Abstract
Acute ischemic stroke has become a major disease burden with high mortality and morbidity rates. There is a lack of evidence-based medicine confirming the efficacy of common treatments. Panax notoginseng saponins, the main active ingredient of radix notoginseng, have a neuroprotective role in ischemic brain injury, and have been popularized as a maintenance treatment for acute cerebral infarction and its sequelae. We conducted literature searches on the Web of Science, ClinicalTrials.gov, Cochrane Collaboration, CNKI, Wanfang and the China Scientific & Technological Achievements Database and analyzed the experimental and clinical outcomes of studies investigating the use of radix notoginseng in the treatment of ischemic brain injury to improve the understanding of relevant research trends and existing problems. We found that over the past 10 years, China has maintained its interest in Panax notoginseng research, while such studies are scarce on the Web of Science. However, Chinese researchers often focus on the neuroprotective role of radix notoginseng in ischemic brain injury, but there are no large-scale clinical data to confirm its efficacy and safety. There remains a need for more rigorous large-sample randomized controlled clinical trials with long-term follow-up, to determine whether radix notoginseng lowers stroke recurrence and improves patient's quality of life.
Keywords: nerve regeneration, neuroprotection, Panax notoginseng, cerebral ischemia, stroke, Panax notoginseng saponins, basic, clinical, neural regeneration
Introduction
Radix notoginseng is the dried root of Panax notoginseng (Araliaceae). It is found in the wild in a narrow zone of southwest China, at 1,500 to 1,800 meters above sea level and at 23.5° north latitude (Jiangxi, Hubei, Guangdong, Guangxi, Sichuan, and Yunnan provinces). However, the majority of radix notoginseng used today is cultivated (Chu et al., 2001; Liu, 2009). According to the Pharmacopoeia of the People's Republic of China, radix notoginseng is used to induce hemostasis, alleviate blood stagnation and relieve pain. Modern pharmacological studies have shown that the main active ingredient of radix notoginseng is Panax notoginseng saponins. Over 20 active saponins and 17 trace elements, as well as proteins, vitamins, and polysaccharides, have been extracted from high-quality radix notoginseng (Ng, 2006). Notoginsenoside R1, a major Panax notoginseng saponin, protects the brain against ischemia and hypoxia (He et al., 2011).
Ischemic stroke or cerebral infarction occurs when local blood circulation in the brain is blocked, resulting in ischemia and hypoxia. Cerebral ischemia-reperfusion injury is considered to be the major pathophysiological process of ischemic cerebrovascular disease (Mergenthaler et al., 2004).
Since 2006, Panax notoginseng saponins have become one of China's fastest-growing drugs used in hospitals. Injectable preparations of radix notoginseng, including freeze-dried Xueshuantong and Xuesetong powders containing over 60% notoginsenoside R1, have been used in the clinic for maintenance treatment of acute cerebral infarction and its sequelae (CAST, 1997). Fourteen manufacturers produce Panax notoginseng saponins approved by the China Food and Drug Administration, and there are approximately 750 approval documents for radix notoginseng preparations and 77 manufacturers of these drugs (Chu et al., 2001).
Data and Methods
Web of Science data analysis
Data source: Science Citation Index (SCI) Expanded, total of 8,765 journals.
Time span: 2009–2014.
Search terms: ts= (sanqi or “panax notoginseng”) & ts= (stroke or “cerebral infarction” or “brain ischemia”).
Inclusion criteria: Peer-reviewed published articles including original research, reviews, meetings, and conference abstracts.
Analysis method: A bibliometric analysis of articles concerning radix notoginseng preparations listed on the Web of Science was performed on the following data: publication year, publication outputs of countries and institutes, document types, and citation frequency.
Wanfang database analysis
Data source: 1,201 medical journals appeared in the Wanfang database (wanfangdata.com.cn).
Time span: 2005–2014.
Keywords [in Chinese]: Radix notoginseng, cerebral ischemia, cerebral infarction and neuroprotection.
Inclusion criteria: Journal articles; academic dissertations; conference papers.
Analysis method: All papers retrieved were subjected to duplicate checking and sorting, before being summarized in combination with manual inspection. This was followed by statistical analysis in Excel. The annual data were analyzed to obtain a trend curve. Discipline distribution was confirmed manually, and the discipline categories were classified according to the source of study object and academic disciplines of the authors.
Funding analysis based on CNKI
Data source: The CNKI database is the largest searchable database of academic literature in China, comprising abstracts and full texts of articles published in 10,112 journals. We conducted an in-depth analysis of the CNKI database according to discipline, publishing year, funding, research level, authors and agencies.
Time span: 2005–2014.
Keywords [in Chinese]: Radix notoginseng, cerebral ischemia, cerebral infarction and neuroprotection.
Inclusion criteria: All articles included in the CNKI.
Analysis method: Funded articles were sorted and summarized in combination with manual inspection followed by statistical analysis in Excel.
Relevant achievement analysis based on the China Scientific & Technological Achievements Database (CSTAD)
Data source: CSTAD is a database of achievements using new technology, which has become the most authoritative technical achievement database in China because of its level of accuracy and detail. In addition to new achievements and technology transfer, this database also provides technical advice and information sources for use in technical reform, product development and process innovation. There are 815,971 items in various disciplines of natural science, which include national-level scientific and technological achievements, with a scope of new technologies, products, processes, materials, and designs.
Time span: 2009–2014.
Keywords [in Chinese]: Radix notoginseng, cerebral ischemia, cerebral infarction and neuroprotection.
ClinicalTrials.gov data analysis
Data source: ClinicalTrials.gov is a website that provides patients, family members, healthcare professionals, and members of the public easy access to information on clinical trials sponsored by the National Institutes of Health, other federal agencies, and the pharmaceutical industry, for a wide range of diseases and conditions. The US National Institutes of Health (NIH) developed the site through its National Library of Medicine in collaboration with the Food and Drug Administration and all NIH institutes. The site was made available to the public in February 2000 and currently contains 173,477 clinical studies conducted in 187 countries.
Time span: 2000–′2014.
Search terms: (sanqi or “panax notoginseng”) and (stroke or “cerebral infarction” or “brain ischemia”).
Inclusion criteria: (1) study participants are not yet being recruited; (2) participants are currently being recruited; (3) participants are being (or will be) selected from a predetermined population; (4) study is ongoing, but participants are not currently being recruited or enrolled; (5) the study has concluded normally and participants are no longer being examined or treated; (6) recruiting or enrolling of participants has halted prematurely but may resume; (7) recruiting or enrolling of participants has halted prematurely and will not resume and participants are no longer being examined or treated; (8) study halted prematurely, prior to enrollment of first participant.
Meta-analysis of the Cochrane Library
The Cochrane Library (http://www.thecochranelibrary.com/) is a collection of databases in medicine and other healthcare specialties provided by the Cochrane Collaboration from 1992. Its aim is to make the results of well-conducted controlled trials readily available and is a key resource in evidence-based medicine. To date, 8,616 systemic reviews have been checked by the Cochrane Collaboration.
Time span: 1993–2014.
Search terms: (sanqi or “panax notoginseng”) and (stroke or “cerebral infarction” or “brain ischemia”).
Results
Web of Science
The Web of Science contains 783 papers addressing radix notoginseng as a treatment for ischemic brain injury, focusing mainly on pharmaceutical and chemical analysis. Only 26 out of the 783 papers are associated with the neuroprotective role of radix notoginseng; these have been cited 166 times in 141 articles (average times cited, 6.38; h-index, 9). These papers are mainly from China (22/26), as well as Korea and Japan.
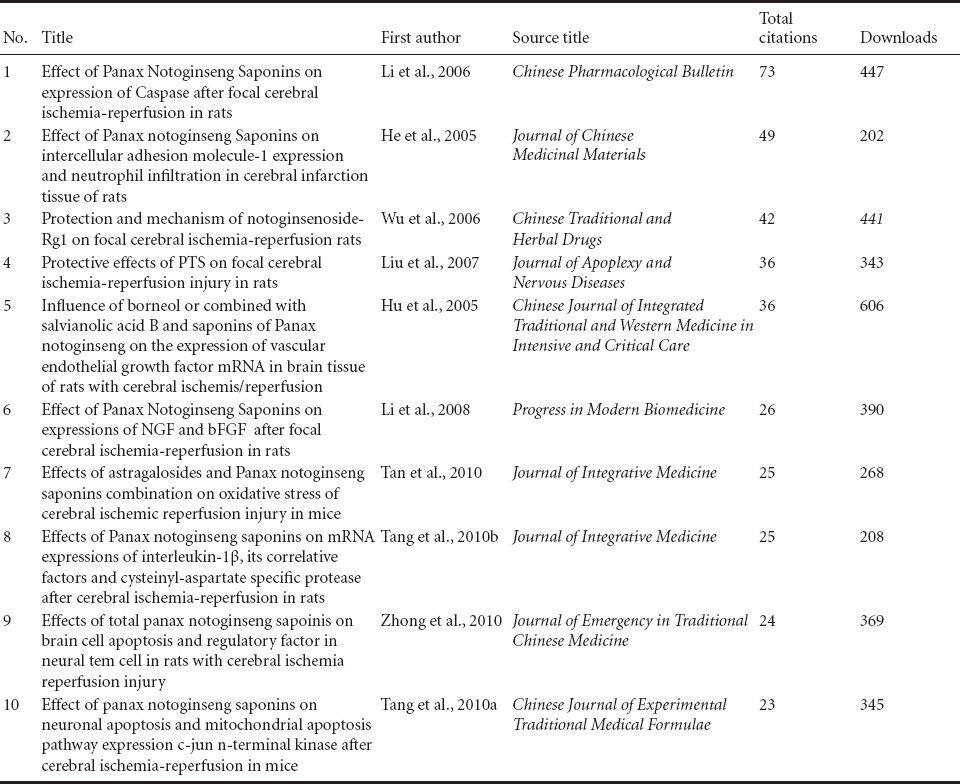
Table 1 shows the most cited original articles addressing the use of radix notoginseng in the treatment of ischemic brain injury on the Web of Science from 2005 to 2014.
Table 1.
Top 10 citations of original articles addressing the use of radix notoginseng in the treatment of ischemic brain injury on the Web of Science, from 2005 to 2014
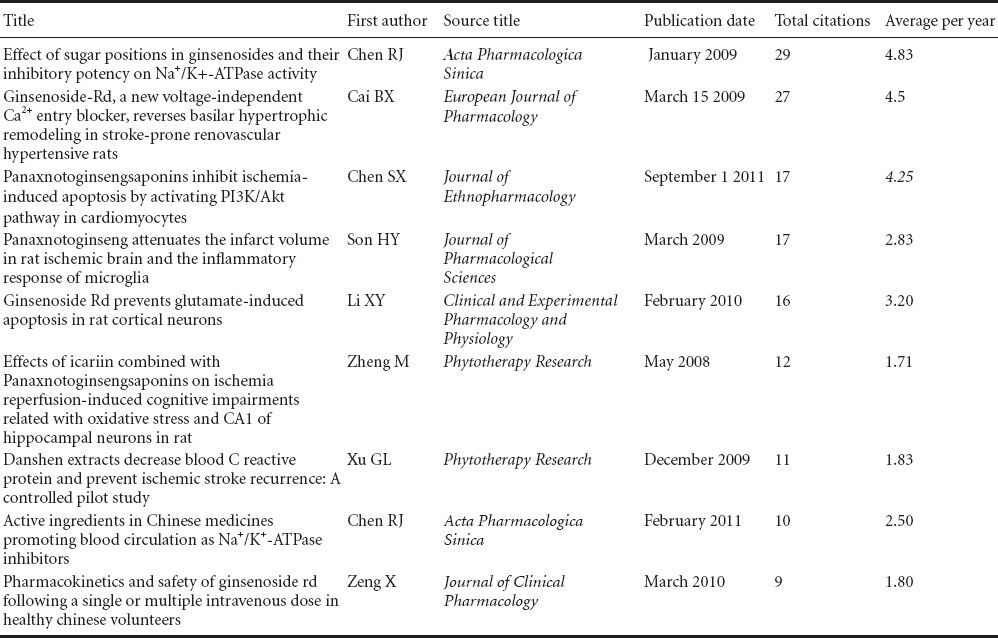
The top cited paper (Chen et al., 2009) is from the National Chung Hsing University, Graduate Institute of Biotechnology, Taiwan, China. The main research focus of the laboratory of Jason T. C. Tzen, the corresponding author, is the active ingredients of radix notoginseng and the molecular mechanisms underlying its effects in the human body.
The second and fifth most popular papers were both from the Department of Pharmacology, Sun Yat-sen University, China. The relevant studies were funded by the National Natural Science Foundation of China (No. 30730105), National Basic Research Program of China (Program 973; No. 2009CB521903), and Science Foundation of Guangzhou in China. The corresponding author, Yongyuan Guan, works in a laboratory that studies the effects of radix notoginseng on ion channels and neuroprotection. In these two papers addressing the purified ingredients of Panax notoginseng saponins, the authors propose that ginsenoside Rd can significantly inhibit calcium overload following middle cerebral artery occlusion, prevent Ca2+ influx in vascular smooth muscle, dilate the cerebral blood vessels, and improve cerebral circulation; moreover, radix notoginseng can be used against glutamate-induced apoptosis in rat cortical neurons.
The third most popular paper comes from Guangdong General Hospital, Guangdong Academy of Medical Science, China. In this paper, Panax notoginseng saponins are shown to protect local ischemia-induced apoptosis in vitro and in vivo by activating the PI3K/Akt signaling pathway (Chen et al., 2011).
The fourth most popular paper came from Dongguk University, Korea (Son et al., 2009). Park and co-workers found that radix notoginseng eased inflammation in the infarcted region and reduced the infarct volume, providing sound evidence that Panax notoginseng saponins are effective against inflammation.
The above papers were mainly published in the following journals listed in the SCI: Journal of Ethnopharmacology; Phytotherapy Research; Neural Regeneration Research; Journal of the Neurological Sciences; Clinical and Experimental Pharmacology and Physiology.
Comparative analysis of Chinese databases
Research trends
Between 2005 and 2014, 1,320 papers addressing radix notoginseng in the treatment of ischemic brain injury were retrieved from the CNKI database, and 1,134 from the Wanfang database. Research trends are consistent between the two databases. The annual publication output was highest in 2008, with 169 and 148 papers included in CNKI and Wanfang, respectively. The number of publications increased fastest in 2006, with 150 and 145 papers included in CNKI and Wanfang that year, respectively, a growth of 27.12% and 47.96%. Publication volume decreased most in 2009, with only 140 and 118 new articles included in CNKI and Wanfang, respectively, or a decrease of 17.16% and 20.27% (Figure 1).
Figure 1.
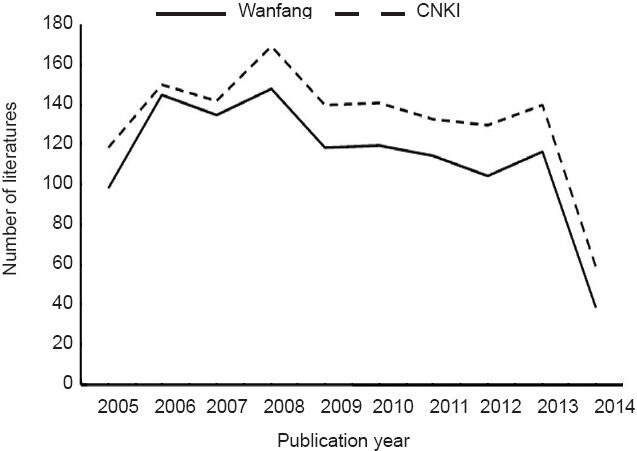
Comparison of the number of articles included in the CNKI and Wanfang databases between 2005 and 2014.
Main journal output
A total of 1 320 articles concerning the use of radix notoginseng for ischemic brain injury were included in CNKI from 2005 to 2014, published mainly in the journals shown in Figure 2 and discussed below.
Figure 2.
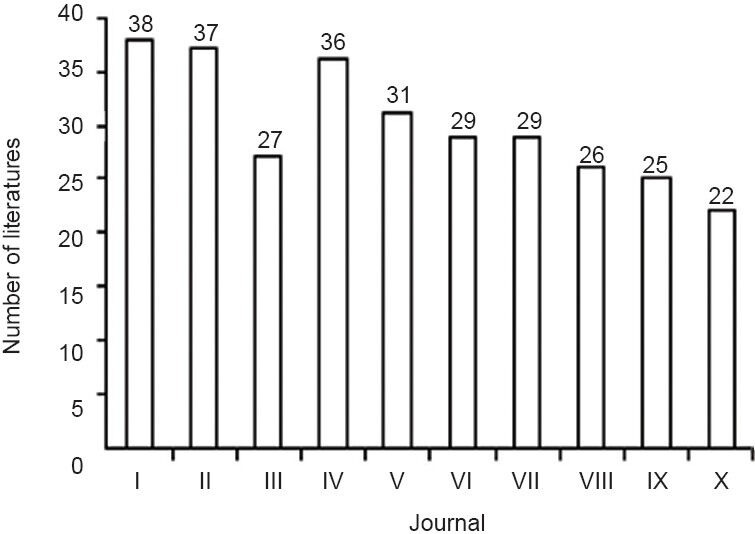
Top 10 journals with the most articles concerning the use of radix notoginseng for ischemic brain injury from 2005 to 2014.
I: Chinese Traditional Patent Medicine; II: Journal of Beijing University of Traditional Chinese Medicine; III: Shaanxi Journal of Traditional Chi-nese Medicine; IV: Journal of Emergency in Traditional Chinese Medicine; V: Chinese Journal of Integrative Medicine on Cardio-/Cerebrovascular Disease; VI: Chinese Journal of Practical Nervous Diseases; VII: Chinese Journal of Tissue Engineering Research; VIII: Chinese Journal of Ex-perimental Traditional Medical Formulae; IX: Lishizhen Medicine and Materia Medica Research; X: Chinese Traditional and Herbal Drugs.
In the past 10 years, the Chinese Traditional Patent Medicine has published the most articles (38), among which the three most-cited are “Effect of Panax notoginseng on cerebral ischemia-reperfusion injury in rats” (Jiang et al., 1995; 63 citations), “Effect of Panax ginseng, Panax pseudoginseng, Acanthopanax and Schisandra on protein biosynthesis in mouse brain” (Ye et al., 1993; 30 citations), and “Effect of Panax notoginseng saponins on the expression of BNDF in rats with cerebral ischemia reperfusion” (Zhang et al., 2008; 23 citations). The top three most downloaded articles are “Quantitative determination of notoginsenoside R1 and ginsenoside Rg1, Rb1 contents in Xinnao Guantong Guttate Pill by HPLC gradient elution method” (Liu et al., 2007; 240 downloads), “Effect of Sanqi Tongshu Capsule on Syp and PSD-95 expression of different periods of cerebral infarction” (Cui et al., 2008; 243 downloads), and “Advanced development of new preparation and component of Salvia and Panax notoginseng” (Zhang et al., 2011; 63 downloads).
Additionally, the three most-published journals in the non-TCM field are the Chinese Journal of Integrative Medicine on Cardio/Cerebrovascular Disease, Chinese Journal of Practical Nervous Diseases, and Chinese Journal of Tissue Engineering Research, with about 30 papers on radix notoginseng for ischemic brain injury (Figure 2).
Research hotspots
High-frequency keywords in the databases reflect the direction of research and commonly covered subjects, as well as the hot topics and research questions across different time periods. The 10 most popular keywords listed in articles related to the use of radix notoginseng for ischemic brain injury in the CNKI and Wanfang databases over the past 10 years are listed in Table 2.
Table 2.
Most popular keywords in articles addressing the use of radix notoginseng for ischemic brain injury in the CNKI and Wanfang databases
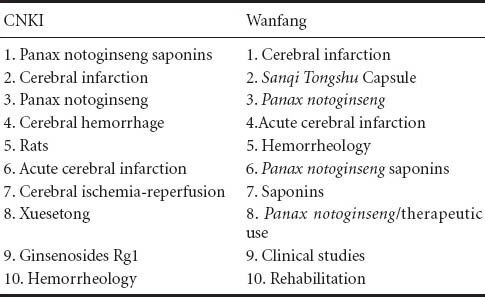
Research fronts
The most relevant keywords relating to the treatment of ischemic brain injury with radix notoginseng occur in the Wanfang database, reflecting the research direction of cross-sectional studies in this field. The 10 most popular keywords searched by the researchers are: risk factors; hemorrheology; extraction process; ginseng; high performance liquid chromatography; thin layer chromatography; ginsenosides; Salvia mittiorrhiza; chemical composition; content determination. Based on these words, we can conclude that the researchers were more interested on the main active ingredients of radix notoginseng and the quality of its preparation, as well as the mechanisms underlying its effects in the treatment of ischemic brain injury.
Most-cited articles and authors
The most-cited articles in CNKI between 2005 and 2014, which address radix notoginseng in the treatment of ischemic brain injury, are listed in Table 3.
Table 3.
The most-cited articles in the CNKI database, from 2005 to 2014, addressing radix notoginseng treatment of ischemic brain injury
Changqing Deng (Li et al., 2006) is the corresponding author with the most citations and comes from Hunan University of Chinese Medicine in China. He is engaged in basic research on Buyang Huanwu Tang and Panax notoginseng saponins in the treatment of cerebral ischemia-reperfusion. His 34 articles are included in the Wanfang database and cited 256 times, with an h-index of 9.
Changqing Deng is a prolific author, publishing every year for the past decade. In 2012, he published eight articles (Figure 3). This author collaborates with the authors shown in Figure 4, who study radix notoginseng and neurological diseases, and has been repeatedly funded by the National Natural Science Foundation of China and the Chinese Academy of Sciences.
Figure 3.
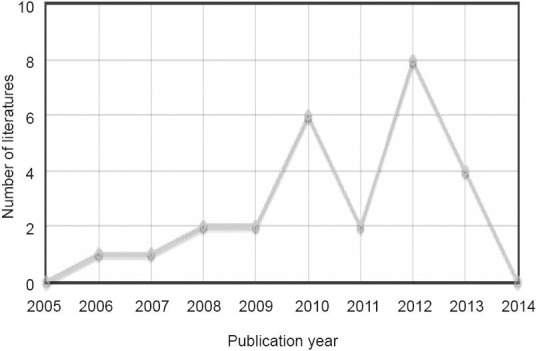
Articles by Changqing Deng in CNKI from 2005 to 2014.
Figure 4.
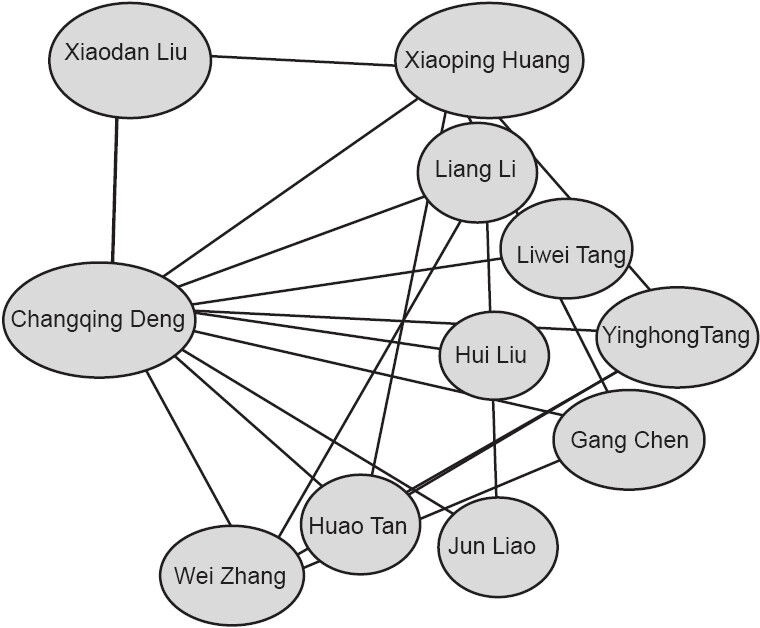
Distribution of authors collaborating with Changqing Deng from 2005 to 2014, according to the CNKI database.
Most productive institutions
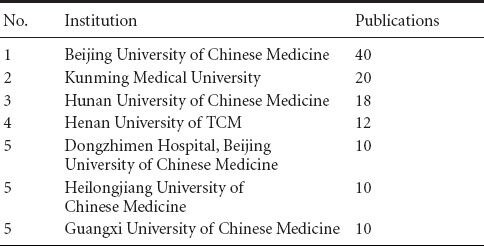
Based on data from the CNKI database (2005–2014), the most productive institutions in this 10-year period are the Beijing University of Chinese Medicine with 40 articles and Kunming Medical University with 20 articles (Table 4).
Table 4.
Most productive institutions in CNKI from 2005 to 2014
Main funding projects
There are 39 funds across 1,320 articles in the CNKI database (2005–2014), including five national-level funds, 28 provincial-level and ministerial-level funds, 6 city-level funds. The national-level funds include the National Natural Science Foundation of China (43 items), China Postdoctoral Fund (five items), National Key Technology R&D Program of China (four items), National Basic Research Program of China, Program 973 (three items), National Key Science and Technology Program of China (one item), Scientific Research Fund of the State Administration of Traditional Chinese Medicine of China (one item), and Key Program for International S&T Cooperation Projects (one item). Additionally, a total of 88 projects with published articles are funded by the 28 provincial-level and ministerial-level funds.
Technological achievements
We retrieved 92 rewarded or planned achievements concerning radix notoginseng in the treatment of ischemic brain injury at provincial-, municipal-level, and ministerial-levels from CSTAD between 2009 and 2014. Among these 92 achievements, 70 are related to medicine and health, and 22 to industrial technology. In 2008, the most funded project in China was supported by 17 funds, but this number shows a declining trend since 2008 (Figure 5).
Figure 5.
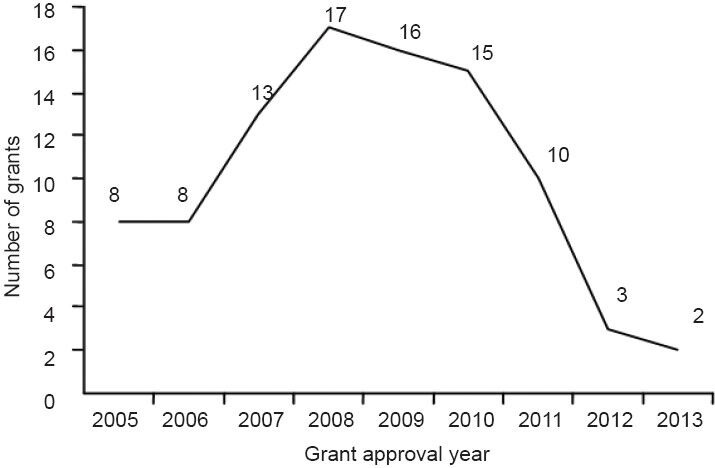
Achievements concerning radix notoginseng in the treatment of ischemic brain injury in CSTAD from 2005 to 2014.
ClinicalTrials.gov database
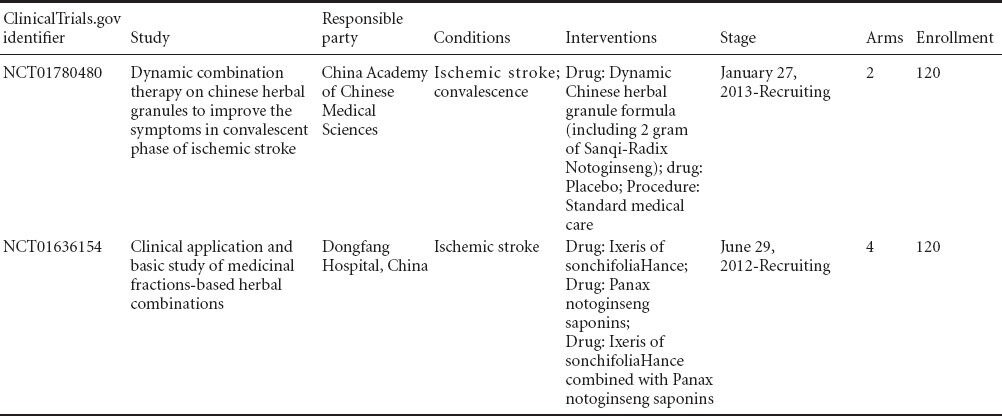
To date, only two clinical trials concerning radix notoginseng for ischemic brain injury have been registered in ClinicalTrials.gov, both of which come from China (Table 5).
Table 5.
Clinical trials of radix notoginseng as a treatment for ischemic brain injury listed in ClinicalTrials.gov.
Cochrane Library
Only one study, “Sanchi for acute ischaemic stroke”, classifiable as a randomized or quasi-randomized controlled clinical trial of Panax preparations for the treatment of acute ischemic stroke, was retrieved in the Cochrane Library (http://www.thecochranelibrary.com/). The study was reported by Chen et al. (2008) from Huaxi Hospital of Sichuan University in China, and the relevant article was included by the Cochrane Stroke Group in 2008. The study focused on the evaluation of the therapeutic effect and safety of radix notoginseng in the treatment of acute ischemic stroke, based on data from published literature retrieved from 15 clinical trial registers and bibliographic databases, including the Cochrane Stroke Group Trials Register, Chinese Clinical Trial Registry, Cochrane Central Register of Controlled Trials, Cochrane Complementary Medicine Field Registry, EMBASE, CNKI, VIP, and the Wanfang database.
The authors screened a total of eight randomized controlled trials. A significant difference was found between radix notoginseng and placebo or no treatment in the therapeutic efficacy against acute ischemic stroke within 30 days of treatment. There were 660 participants enrolled in the studies reported in these eight articles. Seven of the articles show poor quality, six of which have a follow-up period of less than 1 month. Only two of the eight articles show that if treatment continues for 28 days, the use of radix notoginseng can significantly reduce the mortality and disability of patients after acute ischemic stroke. One of these two articles reports a higher Barthel index in the patients undergoing radix notoginseng treatment. Comprehensive analysis of seven studies shows that radix notoginseng can significantly improve neurological deficits. The overall mortality rate is less than 1%, indicating that the participants mostly appear to have mild stroke and fewer adverse reactions. However, there is little information about the quality of life or stroke recurrence. Based on the available evidence, the authors conclude that radix notoginseng may be effective for the treatment of acute ischemic stroke. However, the low number and poor quality of the included studies results in considerable bias, so we cannot be certain of the efficacy and safety of radix notoginseng in the treatment of acute ischemic stroke.
Discussion
The majority of the 1 320 articles from CNKI focus on experimental research to explore the mechanism of radix notoginseng in the treatment of ischemic brain injury, while the 1 134 articles from Wanfang mainly address its clinical use. Researchers are also concerned with the main active ingredients of Panax notoginseng, the quality of Panax notoginseng preparations, and relevant clinical results.
Animal studies have found that Panax notoginseng saponins can significantly improve behavior symptoms, relieve brain edema, and reduce infarct size in rat models of cerebral ischemia. Cytological, morphological and lipid oxidation studies have shown that Panax notoginseng saponins can reduce serum malondialdehyde content and increase superoxide dismutase activity in the brain, suggesting that they protect against nerve cell damage from energy metabolism disorders, and also function as cerebrovascular regulators.
Literature on the mechanism of Panax notoginseng saponins has mainly concentrated on the following subjects: (1) the role of Panax notoginseng saponins in mouse global cerebral ischemia and rat focal cerebral ischemia; (2) their protective role in primary rat cerebral cortical neurons; (3) their effect on brain tissue blood supply; (4) their effect on energy metabolism; (5) their effect on intracellular free calcium concentration; (6) their effect on the calcium intake capacity of synaptosomes; (7) their effect on the release of excitatory amino acids and specific binding to the receptor; (8) their inhibitory effect on the oxidation of rat liver and brain homogenates and lipid peroxidation induced by the Fe2+/ascorbic acid system; (9) their effect on platelet function.
Only two trials on the use of radix notoginseng in the treatment of ischemic brain injury have been registered on ClinicalTrials.gov. Both are in the recruitment period and expected to recruit 120 cases, with no results announced yet.
Conclusion
In China, Panax notoginseng has been used for a long time and is considered to be an effective treatment for ischemic stroke. Relevant articles about its clinical use, however, are lacking in quality. There remains a need for more rigorous large-scale randomized controlled clinical trials with extended follow-up periods to determine the clinical efficacy of Panax notoginseng in improving the quality of life and reducing stroke recurrence after acute ischemic stroke.
Footnotes
Funding: This study was supported by a grant from the Scientific Research Project during the Twelvth Five-Year Period of Jilin Provincial Educational Bureau in China, No. 2013-441; a grant from the Scientific Research Project of Jilin Provincial Health Bureau in China, No. 2012Z102.
Copyedited by Jackson C, Li CH, Wang L, Song LP, Zhao M
References
- 1.Cai BX, Li XY, Chen JH, Tang YB, Wang GL, Zhou JG, Qui QY, Guan YY. Ginsenoside-Rd a new voltage-independent Ca2+ entry blocker reverses basilar hypertrophic remodeling in stroke-prone renovascular hypertensive rats. Eur J Pharmacol. 2009;606:142–149. doi: 10.1016/j.ejphar.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 2.Chen RJ, Chung TY, Li FY, Lin NH, Tzen JT. Effect of sugar positions in ginsenosides and their inhibitory potency on Na+/K+-ATPase activity. Acta Pharmacol Sin. 2009;30:61–69. doi: 10.1038/aps.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen RJ, Jinn TR, Chen YC, Chung TY, Yang WH, Tzen JT. Active ingredients in Chinese medicines promoting blood circulation as Na+/K+ -ATPase inhibitors. Acta Pharmacol Sin. 2011;32:141–151. doi: 10.1038/aps.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Liu J, Liu X, Fu Y, Zhang M, Lin Q, Zhu J, Mai L, Shan Z, Yu X, Yang M, Lin S. Panax notoginseng saponins inhibit ischemia-induced apoptosis by activating PI3K/Akt pathway in cardiomyocytes. J Ethnopharmacol. 2011;137:263–270. doi: 10.1016/j.jep.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Zhou M, Li Q, Yang J, Zhang Y, Zhang D, Kong S, Zhou D, He L. Sanchi for acute ischaemic stroke. Cochrane Database Syst Rev. 2008:CD006305. doi: 10.1002/14651858.CD006305.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Chu JJ, Liu L, Hua XG. Overview of Sanchi and Sanchi industry. Dangdai Shengtai Nongye. 2001;10:116–121. [Google Scholar]
- 7.Cui FF, Zhai JY, Zou WM, Wang XL, Zou YH, Zhu LQ. Effect of Sanqi Tongshu Capsule on Syp and PSD-95 expression of different periods of cerebral infarction. Zhong Cheng Yao. 2008;1:31–34. [Google Scholar]
- 8.CAST (Chinese Acute Stroke Trial) Collaborative Group. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. Lancet. 1997;349:1641–1649. [PubMed] [Google Scholar]
- 9.He L, Chen X, Zhou M, Zhang D, Yang J, Yang M, Zhou D. Radix/rhizoma notoginseng extract (sanchitongtshu) for ischemic stroke. Lancet. 2011;349:9. doi: 10.1016/j.phymed.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 10.He W, Zhu ZP. Effect of Panax notoginseng Saponins on intercellular adhesion molecule-1 expression and neutrophil infiltration in cerebral infarction tissue of rats. Zhong Yao Cai. 2006;5:403–405. [PubMed] [Google Scholar]
- 11.Hu LM, Zhang YJ, Wang W, Wu Y, Fan X, Gao XM, Zhang BL. Influence of borneol or combined with salvianolic acid B and saponins of panax notoginseng on the expression of vascular endothelial growth factor mRNA in brain tissue of rats with cerebral ischemia/reperfusion. Zhongguo Zhongxiyi Jiehe Jijiu Zazhi. 2005;12:263–266. [Google Scholar]
- 12.Jiang KY, Qian ZN. Effect of panax notoginseng on cerebral ischemia-reperfusion injury in rats. Zhong Cheng Yao. 1995;7:32–33. [Google Scholar]
- 13.Li H, Deng CQ, Chen BY, Chen RF, Zhang SP, Liang Y. Effects of Panax Notoginseng Saponins on expression of Caspase after focal cerebral ischemia-reperfusion in rats. Zhongguo Yaolixue Tongbao. 2006;2:189–193. [Google Scholar]
- 14.Li H, Deng CQ, Xiong AJ, Chen BY. Effect of Panax Notoginseng Saponins on expressions of NGF and bFGF after focal cerebral ischemia-reperfusion in rats. Xiandai Shengwu Yixue Jinzhan. 2008;2:219–221. [Google Scholar]
- 15.Li XY, Liang J, Tang YB, Zhou JG, Guan YY. Ginsenoside Rd prevents glutamate-induced apoptosis in rat cortical neurons. Clin Exp Pharmacol Physiol. 2009;37:199–204. doi: 10.1111/j.1440-1681.2009.05286.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu CF, Wen YQ, Wang H, Chen SZ. Quantitive determination of notogin senoside R1 and ginsenoside Rg1, Rb1 contents in Xinnao Guantong Guttate Pill by HPLC gradient elution method. Zhong Cheng Yao. 2008;29:1167–1169. [Google Scholar]
- 17.Liu XQ. Clinical application of Sanchi. Linchuang Heli Yongyao Zazhi. 2009;2:88–89. [Google Scholar]
- 18.Liu ZC, Zhou GE, Zhao KJ, Rao Ml. Protective effects of PTS on focal cerebral ischemia-reperfusion injury in rats. J Apoplexy Nerv Dis. 2007;1:38–40. [Google Scholar]
- 19.Mergenthaler P, Dirnagl U, Meisel A. Pathophysiology of stroke: lessons from animal models. Metab Brain Dis. 2004;19:151–167. doi: 10.1023/b:mebr.0000043966.46964.e6. [DOI] [PubMed] [Google Scholar]
- 20.Ng T. Pharmacological activity of sanchi ginseng (Panax notoginseng) J Pharm Pharmacol. 2006;58:13. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- 21.Son HY, Han HS, Jung HW, Park YK. Panax notoginseng attenuates the infarct volume in rat ischemic brain and the inflammatory response of microglia. J Pharmacol Sci. 2009;109:368–379. doi: 10.1254/jphs.08197fp. [DOI] [PubMed] [Google Scholar]
- 22.Tan H, Huang XP, Deng CQ. Effects of astragalosides and Panax notoginseng saponins combination on oxidative stress of cerebral ischemic reperfusion injury in mice. J Chin Integr Med. 2010;5:448–452. doi: 10.3736/jcim20100508. [DOI] [PubMed] [Google Scholar]
- 23.Tang YH, Huang XP, Tan Hua, Chen BY, Deng CQ. Effect of Panax Notoginseng Saponins on neuronal apoptosis and mitochondrial apoptosis pathway expression c-Jun N-terminal kinase after cerebral ischemia-reperfusion in mice. Zhongguo Shiyan Fangjixue Zazhi. 2010a;16:129–132. [Google Scholar]
- 24.Tang YH, Zhang SP, Liang Y, Deng CQ. Effects of Panax notoginseng saponins on mRNA expressions of interleukin-1β its correlative factors and cysteinyl-aspartate specific protease after cerebral ischemia-reperfusion in rats. J Chin Integr Med. 2010b;3:328–332. doi: 10.3736/jcim20070319. [DOI] [PubMed] [Google Scholar]
- 25.Wu LO, Zhan HQ, Yan JL, Cai WF, Wu JP, Yang KK. Protection and mechanism of notoginsenoside-Rg1 on focal cerebral ischemia-reperfusion rats. Zhong Cao Yao. 2006;37:229–233. [Google Scholar]
- 26.Xu G, Zhao W, Zhou Z, Zhang R, Zhu W, Liu X. Danshen extracts decrease blood C reactive protein and prevent ischemic stroke recurrence: a controlled pilot study. Phytother Res. 2009;23:1721–1725. doi: 10.1002/ptr.2819. [DOI] [PubMed] [Google Scholar]
- 27.Ye CY, Liu ZP. Effect of Panax ginseng, Panax pseudoginseng, Acanthopanas and Schisandra on protein biosynthesis in mouse's brain. Zhong Cheng Yao. 1993;16:30–31. [Google Scholar]
- 28.Zeng X, Deng Y, Feng Y, Liu Y, Yang L, Huang Y, Sun J, Liang W, Guan Y. Pharmacokinetics and safety of ginsenoside Rd following a single or multiple intravenous dose in healthy Chinese volunteers. J Clin Pharmacol. 2010;50:285–292. doi: 10.1177/0091270009344334. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Chen G, Mu L. Advanced development of new preparation and component of Salvia and Panax notoginseng. Zhong Cheng Yao. 2011;33:1568–1570. [Google Scholar]
- 30.Zhang YQ, Mo GH, Chen M, Zeng XF, Lu H. Effect of panax notoginseng saponinsa on the expression of BDNF in rats with cerebral ischemia reperfusion. Zhong Cheng Yao. 2008;7:958–961. [Google Scholar]
- 31.Zheng M, Qu L, Lou Y. Effects of icariin combined with Panax notoginseng saponins on ischemia reperfusion-induced cognitive impairments related with oxidative stress and CA1 of hippocampal neurons in rat. Phytother Res. 2008;22:597–604. doi: 10.1002/ptr.2276. [DOI] [PubMed] [Google Scholar]
- 32.Zhong S, Chen WC, Xu YQ, Huang YZ, Yang JY, Zhang XY. Effects of Total Panax Notoginseng Sapoinis on Brain Cell Apoptosis and Regulatory Factor in Neural tem Cell in Rats with Cerebral Ischemia Reperfusion Injury. Zhongguo Zhongyi Jizheng. 2010;2:279–282. [Google Scholar]


