Abstract
Among the various treatment methods for stroke, increasing attention has been paid to traditional Chinese medicines. Buyang Huanwu decoction is a commonly used traditional Chinese medicine for the treatment of stroke. This paper summarizes the active components of the Chinese herb, which is composed of Huangqi (Radix Astragali seu Hedysari), Danggui (Radix Angelica sinensis), Chishao (Radix Paeoniae Rubra), Chuanxiong (Rhizoma Ligustici Chuanxiong), Honghua (Flos Carthami), Taoren (Semen Persicae) and Dilong (Pheretima), and identifies the therapeutic targets and underlying mechanisms that contribute to the neuroprotective properties of Buyang Huanwu decoction.
Keywords: nerve regeneration, Buyang Huanwu decoction, traditional Chinese medicine, cerebral ischemia, clinical application, neuroprotection, review, neural regeneration
Introduction
Cerebrovascular diseases are ranked as the third leading cause of death and disability after cancer and heart disease (Feigin et al., 2003; Pandya et al., 2011). Both ischemia and hemorrhage are pathologic causes of cerebrovascular disease, with ischemic injury contributing to approximately 85% of all cases. Until recently, tissue plasminogen activator (t-PA) is the only FDA authorized drug that can promote vessel rebuilding after ischemic injury and facilitate neural recovery (Jaffer et al., 2011). However, several disadvantageous limit its clinical application (Bambauer et al., 2006). The therapeutic window is limited to the first 4.5 hours after the indication of symptoms. Only 3–8.5% of patients are treated with t-PA because of its potential to cause hemorrhage and second injury (Bambauer et al., 2006). Moreover, the diffusion of t-PA into the brain parenchyma increases vascular permeability (Yepes et al., 2003) and can cause neurotoxicity (Goto et al., 2007). Therefore, a toxin-free therapeutic method is urgently needed for the treatment of cerebral ischemic injury.
Recent studies have confirmed the beneficial effects of traditional Chinese medicine (TCM) in the treatment of cerebral ischemic injury (Yang et al., 2011a; Zhao et al., 2012a). Among the investigated TCM prescriptions, Buyang Huanwu decoction (BHD) is a well-known Chinese herb prescription which is functionally characterized by Qi supplement, and blood and meridian circulation (Fan et al., 2014). This TCM prescription originated from the old record Yi Lin Gai Cuo (corrections on the errors of medical works), which was compiled by Qingren Wang, a famous doctor in the Qing dynasty. BHD is composed of seven kinds of Chinese herbs, including Huangqi (Radix Astragali seu Hedysari), Danggui (Radix Angelica sinensis), Chishao (Radix Paeoniae Rubra), Chuanxiong (Rhizoma Ligustici Chuanxiong), Honghua (Flos Carthami), Taoren (Semen Persicae), and Dilong (Pheretima). Because of drug-like properties of each herb, BHD is the primary prescription for the treatment of symptoms for hemiplegia and paraplegia (Wang and Jiang, 2009). In particular, BHD has been extensively used for the treatment of cerebral ischemic injury (Sun et al., 2007a), with accumulating experimental evidence indicating that BHD can improve recovery of behavioral scores, reduce the rate and area of infarction, and decrease ischemia-reperfusion injury (Yang et al., 2011a; Zhao et al., 2012a). Additionally, BHD has the ability to promote neurogenesis, increase vascular endothelial growth factor (VEGF) expression (Cai et al., 2007) and neural growth and differentiation, and inhibit apoptosis (Chen et al., 2008; Wang and Jiang, 2009). Although the neuroprotective properties of BHD are known, a systematic review of the mechanisms underlying this neuroprotective effect is still lacking. Here, the active components, the therapeutic targets, the clinical application, and the mechanisms underlying the neuroprotective properties of BHD in stroke are reviewed.
Active components in BHD and their therapeutic targets
BHD is a combination of several Chinese herbs and each herb has their own bioactive components. Although the effect of the active components of BHD in other diseases has been widely reported (Chun-sheng et al., 1978; Grdisa et al., 2001; Fang et al., 2002; Cheng et al., 2006, 2007; Ren et al., 2006; Chen et al., 2008; Chi et al., 2009; Wei et al., 2009; Chang et al., 2011; Li et al., 2012; Liu et al., 2012; Tang et al., 2012; Zhang et al., 2012, 2014a, b; Zhao et al., 2012b; Jin et al., 2013; Li et al., 2013; Gong et al., 2014; Kim et al., 2014; Koushki et al., 2014; Qi et al., 2014; Yan et al., 2014; Yang et al., 2014; Zeng et al., 2014), systematic research regarding the effective components of BHD in the treatment of cerebral ischemic injury is still lacking. The major active components are listed in Figure 1. More than one hundred compounds exist in Huangqi (Zhao et al., 2012b), and these compounds can be separated into saponins, flavonoids, polysaccharides and amino acids according to their structural properties. Astragalus polysaccharide has anti-oxidative (Li et al., 2012), anti-inflammatory and neuroprotective properties (Zhang et al., 2012).
Figure 1.
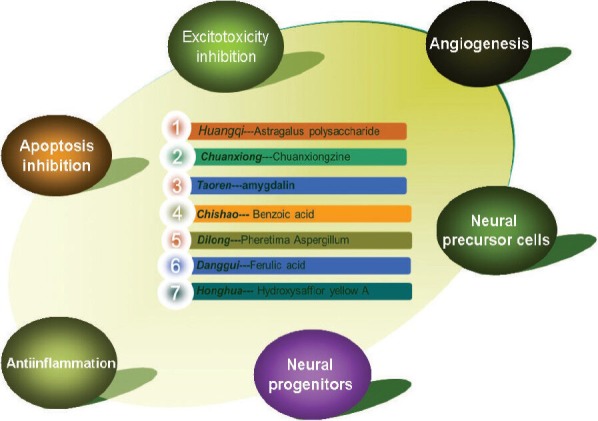
The seven active compounds in Buyang Huangwu Decoction and the possible mechanisms involved in its neuroprotective effect against cerebral ischemic injury.
Attenuating glutamate-induced excitotoxicity is one strategy to fight against cerebral ischemic injury (Jin et al., 2013). Interestingly, astragalus polysaccharide can reduce the accumulation of excitatory amino acids (Zhang et al., 2012). The permeability changes to the brain-blood barrier possibly lead to vasogenic brain edema and causes detrimental chronic injury (Chi et al., 2009). Accordingly, astragaloside A was reported to ameliorate edema in cerebral ischemia-reperfusion injury through regulating matrix metalloproteinase-9 and aquaporin 4 expression (Li et al., 2013).
Chuanxiong has been used for the treatment of cardiac-cerebral vascular disease, and Chuanxiongzine is one of the active components of BHD (Chun-sheng et al., 1978). The experimental evidence suggests that Chuanxiongzine exerts neuroprotective effects possibly through inhibiting calcium overload and inhibiting the anti-inflammatory response (Gong et al., 2014; Kim et al., 2014; Koushki et al., 2014; Yang et al., 2014; Zhang et al., 2014a; Zhang et al., 2014b). There is also evidence indicating that the neuroprotection afforded by Chuanxiongzine is because of inhibition of Bcl-2 and caspase-dependent apoptosis, as observed in PC12 cells subjected to oxidative stress (Cheng et al., 2007) and in animal models of cerebral ischemic injury (Cheng et al., 2006).
Pheretima aspergillum (PA) is one type of Dilong and stroke treatment with PA has been confirmed previously (Fang et al., 2002; Ren et al., 2006). Wei et al. (2009) reported that PA possesses pharmacological activity to promote regeneration of the peripheral nervous system after injury. Several studies have demonstrated that PA has anticoagulant and antioxidative properties (Grdisa et al., 2001) and promotes the growth of Schwann cells (Chang et al., 2011). Liu et al. (2012) reported that oral application of PA could ameliorate cerebral ischemic injury through decreasing the expression of glial fibrillary acidic protein (GFAP) and S-100B. Additionally, ferulic acid in Danggui (Zeng et al., 2014), hydroxysafflor yellow A in Honghua (Qi et al., 2014), benzoic acid in Chishao (Tang et al., 2012), and amygdalin in Taoren (Yan et al., 2014) also have beneficial effects on cerebral ischemic injury. Although the active compounds in BHD are not completely known, the active components already identified contribute to the multiple therapeutic targets of BHD against cerebral ischemic injury. This multi-targeted therapy most likely enhances the efficacy of BHD in fighting against cerebral ischemic injury.
Clinical application of BHD in cerebral ischemic stroke
BHD has been used for the treatment of several diseases, especially paralysis (Wang and Jiang, 2009) and stroke (Sun et al., 2007a) for many years because the formula was formed in the Qing dynasty (approximately 400 years ago). Based on the theory of TCM, BHD has advantages in invigorating the body, blood circulation, Qi supplement, and blood and meridian activation (Liu and Zhou, 1993; Zhang et al., 2010a; Ren et al., 2011). The hundreds of years of clinical experience, as well as modern experimental research, indicates the neuroprotective activity of BHD (Zhao et al., 2012a). In a clinical study, Cai and Lui (2010) found that BHD could promote functional recovery, enhance serum VEGF content, and ameliorate patient's quality of life during the recovery period after stroke. BHD was also effective in treating coronary disease and syndrome of Qi deficiency and blood stasis by decreasing blood viscosity and plasma fibrinogen. For example, Wang et al. (2011b) reported that BHD ameliorated coronary disease through increasing blood circulation and energy metabolism. Zhang et al. (2010a) verified that BHD could inhibit C-reactive protein and cluster of differentiation 40 (CD40L) in white blood cells to treat coronary disease. In addition, BHD also has the ability to maintain blood glucose levels (Wang et al., 2011b).
BHD inhibits excitotoxicity following cerebral ischemic injury
Excitatory amino acids are up-regulated in blood serum and cerebrospinal fluid after ischemic injury, which suggests that inhibiting excitotoxicity may be an effective strategy to inhibit neurological deficits after stroke (Castillo et al., 1996; Oja and Saransaari, 2013). Glutamate is the most important excitatory amino acid, performing critical roles in sustaining neuronal function. However, excitotoxicity due to over-release of glutamate is one of the pathological mechanisms of stroke (Eweka et al., 2010). Under normal physiological conditions, intracellular glutamate is at a resting state (Danbolt, 2001). However, following over-release, a large amount of glutamate is released outside the cell and binds to its receptors to cause depolarization and cell death during ischemic injury (Bonde et al., 2005). In a rat model of middle cerebral artery occlusion (MCAO), Wang et al. (2013) measured the content of excitatory amino acids in cerebrospinal fluid using microdialysis-high performance liquid chromatography-fluorescence detection. They showed that glutamate and aspartic acid were released 40 minutes post ischemia and peaked at 120 and 80 minutes after ischemia, respectively. Glycine, taurine and γ-aminobutyric acid also increased after ischemia and peaked at 120 minutes. By contrast, BHD application could decrease the levels of these excitatory amino acids and increase inhibitory amino acids to neutralize excitotoxicity. Consistently, Zhao et al. (2012a) also found that BHD inhibited ischemic injury-induced elevations of excitatory amino acids. Additionally, BHD also neutralized the increase of metabotropic glutamic acid receptor-1 (m-GluR1) expression in a rat MCAO model (Zhao et al., 2012a). Importantly, the inhibition was related to neurological recovery and a decrease in infarct area. This evidence suggests that inhibition of excitotoxicity is one of the mechanisms involved in the neuroprotective effect of BHD against cerebral ischemic injury.
BHD promotes angiogenesis after cerebral ischemic injury
Induction of angiogenesis, especially in the ischemic boundary area, enhances oxygen and nutrient supply to the infarcted tissue (Wei et al., 2001). Generation of new blood vessels facilitates highly coupled neurorestorative processes including neurogenesis and synaptogenesis, which in turn leads to improved functional recovery (Chen and Chopp, 2006; Beck and Plate, 2009). Therefore, promoting angiogenesis represents an effective way to facilitate neurological functional recovery. Although angiogenesis is not sufficient to satisfy the requirement of new blood vessels in an MCAO model, BHD administration before modeling not only elevates Ang-1 expression, but also extends the expression period (Shen et al., 2014). The changes in Ang-1 levels following BHD administration increase blood vessel density, which contribute to the decrease in infarct area and recovery of the nervous system. Hence, angiogenesis is a mechanism underlying the effect of BHD on neurological recovery after ischemic injury. Consistently, BHD administration also increases the expression of angiogenesis-related proteins (ARP), such as VEGF and its receptor and F1K1 at later recovery phases after ischemic injury (Cai et al., 2007). Although there was a report indicating that in the early phase after injury, BHD restricts the expression of angiogenesis-related proteins (Wang et al., 2011a), further studies on how BHD regulates these proteins is required. The up-regulation of ARPs provides a basis for new blood vessel generation at later recovery phases. The increase in expression of VEGF at the early phase after ischemic injury increases the permeability of the blood-brain barrier and elicits secondary damage (Vandenbroucke et al., 2008). Based on these results, we infer that like VGA1155 (Chiba et al., 2008), an antagonist of VEGF, BHD may also restrict ARP expression to avoid secondary damage following cerebral ischemic injury.
BHD promotes migration of neural precursor cells (NPCs) to the infract zone
NPCs, located in the subventricular zone (SVZ) and subgranular zone (SGZ), have the potential to renew and differentiate into various types of neuronal cells in adult animals (Gage, 2000; Ma et al., 2009). After ischemic injury, endogenous NPCs proliferate, migrate to the ischemic zone and differentiate into neurons (Nakatomi et al., 2002). This process appears to be a means of neurological functional recovery after ischemic injury because newborn neurons replace the damaged cells. However, the newborn neurons are insufficient to facilitate recovery of the injured tissue. Interestingly, advanced studies indicate that proliferation, migration and differentiation of neural precursors can be up-regulated by exogenous interference, which promotes neurological recovery following ischemic injury (Bonde et al., 2005; Nakano-Doi et al., 2010; Osman et al., 2011; Sejersted et al., 2011; Zhuang et al., 2012; Ara and De Montpellier, 2013). In an MCAO model, Kong et al. (2014) verified that BHD could promote proliferation of neural precursors in the SVZ, SGZ and corpus striatum of the infarcted brain. Additionally, expression of migration-related proteins such as stromal cell-derived factor 1 and chemokine receptor type 4 were also up-regulated after BHD administration. These data provide evidence that BHD may exert its neuroprotective effect partially by promoting NPC migration to ischemic brain areas.
BHD facilitates the proliferation and differentiation of NPCs
BHD may facilitate NPC proliferation in a mouse ischemic model (Cai et al., 2007). Cellular calcium concentration is critical for neuronal proliferation and differentiation (Catterall, 2000). Although calcium overload could lead to cell death following cerebral ischemic injury, a low calcium concentration by contrast is beneficial for axon growth (Sun et al., 2007a). With the assistance of serum pharmacological method, the effects of BHD on the growth of hippocampal NPCs was investigated (Sun et al., 2007a, b). Compared with controls, BHD could clearly increase the length of axons, and the expression of neurofilament and GFAP. Consistently, calcium concentrations decreased after application of BHD-containing serum. Extracellular signal regulated kinase 2 (ERK2) is an important component of the MAPK signaling pathway. The ERK2-mediated signaling pathway is known to regulate neural regeneration, neural growth, and differentiation and restoration after neurological injury (Nishimoto and Nishida, 2006; Berwick et al., 2009; Huang et al., 2011; Duan et al., 2013; Ishii et al., 2013). For example, Jinglong et al. (2013) verified that chronic BHD treatment for 30 days could activate ERK2 expression and promote neuronal growth and differentiation in the ischemic area. Based on these results, inhibition of calcium concentrations, as well as activation of ERK2 expression may underlie the effects of BHD on growth and differentiation of NPCs. Additionally, Wang et al. (2011a) employed gene set enrichment analysis and confirmed that BHD enhanced the expression of neural regeneration-related genes (Dcx, Fgfr3, Cttnbp2, Rorb, Abi2 and Miat) and neural development-related genes (Ptprf, Ift172 and Nfib). Hence, promoting NPC regeneration is a potential mechanism underlying the neuroprotective effects of BHD against cerebral ischemic injury.
BHD inhibits inflammation in cerebral ischemic injury
Diapedesis and proinflammatory cytokine release in the ischemic region elicits an inflammatory reaction, which leads to early functional defects to the blood-brain barrier (Jin et al., 2010). Transcription factors, such as nuclear factor-kappaB play critical roles in regulating the post-ischemic inflammatory reaction (Nurmi et al., 2004; Zhang et al., 2005). The up-regulation of related inflammatory cytokines determines neuronal fate. BHD application effectively inhibits cerebral ischemic injury-activated TLR4 expression (Wang et al., 2011a). Additionally, gene expression-mediated diapedesis is significantly attenuated after BHD administration. This evidence suggests that BHD not only antagonizes the inflammation-related signaling pathway, but also inhibits the diapedesis-regulated inflammatory reaction in the cerebral ischemic region, thus preventing cell death.
BHD inhibits apoptosis in ischemia injury
Apoptosis has been reported to contribute to cell death following cerebral ischemic injury (Chen et al., 1998; Lee et al., 2000; Zeng and Xu, 2000; Sugawara et al., 2002). Caspases are a family of cysteine proteases that play an important role in apoptosis, particularly the “initiator” (caspase-9) and “effector” (caspase-3) caspases (Hengartner, 2000). Caspase 3 is the “effector” protease in apoptosis (Deshmukh et al., 1996; Schulz et al., 1996) and is activated during nutrient deficiency, potassium loss and glutamate elicited excitotoxicity (Chen et al., 1998; Sugawara et al., 2002). Accordingly, regulation of caspase 3 though gene deletion or antagonists decreases ischemic injury-induced cell death.
In a rat model of transient ischemic injury produced by the four-vessel occlusion method, neurological function deficits were coupled with damage to neurons and cell loss (Li et al., 2003). Additionally, transferase-mediated biotin-dUTP nick-end labeling identified apoptotic cells in the model group (Gavrieli et al., 1992; Chen et al., 1997). Interestingly, BHD administration post-ischemia markedly reversed the extent of apoptosis and rescued neural function deficits. Concomitantly, ischemic injury-induced caspase-3 activation was attenuated by BHD administration. Therefore, the blockade effect of BHD on ischemic injury-induced apoptosis is an effective way to rescue neuronal deficits. Using genome-wide transcriptome analysis, Wang et al. (2011a) screened 15 genes that may be involved in the protective effect of BHD on ischemic injury-induced apoptosis.
Conclusion
The protective effects of BHD on ischemic injury were confirmed by various experimental models (Zhang et al., 2001, 2007, 2010b, 2011; Deng et al., 2002; Lai et al., 2002; Shao et al., 2003; Liao et al., 2004; Qu et al., 2004, 2014; Fan et al., 2006; Tan et al., 2006; Tang et al., 2006; Tong et al., 2007; Wu et al., 2008, 2011, 2012; Zhou et al., 2008, 2011, 2012; Wang and Jiang, 2009; Yi et al., 2010; Zhao et al., 2010; Ren et al., 2011; Yang et al., 2011a; Gu et al., 2013; Wang et al., 2013; Kong et al., 2014). Most investigators preferred the SD rat model of MCAO. The effect of time after BHD administration and the dose of BHD administered were also studied (Zhao et al., 2012a). A dose of 40 mg/kg BHD had a greater effect than 20 mg/kg BHD. Additionally, the therapeutic window was also important, as application of BHD 2 hours after injury had a more prominent effect than application at 4 or 6 hours. Therefore, the therapeutic window of BHD administration is critical for effective treatment. Due to the limited number of studies on BHD, further research related to the time and dose of BHD required for the treatment of ischemic injury is required.
Based on literature, BHD has a therapeutic effect on ischemic injury, primarily through ameliorating blood circulation, reducing calcium overload, promoting neural precursor migration, increasing growth of NPCs, reducing the inflammatory response and inhibiting neuronal apoptosis. In addition to the above mechanisms, BHD has also been reported to ameliorate ischemic injury in cardiac tissue, the spinal cord and the peripheral nervous system via its antioxidant properties (Fan et al., 2006; Yang et al., 2011b). To the best of our knowledge, there have been no studies regarding the anti-oxidation of BHD in cerebral ischemia injury. Additionally, an in-depth investigation on blood circulation after BHD application is required to further clarify its vessel rebuilding properties.
Footnotes
Funding: This work was supported by grants from Health and Family Planning Commission of Heilongjiang Province Research Projects, No. 2014-195 and Science and Technology Research Projects of Mudanjiang Medical University, No. ZS201305.
Conflicts of interest: None declared.
Copyedited by Diwakarla S, Robert J, Li CH, Song LP, Zhao M
References
- 1.Ara J, De Montpellier S. Hypoxic-preconditioning enhances the regenerative capacity of neural stem/progenitors in subventricular zone of newborn piglet brain. Stem Cell Res. 2013;11:669–686. doi: 10.1016/j.scr.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Bambauer KZ, Johnston SC, Bambauer DE, Zivin JA. Reasons why few patients with acute stroke receive tissue plasminogen activator. Arch Neurol. 2006;63:661–664. doi: 10.1001/archneur.63.5.661. [DOI] [PubMed] [Google Scholar]
- 3.Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117:481–496. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
- 4.Berwick DC, Calissano M, Corness JD, Cook SJ, Latchman DS. Regulation of Brn-3a N-terminal transcriptional activity by MEK1/2-ERK1/2 signalling in neural differentiation. Brain Res. 2009;1256:8–18. doi: 10.1016/j.brainres.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Bonde C, Noraberg J, Noer H, Zimmer J. Ionotropic glutamate receptors and glutamate transporters are involved in necrotic neuronal cell death induced by oxygen-glucose deprivation of hippocampal slice cultures. Neuroscience. 2005;136:779–794. doi: 10.1016/j.neuroscience.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Cai G, Liu B, Liu W, Tan X, Rong J, Chen X, Tong L, Shen J. Buyang Huanwu Decoction can improve recovery of neurological function, reduce infarction volume, stimulate neural proliferation and modulate VEGF and Flk1 expressions in transient focal cerebral ischaemic rat brains. J Ethnopharmacol. 2007;113:292–299. doi: 10.1016/j.jep.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Cai GX, Liu BY. Effect of ultra-micronized Buyang Huanwu decoction on neurological function, quality of life, and serum vascular endothelial growth factor in patients convalescent from cerebral infarction. Zhongguo Weizhong Bing Jijiu Yixue. 2010;22:591–594. [PubMed] [Google Scholar]
- 8.Castillo J, Davalos A, Naveiro J, Noya M. Neuroexcitatory amino acids and their relation to infarct size and neurological deficit in ischemic stroke. Stroke. 1996;27:1060–1065. doi: 10.1161/01.str.27.6.1060. [DOI] [PubMed] [Google Scholar]
- 9.Catterall WA. Structure and regulation of voltage-gated Ca 2+ channels. Annu Rev Cell Dev Biol 2000. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 10.Chang YM, Kuo WH, Lai TY, Shih YT, Tsai FJ, Tsai CH, Shu WT, Chen YY, Chen YS, Kuo WW, Huang CY. RSC96 schwann cell proliferation and survival induced by Dilong through PI3K/Akt signaling mediated by IGF-I. Evid Based Complement Alternat Med 2011. 2011:216148. doi: 10.1093/ecam/nep216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen A, Wang H, Zhang J, Wu X, Liao J, Li H, Cai W, Luo X, Ju G. BYHWD rescues axotomized neurons and promotes functional recovery after spinal cord injury in rats. J Ethnopharmacol. 2008;117:451–456. doi: 10.1016/j.jep.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Chopp M. Neurorestorative treatment of stroke: cell and pharmacological approaches. NeuroRx. 2006;3:466–473. doi: 10.1016/j.nurx.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Jin K, Chen M, Pei W, Kawaguchi K, Greenberg DA, Simon RP. Early detection of DNA strand breaks in the brain after transient focal ischemia: implications for the role of DNA damage in apoptosis and neuronal cell death. J Neurochem. 1997;69:232–245. doi: 10.1046/j.1471-4159.1997.69010232.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Nagayama T, Jin K, Stetler RA, Zhu RL, Graham SH, Simon RP. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci. 1998;18:4914–4928. doi: 10.1523/JNEUROSCI.18-13-04914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng CY, Sue YM, Chen CH, Hou CC, Chan P, Chu YL, Chen TH, Hsu YH. Tetramethylpyrazine attenuates adriamycin-induced apoptotic injury in rat renal tubular cells NRK-52E. Planta Med. 2006;72:888–893. doi: 10.1055/s-2006-946695. [DOI] [PubMed] [Google Scholar]
- 16.Cheng XR, Zhang L, Hu JJ, Sun L, Du GH. Neuroprotective effects of tetramethylpyrazine on hydrogen peroxide-induced apoptosis in PC12 cells. Cell Biol Int. 2007;31:438–443. doi: 10.1016/j.cellbi.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Chi OZ, Hunter C, Liu X, Weiss HR. Effects of exogenous excitatory amino acid neurotransmitters on blood-brain barrier disruption in focal cerebral ischemia. Neurochem Res. 2009;34:1249–1254. doi: 10.1007/s11064-008-9902-7. [DOI] [PubMed] [Google Scholar]
- 18.Chiba Y, Sasayama T, Miyake S, Koyama J, Kondoh T, Hosoda K, Kohmura E. Anti-VEGF receptor antagonist (VGA1155) reduces infarction in rat permanent focal brain ischemia. Kobe J Med Sci. 2008;54:E136–146. [PubMed] [Google Scholar]
- 19.Chun-sheng L, Hsiao-meng Y, Yun-hsiang H, Chun P, Chi-fen S. Radix salviae miltiorrhizae and Rhizoma ligustici wallichii in coronary heart disease. Chin Med J (Engl) 1978;4:43–46. [PubMed] [Google Scholar]
- 20.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 21.Deng CQ, Wang M, He FY. Effect of buyang huanwu decoction and its active regions combination on brain heat shock protein 70 expression in gerbils after cerebral ischemia/reperfusion. Zhongguo Zhongxiyi Jiehe Zazhi. 2002;22:193–195. 210. [PubMed] [Google Scholar]
- 22.Deshmukh M, Vasilakos J, Deckwerth TL, Lampe PA, Shivers BD, Johnson EM., Jr Genetic and metabolic status of NGF-deprived sympathetic neurons saved by an inhibitor of ICE family proteases. J Cell Biol. 1996;135:1341–1354. doi: 10.1083/jcb.135.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan Z, Zhang X, Zhu GX, Gao Y, Xue X. Activation of mGluR4 promotes proliferation of rat neural progenitor cells while mediating activation of ERK1/2 signaling pathway. Cell Mol Biol (Noisy-le-grand) 2013;59(Suppl):OL1809–1817. [PubMed] [Google Scholar]
- 24.Eweka AO, Eweka A, Om’iniabohs FA. Histological studies of the effects of monosodium glutamate of the fallopian tubes of adult female Wistar rats. N Am J Med Sci. 2010;2:146–149. doi: 10.4297/najms.2010.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan L, Wang K, Cheng B. Effects of buyang huanwu decoction on apoptosis of nervous cells and expressions of Bcl-2 and bax in the spinal cord of ischemia-reperfusion injury in rabbits. J Tradit Chin Med. 2006;26:153–156. [PubMed] [Google Scholar]
- 26.Fan XH, Shi WZ, Cheng YX, Yang XF. Effects of Buyang Huanwu Decoction on antioxidant and drug-metabolizing enzymes in rat liver. Chin J Nat Med. 2014;12:449–454. doi: 10.1016/S1875-5364(14)60070-4. [DOI] [PubMed] [Google Scholar]
- 27.Fang T, Yang C, Su W. Study on TLC profile of Pheretima aspergillum and Pheretima (Dilong) injection. Zhong Yao Cai. 2002;25:813–815. [PubMed] [Google Scholar]
- 28.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 29.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 30.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong X, Ivanov VN, Davidson MM, Hei TK. Tetramethylpyrazine (TMP) protects against sodium arsenite-induced nephrotoxicity by suppressing ROS production, mitochondrial dysfunction, pro-in-flammatory signaling pathways and programed cell death. Arch Toxicol [Epub ahead of print] 2014 doi: 10.1007/s00204-014-1302-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goto H, Fujisawa H, Oka F, Nomura S, Kajiwara K, Kato S, Fujii M, Maekawa T, Suzuki M. Neurotoxic effects of exogenous recombinant tissue-type plasminogen activator on the normal rat brain. J Neurotrauma. 2007;24:745–752. doi: 10.1089/neu.2006.0183. [DOI] [PubMed] [Google Scholar]
- 33.Grdisa M, Popovic M, Hrzenjak T. Glycolipoprotein extract (G-90) from earthworm Eisenia foetida exerts some antioxidative activity. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:821–825. doi: 10.1016/s1095-6433(00)00323-8. [DOI] [PubMed] [Google Scholar]
- 34.Gu YP, Liao YL, Zhang C, Guo W, Wei HC, Lu R. Effects of buyang huanwu decoction on the sarcoplasmic reticulum calcium uptake in abdominal aortic constriction induced myocardial hypertrophic rats. Zhongguo Zhongxiyi Jiehe Zazhi. 2013;33:627–631. [PubMed] [Google Scholar]
- 35.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 36.Huang X, Zhu LL, Zhao T, Wu LY, Wu KW, Schachner M, Xiao ZC, Fan M. CHL1 negatively regulates the proliferation and neuronal differentiation of neural progenitor cells through activation of the ERK1/2 MAPK pathway. Mol Cell Neurosci. 2011;46:296–307. doi: 10.1016/j.mcn.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Ishii A, Furusho M, Bansal R. Sustained activation of ERK1/2 MAPK in oligodendrocytes and schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. J Neurosci. 2013;33:175–186. doi: 10.1523/JNEUROSCI.4403-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaffer H, Morris VB, Stewart D, Labhasetwar V. Advances in stroke therapy. Drug Deliv Transl Res. 2011;1:409–419. doi: 10.1007/s13346-011-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin AY, Tuor UI, Rushforth D, Kaur J, Muller RN, Petterson JL, Boutry S, Barber PA. Reduced blood brain barrier breakdown in P-selectin deficient mice following transient ischemic stroke: a future therapeutic target for treatment of stroke. BMC Neurosci. 2010;11:12. doi: 10.1186/1471-2202-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin M, Huang Q, Zhao K, Shang P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int J Biol Macromol. 2013;54:16–23. doi: 10.1016/j.ijbiomac.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 41.Jinglong T, Weijuan G, Jun L, Tao Q, Hongbo Z, Shasha L. The molecular and electrophysiological mechanism of buyanghuanwu decoction in learning and memory ability of vascular dementia rats. Brain Res Bull. 2013;99:13–18. doi: 10.1016/j.brainresbull.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Kim M, Kim SO, Lee M, Lee JH, Jung WS, Moon SK, Kim YS, Cho KH, Ko CN, Lee EH. Tetramethylpyrazine, a natural alkaloid, attenuates pro-inflammatory mediators induced by amyloid beta and interferon-gamma in rat brain microglia. Eur J Pharmacol. 2014:S0014–2999. doi: 10.1016/j.ejphar.2014.06.037. (14)00488-9. [DOI] [PubMed] [Google Scholar]
- 43.Kong X, Su X, Zhu J, Wang J, Wan H, Zhong M, Li L, Lin N. Neuroprotective effect of buyang huanwu decoction on rat ischemic/reperfusion brain damage by promoting migration of neural precursor cells. Rejuvenation Res. 2014;17:264–275. doi: 10.1089/rej.2013.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koushki D, Latifi S, Javidan AN, Matin M. Efficacy of some non-conventional herbal medications (sulforaphane, tanshinone IIA, and tetramethylpyrazine) in inducing neuroprotection in comparison with interleukin-10 after spinal cord injury: A meta-analysis. J Spinal Cord Med [Epub ahead of print] 2014 doi: 10.1179/2045772314Y.0000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai Z, Wang SY, Geng XY, Deng CQ, Zhang RZ. Effects of bu yang huan wu decoction on astrocytes after cerebral ischemia and reperfusion. Zhongguo Zhongyao Zazhi. 2002;27:763–765. [PubMed] [Google Scholar]
- 46.Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest. 2000;106:723–731. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li M, Ma RN, Li LH, Qu YZ, Gao GD. Astragaloside IV reduces cerebral edema post-ischemia/reperfusion correlating the suppression of MMP-9 and AQP4. Eur J Pharmacol. 2013;715:189–195. doi: 10.1016/j.ejphar.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 48.Li XM, Bai XC, Qin LN, Huang H, Xiao ZJ, Gao TM. Neuroprotective effects of Buyang Huanwu Decoction on neuronal injury in hippocampus after transient forebrain ischemia in rats. Neurosci Lett. 2003;346:29–32. doi: 10.1016/s0304-3940(03)00522-6. [DOI] [PubMed] [Google Scholar]
- 49.Li XT, Zhang YK, Kuang HX, Jin FX, Liu DW, Gao MB, Liu Z, Xin XJ. Mitochondrial protection and anti-aging activity of Astragalus polysaccharides and their potential mechanism. Int J Mol Sci. 2012;13:1747–1761. doi: 10.3390/ijms13021747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liao CL, Tong L, Chen YY. Effect of Buyanghuanwu decoction on neuronal nitric oxide synthase expression after permanent focal cerebral ischemia in rats. Di Yi Jun Yi Da Xue Xue Bao. 2004;24:864–868. 891. [PubMed] [Google Scholar]
- 51.Liu CH, Lin YW, Tang NY, Liu HJ, Huang CY, Hsieh CL. Effect of oral administration of Pheretima aspergillum (earthworm) in rats with cerebral infarction induced by middle-cerebral artery occlusion. Afr J Tradit Complement Altern Med. 2012;10:66–82. [PMC free article] [PubMed] [Google Scholar]
- 52.Liu H, Zhou JF. Xianbai buyang Huanwu decoction used for treating hypertension with kidney qi deficiency and blood stasis. Zhongguo Zhongxiyi Jiehe Zazhi. 1993;13:714–717. 707. [PubMed] [Google Scholar]
- 53.Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19:672–682. doi: 10.1038/cr.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakano-Doi A, Nakagomi T, Fujikawa M, Nakagomi N, Kubo S, Lu S, Yoshikawa H, Soma T, Taguchi A, Matsuyama T. Bone marrow mononuclear cells promote proliferation of endogenous neural stem cells through vascular niches after cerebral infarction. Stem Cells. 2010;28:1292–1302. doi: 10.1002/stem.454. [DOI] [PubMed] [Google Scholar]
- 55.Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 56.Nishimoto S, Nishida E. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 2006;7:782–786. doi: 10.1038/sj.embor.7400755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nurmi A, Lindsberg PJ, Koistinaho M, Zhang W, Juettler E, Karjalainen-Lindsberg ML, Weih F, Frank N, Schwaninger M, Koistinaho J. Nuclear factor-kappaB contributes to infarction after permanent focal ischemia. Stroke. 2004;35:987–991. doi: 10.1161/01.STR.0000120732.45951.26. [DOI] [PubMed] [Google Scholar]
- 58.Oja SS, Saransaari P. Ischemia induces release of endogenous amino acids from the cerebral cortex and cerebellum of developing and adult mice. J Amino Acids 2013. 2013:839036. doi: 10.1155/2013/839036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osman AM, Porritt MJ, Nilsson M, Kuhn HG. Long-term stimulation of neural progenitor cell migration after cortical ischemia in mice. Stroke. 2011;42:3559–3565. doi: 10.1161/STROKEAHA.111.627802. [DOI] [PubMed] [Google Scholar]
- 60.Pandya RS, Mao L, Zhou H, Zhou S, Zeng J, Popp AJ, Wang X. Central nervous system agents for ischemic stroke: neuroprotection mechanisms. Cent Nerv Syst Agents Med Chem. 2011;11:81–97. doi: 10.2174/187152411796011321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qi Z, Yan F, Shi W, Zhang C, Dong W, Zhao Y, Shen J, Ji X, Liu KJ, Luo Y. AKT-related autophagy contributes to the neuroprotective efficacy of hydroxysafflor yellow a against ischemic stroke in rats. Transl Stroke Res. 2014;5:501–509. doi: 10.1007/s12975-014-0346-x. [DOI] [PubMed] [Google Scholar]
- 62.Qu HD, Tong L, Shen JG. Effect of buyang huanwu decoction drug serum on expression of p53 and p21 genes in cultured rat's cerebral cortical neuron after hypoxia in vitro. Zhongguo Zhongxiyi Jiehe Zazhi. 2004;24:133–135. [PubMed] [Google Scholar]
- 63.Qu TB, Yu TH, Liu ZT, Li L, Chu LS. Effect of Buyang Huanwu Decoction and its disassembled recipes on rats’ neurogenesis after focal cerebral ischemia. Zhongguo Zhongxiyi Jiehe Zazhi. 2014;34:342–347. [PubMed] [Google Scholar]
- 64.Ren J, Lin C, Liu J, Xu L, Wang M. Experimental study on Qi deficiency and blood stasis induced by muti-factor stimulation in rats. Zhongguo Zhongyao Zazhi. 2011;36:72–76. [PubMed] [Google Scholar]
- 65.Ren Y, Houghton P, Hider RC. Relevant activities of extracts and constituents of animals used in traditional Chinese medicine for central nervous system effects associated with Alzheimer's disease. J Pharm Pharmacol. 2006;58:989–996. doi: 10.1211/jpp.58.7.0015. [DOI] [PubMed] [Google Scholar]
- 66.Schulz JB, Weller M, Klockgether T. Potassium deprivation-induced apoptosis of cerebellar granule neurons: a sequential requirement for new mRNA and protein synthesis, ICE-like protease activity, and reactive oxygen species. J Neurosci. 1996;16:4696–4706. doi: 10.1523/JNEUROSCI.16-15-04696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sejersted Y, Hildrestrand GA, Kunke D, Rolseth V, Krokeide SZ, Neurauter CG, Suganthan R, Atneosen-Asegg M, Fleming AM, Saugstad OD, Burrows CJ, Luna L, Bjoras M. Endonuclease VIII-like 3 (Neil3) DNA glycosylase promotes neurogenesis induced by hypoxia-ischemia. Proc Natl Acad Sci U S A. 2011;108:18802–18807. doi: 10.1073/pnas.1106880108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shao SJ, Shan BZ, Jiang J, Yan ZG. Comparative experimental study on treatment of rat's injured sciatic nerve with electroacupuncture and Buyang Huanwu Decoction. Zhong Xi Yi Jie He Xue Bao. 2003;1:54–56. doi: 10.3736/jcim20030123. [DOI] [PubMed] [Google Scholar]
- 69.Shen J, Zhu Y, Yu H, Fan ZX, Xiao F, Wu P, Zhang QH, Xiong XX, Pan JW, Zhan RY. Buyang Huanwu decoction increases angiopoietin-1 expression and promotes angiogenesis and functional outcome after focal cerebral ischemia. J Zhejiang Univ Sci B. 2014;15:272–280. doi: 10.1631/jzus.B1300166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sugawara T, Noshita N, Lewen A, Gasche Y, Ferrand-Drake M, Fujimura M, Morita-Fujimura Y, Chan PH. Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation. J Neurosci. 2002;22:209–217. doi: 10.1523/JNEUROSCI.22-01-00209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun J, Bi Y, Guo L, Qi X, Zhang J, Li G, Tian G, Ren F, Li Z. Buyang Huanwu Decoction promotes growth and differentiation of neural progenitor cells: using a serum pharmacological method. J Ethnopharmacol. 2007a;113:199–203. doi: 10.1016/j.jep.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 72.Sun JH, Gao YM, Yang L, Wang X, Bao LH, Liu WJ, Yew D. Effects of Buyang Huanwu Decoction on neurite outgrowth and differentiation of neuroepithelial stem cells. Zhongguo Shenglixue Zazhi. 2007b;50:151–156. [PubMed] [Google Scholar]
- 73.Tan XH, Qu HD, Peng K, Chen YY, Tong L, Shen JG, Zhu CW. Effects of Buyanghuanwu decoction on nerve proliferation in rats with sequelae of ischemic stroke. Nan Fang Yi Ke Da Xue Xue Bao. 2006;26:189–192. [PubMed] [Google Scholar]
- 74.Tang YH, Li H, Chen BY. Effect of active fraction of buyang huanwu decoction on caspase expression in rats after focal cerebral ischemic reperfusion. Zhongguo Zhongxiyi Jiehe Zazhi. 2006;26:533–537. [PubMed] [Google Scholar]
- 75.Tang YP, Huang MY, Zhang YH. Comparison of in vitro anti-oxidative activities among Siwu Decoction Serial Recipes, their composed crude herbs, and main aromatic acids, as well as their dose-effect correlation. Zhongguo Zhongxiyi Jiehe Zazhi. 2012;32:64–67. [PubMed] [Google Scholar]
- 76.Tong L, Tan XH, Shen JG. Comparative study of Buyang Huanwu Decoction and the different combinations of its ingredients on neurogenesis following ischemic stroke in rats. Zhongguo Zhongxiyi Jiehe Zazhi. 2007;27:519–522. [PubMed] [Google Scholar]
- 77.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 78.Wang HW, Liou KT, Wang YH, Lu CK, Lin YL, Lee IJ, Huang ST, Tsai YH, Cheng YC, Lin HJ, Shen YC. Deciphering the neuroprotective mechanisms of Bu-yang Huan-wu decoction by an integrative neurofunctional and genomic approach in ischemic stroke mice. J Ethnopharmacol. 2011a;138:22–33. doi: 10.1016/j.jep.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 79.Wang L, Jiang DM. Neuroprotective effect of Buyang Huanwu Decoction on spinal ischemia/reperfusion injury in rats. J Ethnopharmacol. 2009;124:219–223. doi: 10.1016/j.jep.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 80.Wang L, Huang Y, Wu J, Lv G, Zhou L, Jia J. Effect of Buyang Huanwu decoction on amino acid content in cerebrospinal fluid of rats during ischemic/reperfusion injury. J Pharm Biomed Anal. 2013;86:143–150. doi: 10.1016/j.jpba.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 81.Wang WR, Lin R, Zhang H, Lin QQ, Yang LN, Zhang KF, Ren F. The effects of Buyang Huanwu Decoction on hemorheological disorders and energy metabolism in rats with coronary heart disease. J Ethnopharmacol. 2011b;137:214–220. doi: 10.1016/j.jep.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 82.Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001;32:2179–2184. doi: 10.1161/hs0901.094282. [DOI] [PubMed] [Google Scholar]
- 83.Wei S, Yin X, Kou Y, Jiang B. Lumbricus extract promotes the regeneration of injured peripheral nerve in rats. J Ethnopharmacol. 2009;123:51–54. doi: 10.1016/j.jep.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 84.Wu L, Zhang W, Li H, Zhang GM, Chen BY, Tang YH, Deng CQ. Effects of Buyang Huanwu Decoction and its alkaloids and glycosides on aortic intimal hyperplasia and expression of proliferating cell nuclear antigen in rats with aortic intimal injuries. Zhong Xi Yi Jie He Xue Bao. 2008;6:836–842. doi: 10.3736/jcim20080813. [DOI] [PubMed] [Google Scholar]
- 85.Yan T, Fu Q, Wang J, Ma S. UPLC-MS/MS determination of ephedrine, methylephedrine, amygdalin and glycyrrhizic acid in Beagle plasma and its application to a pharmacokinetic study after oral administration of Ma Huang Tang. Drug Test Anal. 2014 doi: 10.1002/dta.1635. doi: 10.1002/dta.1635. [DOI] [PubMed] [Google Scholar]
- 86.Yang G, Fang Z, Liu Y, Zhang H, Shi X, Ji Q, Lin Q, Lin R. Protective effects of Chinese traditional medicine buyang huanwu decoction on myocardial injury. Evid Based Complement Alternat Med 2011. 2011a:930324. doi: 10.1093/ecam/nep013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang S, Gao Q, Xing S, Feng X, Peng L, Dong H, Bao L, Zhang J, Hu Y, Li G, Song T, Li Z, Sun J. Neuroprotective effects of Buyang Huanwu decoction against hydrogen peroxide induced oxidative injury in Schwann cells. J Ethnopharmacol. 2011b;137:1095–1101. doi: 10.1016/j.jep.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 88.Yang Y, Li ZH, Liu H, Shi WD, Zhang J. Inhibitory effect of tetramethylpyrazine preconditioning on overload training-induced myocardial apoptosis in rats. Chin J Integr Med [Epub ahead of print] 2014 doi: 10.1007/s11655-014-1752-3. [DOI] [PubMed] [Google Scholar]
- 89.Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yi J, Huang X, Yu Y, Cai GX, Liu BY. Effect of Buyang Huanwu decoction on interleukin-1beta and tumor necrosis factor-alpha expression in rats after cerebral infarction. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2010;22:599–601. [PubMed] [Google Scholar]
- 91.Zeng M, Zhang J, Yang Y, Jin Y, Xiao W, Wang Z, Ding G, Yan R. An automated dual-gradient liquid chromatography-MS/MS method for the simultaneous determination of ferulic acid, ligustrazine and ligustilide in rat plasma and its application to a pharmacokinetic study. J Pharm Biomed Anal. 2014;88:354–363. doi: 10.1016/j.jpba.2013.08.038. [DOI] [PubMed] [Google Scholar]
- 92.Zeng YS, Xu ZC. Co-existence of necrosis and apoptosis in rat hippocampus following transient forebrain ischemia. Neurosci Res. 2000;37:113–125. doi: 10.1016/s0168-0102(00)00107-3. [DOI] [PubMed] [Google Scholar]
- 93.Zhang H, Wang WR, Lin R, Zhang JY, Ji QL, Lin QQ, Yang LN. Buyang Huanwu decoction ameliorates coronary heart disease with Qi deficiency and blood stasis syndrome by reducing CRP and CD40 in rats. J Ethnopharmacol. 2010a;130:98–102. doi: 10.1016/j.jep.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 94.Zhang H, Pan N, Xiong S, Zou S, Li H, Xiao L, Cao Z, Tunnacliffe A, Huang Z. Inhibition of polyglutamine-mediated proteotoxicity by Astragalus membranaceus polysaccharide through the DAF-16/FOXO transcription factor in Caenorhabditis elegans. Biochem J. 2012;441:417–424. doi: 10.1042/BJ20110621. [DOI] [PubMed] [Google Scholar]
- 95.Zhang J, Li C, Guo X, Wang G. Effect of buyang huanwu decoction on platelet activating factor content in arterial blood pre- and post-arterial thrombosis in rats. J Tradit Chin Med. 2001;21:299–302. [PubMed] [Google Scholar]
- 96.Zhang M, Gao F, Teng F, Zhang C. Tetramethylpyrazine promotes the proliferation and migration of brain endothelial cells. Mol Med Rep. 2014a;10:29–32. doi: 10.3892/mmr.2014.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang N, Komine-Kobayashi M, Tanaka R, Liu M, Mizuno Y, Urabe T. Edaravone reduces early accumulation of oxidative products and sequential inflammatory responses after transient focal ischemia in mice brain. Stroke. 2005;36:2220–2225. doi: 10.1161/01.STR.0000182241.07096.06. [DOI] [PubMed] [Google Scholar]
- 98.Zhang P, Guo CF, Luo N, Wang B, Liu JH, Xu XN. Effect of Buyang Huanwut decoction on apoptosis of splenocytes in rats with sepsis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2011;23:486–489. [PubMed] [Google Scholar]
- 99.Zhang X, Zhang F, Kong D, Wu X, Lian N, Chen L, Lu Y, Zheng S. Tetramethylpyrazine inhibits angiotensin II-induced activation of hepatic stellate cells associated with interference of platelet-derived growth factor beta receptor pathways. FEBS J. 2014b;281:2754–2768. doi: 10.1111/febs.12818. [DOI] [PubMed] [Google Scholar]
- 100.Zhang YK, Han XY, Che ZY. Effects of buyang huanwu tang combined with bone marrow mesenchymal stem cell transplantation on the expression of VEGF and Ki-67 in the brain tissue of the cerebral ischemia-reperfusion model rat. J Tradit Chin Med. 2010b;30:278–282. doi: 10.1016/s0254-6272(10)60056-8. [DOI] [PubMed] [Google Scholar]
- 101.Zhang ZQ, Tang T, Luo JK, Huang JF, Yang QD, Li XQ, Jin YQ, Qi Y, Guo CJ, Zhang HX, Xing ZH, Shen DZ. Effect of qi-tonifying and stasis-eliminating therapy on expression of vascular endothelial growth factor and its receptors Flt-1, Flk-1 in the brain of intracerebral hemorrhagic rats. Chin J Integr Med. 2007;13:285–290. doi: 10.1007/s11655-007-0285-4. [DOI] [PubMed] [Google Scholar]
- 102.Zhao LD, Wang JH, Jin GR, Zhao Y, Zhang HJ. Neuroprotective effect of Buyang Huanwu decoction against focal cerebral ischemia/reperfusion injury in rats--time window and mechanism. J Ethnopharmacol. 2012a;140:339–344. doi: 10.1016/j.jep.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 103.Zhao M, Zhang ZF, Ding Y, Wang JB, Li Y. Astragalus polysaccharide improves palmitate-induced insulin resistance by inhibiting PTP1B and NF-kappaB in C2C12 myotubes. Molecules. 2012b;17:7083–7092. doi: 10.3390/molecules17067083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao YN, Wu XG, Li JM, Chen CX, Rao YZ, Li SX. Effect of BuYangHuanWu recipe on cerebral microcirculation in gerbils with ischemia-reperfusion. Sichuan Da Xue Xue Bao Yi Xue Ban. 2010;41:53–56. [PubMed] [Google Scholar]
- 105.Zhou HJ, Tang T, Zhong JH. Effect of buyang huanwu decoction on expressions of angiopoietin-1 and its receptor mRNA in brain of rat after intracerebral hemorrhage. Zhongguo Zhongxiyi Jiehe Zazhi. 2008;28:343–347. [PubMed] [Google Scholar]
- 106.Zhou L, Mei XY, Wu HX, Xie H, Tang XM, Sun HL. Experimental study on Buyang Huanwu decoction [Chinese characters: see text] for promoting functional recovery of crushed common peroneal nerve in rats. Zhongguo Gu Shang. 2011;24:249–252. [PubMed] [Google Scholar]
- 107.Zhou YC, Liu B, Li YJ, Jing LL, Wen G, Tang J, Xu X, Lv ZP, Sun XG. Effects of buyang huanwu decoction on ventricular remodeling and differential protein profile in a rat model of myocardial infarction. Evid Based Complement Alternat Med 2012. 2012:385247. doi: 10.1155/2012/385247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhuang P, Zhang Y, Cui G, Bian Y, Zhang M, Zhang J, Liu Y, Yang X, Isaiah AO, Lin Y, Jiang Y. Direct stimulation of adult neural stem/progenitor cells in vitro and neurogenesis in vivo by salvianolic acid B. PLoS One. 2012;7:e35636. doi: 10.1371/journal.pone.0035636. [DOI] [PMC free article] [PubMed] [Google Scholar]


