended in Dulbecco's modified Eagle's medium (DMEM; Sigma, St. Louis, MO, USA), supplemented with 5% heat-inactivated bovine serum (Biological Industries, Kibbutz Beit-Haemek, Israel), 5% horse serum (Institute of Tianjin Blood, Tianjin, China), 100 U/mL penicillin and cultured at 37°C in a humidified incubator containing 5% CO2. The cells were seeded by 0.15 mL or 1 mL of suspension at a density of 8.3 × 104 or 2.25 × 105 cells per mL on 90- or 24-well plastic plates (Costar, New York, NY, USA) coated with 1% poly-L-lysine (Sigma), respectively. After the cells were seeded for 72 hours, cytosine arabinoside (10 μmol/L) was added into the culture system for 24 hours to inhibit non-neuronal proliferation. The following experiments were conducted 11 days after the cells were seeded.
Potassium cyanide and drug treatment
The primary cultured cortical cells of rats, at 11 days after seeding, were divided into a control group, potassium cyanide-treated group, and groups that received one of three doses of serum containing flavonoids from the stems and leaves of Scutellaria baicalensis Georgi groups (flavonoid from the stems and leaves of Scutellaria baicalensis Georgi group). The potassium cyanide-treated group and each of the three flavonoid groups were exposed to 1 mmol/L potassium cyanide (Anhui Shuguang, Anqing, China) for 60 minutes, as provided in a previous protocol description (Shang et al., 2008). Cells in the flavonoid groups were pre-hatched in 18.98, 37.36, or 75.92 μg/mL concentrations of flavonoids from the stems and leaves of Scutellaria baicalensis Georgi-containing serum (Shang et al., 2006, 2008) for 24 hours prior to potassium cyanide addition. The control cells were given the same content of rat serum without flavonoids. Cell survival, lactate dehydrogenase release, and malondialdehyde, superoxide dismutase, glutathione peroxidase, and Na+-K+-ATPase activities were assayed 60 minutes following cell exposure to 1 mmol/L potassium cyanide. Four or five wells contributed to each independent group. Neuronal morphology was observed with an inverted microscope (Chongqing Optical or Electrical Instrument Co., Chongqing, China).
Cell survival determination
Cell survival was assayed by 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT, Sigma) and lactate dehydrogenase (Nanjing Jiancheng Institute of Biological Engineering, Nanjing, China) release. After primary cortical cells were cultured for 11 days, the cell medium was discarded, and serum containing DMEM or one of the three doses of flavonoids from the stems and leaves of Scutellaria baicalensis Georgi was added, and the cells remained in culture for 24 hours in a 5% CO2 incubator at 37°C. After these cells were applied to 1 mmol/L potassium cyanide for 60 minutes, the cell culture medium was discarded. DMEM was added and proceeded incubation for 24 hours, and cell morphology was observed and photographed with an XDS-1A inverted microscope (Guangzhou Guangxue, Guangzhou, China). The cultured medium was collected for assay of cellular lactate dehydrogenase release. An MTT stock solution was added to each well to a final concentration of 0.5 mg/mL, and then incubated for 4 hours. Finally, the MTT solution was discarded and dimethyl sulfoxide was added to each well and left overnight in the incubator. The yield of formazan was detected by optical density at λ570 nm with a microplate reader (Institute of Beijing New Technology Applications Research, China).
DNA fragment assay
Rat primary cultured cortical cells that were exposed to 1 mmol/L potassium cyanide for 60 minutes in the absence or presence of flavonoids from the stems and leaves of Scutellaria baicalensis Georgi-containing serum were collected. The fragmented DNA of the cortical cells was isolated according to the instructions of DNA and RNA purification kits and the DNA amount obtained from each group was equalized. The DNA pellets were re-dissolved in 20 μL TE buffers (1 mmol/L ethylenediamine tetraacetic acid, 10 mmol/L Tri-HCl, pH 7.6) and electrophoresis was performed on 1.5% agarose gel for 30 minutes at 100 V. Finally, the agarose gel was stained with ethidium bromide and photographed using the Ultra Violet Gel Documentation System (Beijing Maisiqi, Beijing, China).
Free radical system and Na+-K+-ATPase assay
For assaying the level of malondialdehyde and the activities of superoxide dismutase, glutathione peroxidase, and Na+-K+-ATPase, the primary cultured cortical cells of rats were washed with ice-cold PBS and then re-suspended with 0.1 mol/L PBS containing 0.05 mmol/L ethylenediamine tetraacetic acid. The cell suspension was frozen for 2 hours at −85°C in an ultra-low temperature freezer and quickly thawed at 37°C. The same process was repeated three times and the ruptured cell suspension was centrifuged at 10,000 × g at 4°C for 1 hour. The supernatant was used for analyses of malondialdehyde, superoxide dismutase, glutathione peroxidase, and Na+-K+-ATPase. The measured reagent kits of the above targets were provided by Nanjing Jiancheng Bioengineering Institute, Nanjing, China.
Malondialdehyde assay
The thiobarbituric acid method was used to estimate the level of intracellular malondialdehyde in accordance with the method of Ohkawa et al. (1979). Two units of thiobarbituric acid together with one unit sample supernatant were boiled for 40 minutes at 100°C and then centrifuged at 3,000 × g for 10 minutes. The optical density of each sample was determined at λ532 nm using a 752N spectrophotometer (Shanghai Precision & Science Instrument Co., Shanghai, China). Intracellular malondialdehyde levels were calculated based on the manufacturer's instructions provided with the reagent kit (Nanjing Jiancheng Bioengineering Institute).
Superoxide dismutase activity assay
The measurement of superoxide dismutase activity was conducted according to a xanthine-xanthine oxidase method (Yoshioka, 1979). The sample supernatant together with xanthine-xanthine oxidase reagent was incubated for 40 minutes at 37°C for the proceeding enzymatic reaction. The reaction was terminated by adding sodium dodecyl sulfate. The optical density of each sample was detected at λ532 nm and superoxide dismutase activity was evaluated in accordance with a sodium nitrite standard curve: Y = 0.06683X − 0.009373, r = 0.9987, where X represents the concentration of sodium nitrite and Y represents the optical density of sodium nitrite combined with developer. The process for measuring superoxide dismutase followed the manufacturer's instructions provided with the reagent kit (Nanjing Jiancheng Bioengineering Institute).
Glutathione peroxidase activity assay
Glutathione peroxidase was analyzed according to a previous method (Zakowski, 1978). Its activity was detected by determination of the rate from reduced glutathione to oxidized glutathione by hydrogen peroxide. Reduced glutathione combines with 5,5′-dithiobis-(2-nitrobenzoic acid) to form a yellow substance that was detected by optical density at λ412 nm with a spectrophotometer. Measurement was performed in an ice-cold environment following the manufacturer's instructions provided with the reagent kit (Nanjing Jiancheng Bioengineering Institute).
Na+-K+-ATPase activity assay
The ammonium molybdate method was used to calculate Na+-K+-ATPase activity, based on the phosphoric acid was formed from ATP as described by He et al. (1999). The assay was carried out according to the manufacturer's instructions provided with the reagent kit (Nanjing Jiancheng Bioengineering Institute). The Na+-K+-ATPase activity was calculated as follows: Na+-K+-ATPase activity in cell supernatant = (A1–A2) ÷ A3 × C × D × 6 (μmol/ pi/min/mL), where A1, A2 and A3 are the optical density of sample, control and standard, respectively. C is the phosphoric content of standard. D is the dilution times of the sample.
Statistical analysis
All data were expressed as the mean ± SEM, and were analyzed with Statpark statistical software (Oracle Corporation, Redwood Shores, CA, USA). One-way analysis of variance followed by Duncan's multiple-range test was applied to analyze differences of groups for all variables. A P < 0.05 was considered a statistically significant difference between the groups.
Results
Serum containing flavonoids from the stems and leaves of Scutellaria baicalensis Georgi attenuated potassium cyanide-induced damage in primary cortical cells of rats
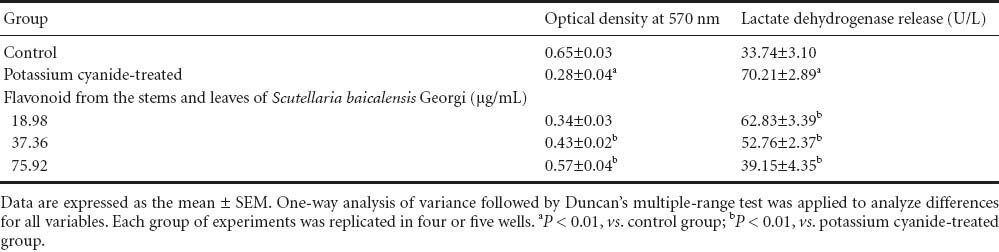
The neurons in the control group exhibited an intact state, resembling normal growth, tight attachment on the surface of a cultured dish, a dipolar/multipolar appearance, an integrated and smooth cell membrane, and a vivid middle cell body, as well as long and thick neurites shaped with a reticular structure (Figure 1A). However, when the cells were exposed to potassium cyanide at 1 mmol/L for 60 minutes, severely damaged cells were observed. The cells in Figure 1B show that the count and volume of cells were notably decreased, the cell neurites and reticular structures disappeared, the cells assumed a rounded shape with rough cell membrane, and there were many dead and fragmented cells. Interestingly, cell injuries by potassium cyanide were significantly lower after treatment with serum containing flavonoids from the stems and leaves of Scutellaria baicalensis Georgi (18.98, 37.36, or 75.92 μg/mL) for 24 hours (Figures 1C–E). Additionally, damage to rat primary cultured neurons was evaluated by assaying survival with the MTT assay and MTT-dependent lactate dehydrogenase release of cells. As displayed in Table 1, compared with the control group, the optical density value at 570 nm decreased by 56.92% (P < 0.01), and lactate dehydrogenase release increased by 108.08% (P < 0.01), in the potassium cyanide-treated group. The optical density value at 570 nm increased by 21.23% in 18.98 μg/mL, by 53.57% (P < 0.01) in 37.36 μg/mL, and by 103.57% (P < 0.01) in 75.92 μg/mL serum containing flavonoids from the stems and leaves of Scutellaria baicalensis Georgi, respectively. Meanwhile, increased lactate dehydrogenase release in the potassium cyanide-treated group was lowered by treatment with serum containing flavonoids from the stems and leaves of Scutellaria baicalensis Georgi for 24 hours, which were 25.24% (P < 0.01) by 18.98 μg/mL, 38.33% (P < 0.01) by 37.36 μg/mL and 49.69% (P < 0.01) by a concentration of 75.92 μg/mL, respectively. The above results show that flavonoids from the stems and leaves of Scutellaria baicalensis Georgi improve cell morphology in parallel with lactate dehydrogenase release, in a dose-dependent manner.
Figure 1.
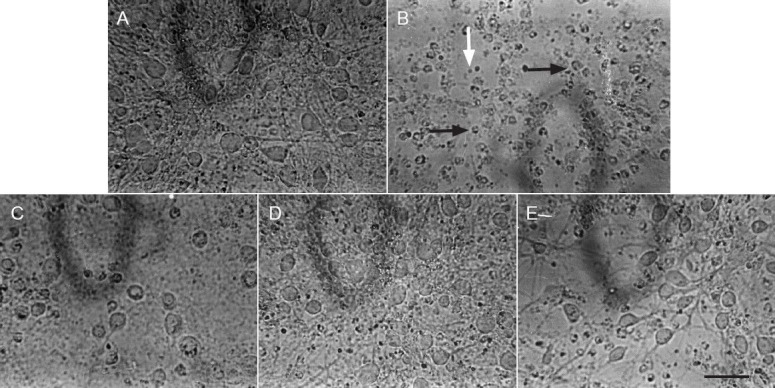
Serum containing flavonoids from the stems and leaves of Scutellaria baicalensis Georgi lessens potassium cyanide-induced injury in primary cultured neurons in rats (× 400).
(A) Primary cortical neurons from the control group. (B) Primary cortical neurons from the potassium cyanide-treated group. Primary cortical neuron exposed to 1 mmol/L potassium cyanide for 60 minutes showed that the count and volume of cells were notably decreased, cell neuritis and reticular structure disappeared and the cells assumed a rounded shape with a rough cell membrane (black arrows), and there were many dead and fragmented cells (white arrow). (C–E) Primary cortical neurons from the groups exposed to flavonoids from the stems and leaves of Scutellaria baicalensis Georgi groups at doses of 18.98, 37.36, 75.92 μg/mL for 24 hours, followed by exposure to potassium cyanide (1 mmol/L) for 60 minutes, respectively. Cell morphology was observed and photographed by reversed microscopy.
Table 1.
Effects of flavonoids from the stems and leaves of Scutellaria baicalensis Georgi on cell viability and lactate dehydrogenase release in primary cortical neurons of injured rats induced by potassium cyanide
Serum containing flavonoids from the stems and leaves of Scutellaria baicalensis Georgi reduced potassium cyanide-induced neuronal apoptosis
To elucidate potassium cyanide induced cell apoptosis, the present studies used the intracellular DNA fragmentation pattern by agarose gel electrophoresis. As shown in Figure 2, chromatin cleavage was visible in primary cortical cells exposed to potassium cyanide at 1 mmol/L for 60 minutes, as a “ladder” pattern representing DNA fragmentation into multiple oligonucleosome-length fragments. Simultaneously, preincubation of these primary cortical cells with flavonoids from the stems and leaves of Scutellaria baicalensis Georgi at a dose of 75.92 μg/mL dramatically attenuated potassium cyanide-induced apoptosis by weakening ladder pattern on agarose gel electrophoresis.
Figure 2.
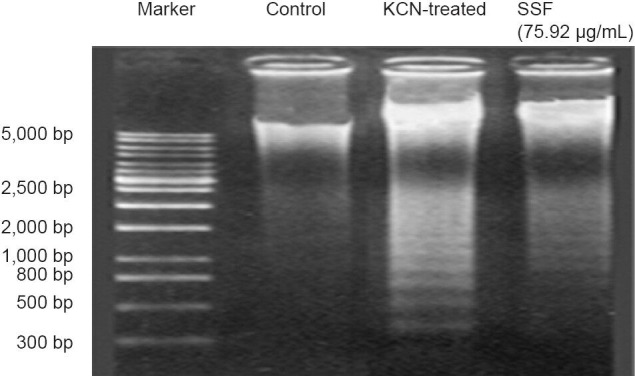
Serum containing flavonoids from the stems and leaves of Scutellaria baicalensis Georgi (SSF) diminishes potassium cyanide (KCN)-induced DNA fragmentations in neuronal nuclei.
The primary cortical neurons were treated with 1 mmol/L KCN for 60 minutes with or without 75.92 μg/mL SSF. Fragmented DNA was isolated by NucleoBond DNA and RNA purification kits, electrophoresed with agarose gel, and finally stained with ethidium bromide.
Serum containing flavonoids from the stems and leaves of Scutellaria baicalensis Georgi decreased malondialdehyde levels and increased superoxide dismutase, glutathione peroxidase and Na+-K+-ATPase activities in rat primary cortical cells with potassium cyanide exposure
The present study shows that damage to primary cultured cortical cells induced by potassium cyanide was related to free radical system and Na+-K+-ATPase activity disturbances. As shown in Figure 3, the intracellular malondialdehyde level in potassium cyanide-treated neurons was 2.66 times higher than that of the control group (P < 0.01). Additionally, superoxide dismutase, glutathione peroxidase, and Na+-K+-ATPase activities in potassium cyanide-treated neurons were 46.26, 56.69, and 61.19% lower, respectively, compared with the control group (P < 0.01). Fortunately, the increase in malondialdehyde levels and the decreases in superoxide dismutase, glutathione peroxidase, and Na+-K+-ATPase activities in neurons caused by potassium cyanide were significantly reversed by treatment with serum containing flavonoids from the stems and leaves of Scutellaria baicalensis Georgi at doses of 18.98, 37.36 and 75.92 μg/mL for 24 hours (P < 0.05, P < 0.01).
Figure 3.
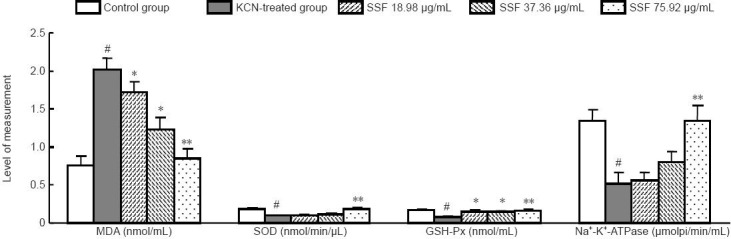
Effects of serum containing flavonoid from the stems and leaves of Scutellaria baicalensis Georgi (SSF) on content of malondialdehyde (MDA) and activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and Na+-K+-ATPase of potassium cyanide (KCN) in primary cortical neurons.
Data are expressed as the mean ± SEM. One-way analysis of variance followed by Duncan's multiple-range test was applied to analyze differences for all variables. Each group of experiments was replicated in four or five wells. #P < 0.01, vs. control group; *P < 0.05, **P < 0.01, vs. KCN-treated group.
Discussion
Many reports demonstrate that brain hypoxia primarily contributes to cerebral damage, including neuronal apoptosis (Lee et al., 1990). A reduced oxygen supply can start a potential, harmful cascade, such as biochemical reactions, glucose phosphorylation interruptions, intracellular adenosine triphosphate exhaustion, lactic acid accumulation, membrane depolarization, excitatory amino acid release, and Na+, Ca2+, water, and free radical retention within neurons. These disturbances of neurons can result in cytotoxicity through apoptosis (Zhang et al., 2012). Many studies provide direct morphological and biochemical evidences that neurons degenerate via apoptosis, including the formation of DNA fragments, nuclear apoptotic bodies, and other markers of apoptosis. Additionally, neuronal hypoxia can reportedly induce apoptosis and this apoptosis has been associated with genetic mutation, which possibly results in neurodegenerative diseases including Alzheimer's disease (Guo et al., 2011). The above studies indicate that neuronal apoptosis plays an important role in neurodegenerative disease, and neuronal hypoxia may be a vital cause of neuronal apoptosis. Therefore, targeting neuronal hypoxia is a reasonable strategy for the development of new treatments for neurodegenerative diseases.
Potassium cyanide, an inhibitor of cytochrome oxidase, can block mitochondria oxidative respiratory chain metabolism and interrupt effective adenosine triphosphate production (Salkowski and Penney, 1994; Kunimoto et al., 1999). The brain is critically susceptible supply from the mitochondria oxidative respiratory chain, because of lower stores of energy in brain tissue compared with other tissues (Wang et al., 2010). In the present study, primary cortical neurons exposed to 1 mmol/L potassium cyanide for 60 minutes underwent typical apoptosis, including cell body condensation and neurite reticular structure dissolution as shown by morphology observation. These results are consistent with a previous study (Zhou et al., 2007). Flavonoids from the stems and leaves of Scutellaria baicalensis Georgi in range of 18–76 μg/mL for 24 hours conferred good protection as assayed by MTT and MTT-dependent lactate dehydrogenase release reductions. When measured with agarose gel electrophoresis, 75.92 μg/mL flavonoids from the stems and leaves of Scutellaria baicalensis Georgi substantially weakened potassium cyanide-induced DNA laddering. These results strongly suggest that flavonoids from the stems and leaves of Scutellaria baicalensis Georgi protect neurons against potassium cyanide-induced apoptosis. These are consistent with our previous studies that showed that flavonoids from the stems and leaves of Scutellaria baicalensis Georgi improved memory dysfunction and neuronal degeneration caused by hypoxia (Markesbery, 1997).
It has been well documented that cellular events are involved in oxidative stress and energy metabolism disorders, which may be mediated by hypoxia. Our results are consistent with the conclusion that potassium cyanide can cause cellular events, such as apoptosis along with dysfunctions in oxidation and energy metabolism (Rayner et al., 2006; Tan et al., 2013). Free radicals are normal products of internal metabolism and some insults, including hypoxia. Excessive free radical production can result in peroxidation of DNA, proteins, fatty acids, and other biomacromolecules, especially proteases. The membrane of cells is extremely vulnerable to insults because of its unsaturated fatty acid structure and likelihood of encountering lipid peroxidation. This can induce cell structure damage and functional disorders, including signal transduction and gene regulation disturbances, which eventually leads to cell death or apoptosis (Miao et al., 2003). Lipid peroxidation is associated with degeneration of neurons, and malondialdehyde as the terminal product of lipid peroxidation, is a highly reactive and cytotoxic species that is responsible for neuron apoptosis. The level of malondialdehyde can be used as a clear signal of neuron states. Additionally, some antioxidants, including superoxide dismutase, catalase, glutathione peroxidase and non-enzymatic antioxidants, such as α-tocopherol, ascorbate, reduced glutathione, and cysteine serve as detoxifying systems to prevent cytotoxic insults.
Many reports have confirmed that superoxide dismutase combined with glutathione peroxidase may thoroughly eliminate lipid hydroperoxides and provide a mechanism for the repair of oxidized membrane components. Thus, when superoxide dismutase and glutathione peroxidase exposed neurons are a feature of a disorder, induced by insults including hypoxia, it may be accompanied by neuronal apoptosis (Maiti et al., 2008; Jomova et al., 2010; Shuangyan et al., 2012). Na+-K+-ATPase activity is sensitive to the supply of adenosine triphosphate. When neurons become hypoxic, intracellular adenosine triphosphate is rapidly depleted, and Na+-K+-ATPase activity decreases. Lower Na+-K+-ATPase activity can induce neural apoptosis (Iijima et al., 2006). In the present study, we found that primary cortical cells exposed to 1 mmol/L potassium cyanide for 60 minutes underwent apoptosis, which was accompanied by raised levels in malondialdehyde and lowered activities in superoxide dismutase, glutathione peroxidase, and Na+-K+-ATPase. Interestingly, flavonoids from the stems and leaves of Scutellaria baicalensis Georgi clearly attenuated the excessive production of malondialdehyde, and superoxide dismutase, glutathione peroxidase, and Na+-K+-ATPase activities decrease. The results of the present study are in accordance with our previous findings that flavonoids from the stems and leaves of Scutellaria baicalensis Georgi inhibit oxidative stress and Na+-K+-ATPase metabolism disorders both in vivo and in vitro (Song et al., 2009; Liu et al., 2011). The polyphenol structure of flavonoids from the stems and leaves of Scutellaria baicalensis Georgi provides protective properties against free radical attack and cytotoxicity induced by hypoxia. That flavonoids from the stems and leaves of Scutellaria baicalensis Georgi ameliorate malondialdehyde, superoxide dismutase, and glutathione peroxidase disturbances in primary cortical cells of hypoxic rats is paralleled by Na+-K+-ATPase disorders, evidently supports the hypothesis that the anti-apoptotic effects of flavonoids from the stems and leaves of Scutellaria baicalensis Georgi are mediated by its strongly anti-oxidative properties.
The above studies therefore confirm that flavonoids may provide new treatments for neurodegenerative diseases, such as Alzheimer disease, characterized by cerebral apoptosis, oxidative stress, and Na+-K+-ATPase metabolism disruptions. Whether the salutary effects of Scutellaria baicalensis on cell survival are a consequence of antioxidant properties versus cell death induced by potassium cyanide requires further mechanistic studies to extend our establishment of its antioxidant effects.
In summary, flavonoids from the stems and leaves of Scutellaria baicalensis Georgi inhibited apoptosis and modulated oxidative stress and Na+-K+-ATPase detriments induced by potassium cyanide in rat primary cortical cells, in a dose-dependent manner. The increasingly beneficial effects of three increasing doses of flavonoid from the stems and leaves of Scutellaria baicalensis Georgi on cell survival and apoptosis paralleled attenuation in malondialdehyde, superoxide dismutase, glutathione peroxidase, and Na+-K+-ATPase deregulation. The present results, together with our previous reports, demonstrate that flavonoids from the stems and leaves of Scutellaria baicalensis Georgi protects against neuronal injuries and cognitive dysfunction in models in vivo, suggesting that flavonoids from the stems and leaves of Scutellaria baicalensis Georgi may make important contributions to the treatment of neurodegenerative disease.
Footnotes
Conflicts of interest: None declared.
Copyedited by Murnane K, Robens J, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Blomgren K, Leist M, Groc L. Pathological apoptosis in the developing brain. Apoptosis. 2007;12:993–1010. doi: 10.1007/s10495-007-0754-4. [DOI] [PubMed] [Google Scholar]
- 2.Chopra K, Misra S, Kuhad A. Neurobiological aspects of Alzheimer's disease. Expert Opin Ther Targets. 2011;15:535–555. doi: 10.1517/14728222.2011.557363. [DOI] [PubMed] [Google Scholar]
- 3.Guo MF, Yu JZ, Ma CG. Mechanisms related to neuron injury and death in cerebral hypoxic ischaemia. Folia Neuropathol. 2011;49:78–87. [PubMed] [Google Scholar]
- 4.He YZ, Li M, Feng GL, Wang YC. Inhibition of pyrethroid insecticides on nerve Na-K-ATPase in house flies (Musca Domestica) Acta Entomol Sin. 1999;42:9–24. [Google Scholar]
- 5.Iijima T, Mishima T, Akagawa K, Iwao Y. Neuroprotective effect of propofol on necrosis and apoptosis following oxygen-glucose deprivation--relationship between mitochondrial membrane potential and mode of death. Brain Res. 2006;1099:25–32. doi: 10.1016/j.brainres.2006.04.117. [DOI] [PubMed] [Google Scholar]
- 6.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 7.Kunimoto S, Nosaka C, Takeuchi T. Stimulation of cellular XTT reduction by cytochrome oxidase inhibitors. Biol Pharm Bull. 1999;22:660–661. doi: 10.1248/bpb.22.660. [DOI] [PubMed] [Google Scholar]
- 8.Lee MM, Hseih MT, Kuo JS, Yeh FT, Huang HM. Magnolol protects cortical neuronal cells from chemical hypoxia in rats. Neuroreport. 1998;9:3451–3456. doi: 10.1097/00001756-199810260-00021. [DOI] [PubMed] [Google Scholar]
- 9.Liu YP, Cao K, Miao H, Cheng JJ, Shang Yazhen. Flavonoids from the stems and leaves of Scutellaria baicalensis Georgi attenuate H2O2-induced oxidative damage to rat cortical cells. Neural Regen Res. 2011;6:2100–2104. [Google Scholar]
- 10.MacMillan VH. Cerebral energy metabolism in cyanide encephalopathy. J Cereb Blood Flow Metab. 1989;9:156–162. doi: 10.1038/jcbfm.1989.23. [DOI] [PubMed] [Google Scholar]
- 11.Maiti P, Singh SB, Mallick B, Muthuraju S, Ilavazhagan G. High altitude memory impairment is due to neuronal apoptosis in hippocampus cortex and striatum. J Chem Neuroanat. 2008;36:227–238. doi: 10.1016/j.jchemneu.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 13.Miao H, Su YF, Cheng JJ, Shang YZ. Effect of SSF on learning and memory in mice. Chengde Yixueyuan Xuebao. 2003;20:299–302. [Google Scholar]
- 14.Mills EM, Gunasekar PG, Pavlakovic G, Isom GE. Cyanide-induced apoptosis and oxidative stress in differentiated PC12 cells. J Neurochem. 1996;67:1039–1046. doi: 10.1046/j.1471-4159.1996.67031039.x. [DOI] [PubMed] [Google Scholar]
- 15.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Rayner BS, Duong TT, Myers SJ, Witting PK. Protective effect of a synthetic anti-oxidant on neuronal cell apoptosis resulting from experimental hypoxia re-oxygenation injury. J Neurochem. 2006;97:211–221. doi: 10.1111/j.1471-4159.2006.03726.x. [DOI] [PubMed] [Google Scholar]
- 17.Salkowski AA, Penney DG. Cyanide poisoning in animals and humans: a review. Vet Hum Toxicol. 1994;36:455–466. [PubMed] [Google Scholar]
- 18.Shang YZ, Cheng JJ, Qi J, Miao H. Scutellaria flavonoid reduced memory dysfunction and neuronal injury caused by permanent global ischemia in rats. Pharmacol Biochem Behav. 2005;82:67–73. doi: 10.1016/j.pbb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Shang YZ, Qin BW, Cheng JJ, Miao H. Prevention of oxidative injury by flavonoids from stems and leaves of Scutellaria baicalensis Georgi in PC12 cells. Phytother Res. 2006;20:53–57. doi: 10.1002/ptr.1802. [DOI] [PubMed] [Google Scholar]
- 20.Shang YZ, Qin BW, Cheng JJ, Miao H. Effect of Scutellaria falvonoids on KCN-induced damages in rat pheochromocytoma PC12 cells. Indian J Med Res. 2008;127:610–615. [PubMed] [Google Scholar]
- 21.Shang YZ, Zhang H, Cheng JJ, Miao H, Liu YP, Cao K, Miao H, Wang H. Falvonoid from Scutellaria baicalensis Georgi is effective to treat cerebral ischemia/reperfusion. Neural Regen Res. 2013;8:514–522. doi: 10.3969/j.issn.1673-5374.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shuangyan W, Ruowu S, Hongli N, Bei Z, Yong S. Protective effects of Rg2 on hypoxia-induced neuronal damage in hippocampal neurons. Artif Cells Blood Substit Immobil Biotechnol. 2012;40:142–145. doi: 10.3109/10731199.2011.611474. [DOI] [PubMed] [Google Scholar]
- 23.Song HR, Cheng JJ, Miao H, Shang Yazhen. Scutellaria flavonoid supplementation reverses ageing-related cognitive impairment and neuronal changes in aged rats. Brain Inj. 2009;23:146–153. doi: 10.1080/02699050802649670. [DOI] [PubMed] [Google Scholar]
- 24.Tan X, Guo X, Liu H. Melatonin attenuates hippocampal neuron apoptosis and oxidative stress during chronic intermittent hypoxia via up-regulating B-cell lymphoma-2 and down-regulating B-cell lymphoma-2-associated X protein. Saudi Med J. 2013;34:701–708. [PubMed] [Google Scholar]
- 25.Wang X, Michaelis ML, Michaelis EK. Functional genomics of brain aging and Alzheimer's disease: focus on selective neuronal vulnerability. Curr Genomics. 2010;11:618–633. doi: 10.2174/138920210793360943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao XQ, Wang R, Tang XC. Huperzine A and tacrine attenuate beta-amyloid peptide-induced oxidative injury. J Neurosci Res. 2000;61:564–569. doi: 10.1002/1097-4547(20000901)61:5<564::AID-JNR11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 27.Yoshioka T, Sugiue A, Shimada T, Utsumi K. Superoxide dismutase activity in the maternal and cord blood. Biol Neonate. 1979;36:173–180. doi: 10.1159/000241224. [DOI] [PubMed] [Google Scholar]
- 28.Zakowski JJ, Tappel AL. A semiautomated system for measurement of glutathione in the assay of glutathione peroxidase. Anal Biochem. 1978;89:430–436. doi: 10.1016/0003-2697(78)90372-x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang SX, Wang Y, Gozal D. Pathological consequences of intermittent hypoxia in the central nervous system. Compr Physiol. 2012;2:1767–1777. doi: 10.1002/cphy.c100060. [DOI] [PubMed] [Google Scholar]
- 30.Zhou GY, Hu XQ, Chen XH. Experimental study on effect of Buchang Naoxintong containing serum for antagonizing hypoxia-induced apoptosis of cortical cells. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27:835–838. [PubMed] [Google Scholar]


