Abstract
Osteosarcomas are relatively rare in jaws and maxillomandibular lesions in children are distinctly uncommon. Telengiactic osteosarcomas in jaws are still a very rare variant and till now just 3 cases are reported in English literature. Herein, we report the first case of telangiectatic osteosarcoma occurring in mandible in pediatric patient. Patient was treated by hemimandibulectomy and is free of disease since seven years with regular follow-up.
Keywords: Aneurysmal bone cyst, mandible, paediatric patient, telangiectatic osteosarcoma
INTRODUCTION
Osteosarcomas (OS) of jaws bone are uncommon and most of them are classical osteosarcoma representing about 6% to 7% of the total osteosarcomas.[1,2] The tumor usually occurs in the long bones with predilection to the distal femoral metaphysis, proximal tibia and humeral metaphysis. The age incidence of OS in long bones is second decade of life.[1] The mortality rate is also varied in these locations with 5 years survival around 20% to 70%.[1,3] Whereas in the maxillofacial bones, the presentation of the tumor is at the later age (a decade later), its higher survival rate and a lower incidence of metastasis are believed to differentiate from OS in other locations.[4] Telangiectatic osteosarcoma (TOS) is a rare variant of OS, accounting for 0.8-2.5% of all OS.[5] Two cases of primary TOS in jaws has been reported by Chan et al.,[6] and Huvos et al.,[7] and one case of secondary metastasis reported by Alves et al.,[8] We believe that this is the fourth case of TOS affecting the mandible and first case affecting the jaw in pediatric patients, under the age of 14 years.
CASE REPORT
A 13-year-old girl reported with a chief complaint of painless swelling in the lower jaw on leftside since 3 weeks. Swelling was asymptomatic with no increase in size. There was no history of trauma to the jaw. Medical history was non-contributory. On local examination a solitary, diffuse, non-tender, bony hard swelling was noted involving left lower border of the mandible. The overlying skin was freely mobile with slight rise in temperature. No numbness or paresthesia of lower lip was noted. The swelling was extending posteriorly 1cm behind the angle of the mandible, anteriorly 1cm behind the angle of the mouth, superiorly 5 cm above the lower border and inferiorly 1.5 cm below the lower border of the mandible [Figure 1]. On intraoral examination, mouth opening was normal with partial obliteration of the buccal vestibule in relation to lower left premolars and molars. Lower second premolar was nonvital. Lymph nodes were not palpable. A provisional diagnosis of ameloblastoma was made. Differential diagnosis of odontogenickeratocyst, dentigerous cyst, benign odontogenictumours, central giant cell granuloma, central cemento-ossifying fibroma and aneurysmal bone cyst were considered.
Figure 1.
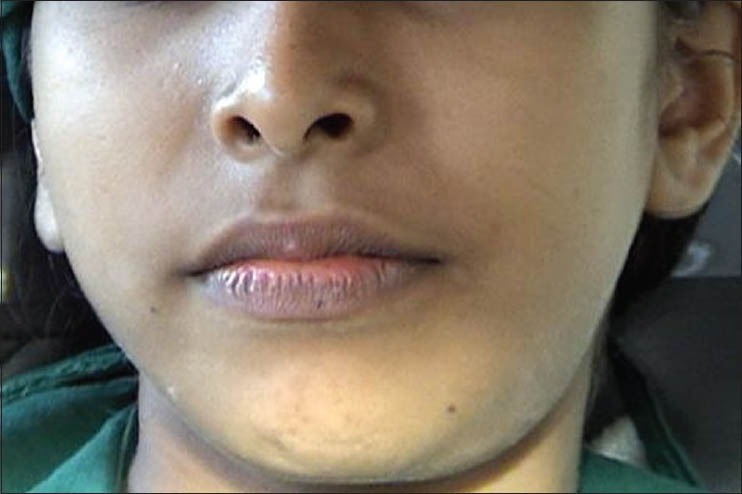
Extra oral photograph showing the swelling in left lower part of the mandible
On radiographic evaluation, an ill-defined radiolucency was noted in the left side of mandible with respect to the peri-apical region of premolars and molars. Radiopaque lines resembling whisps of cotton were noted adjacent to the lower border of the mandible suggesting sun ray appearance [Figure 2]. Axial and coronal computed tomography sections revealed a multi-locular hypodense lesion involving the body of the mandible till the angle, causing expansion and destruction of the lingual and buccal cortices [Figures 3 and 4]. There was blown-out pattern. Differential diagnoses considered after radiographic examinations were osteosarcoma, central hemangioma, chondrosarcoma, Ewing's sarcoma, primary lymphoma and metastatic tumor.
Figure 2.
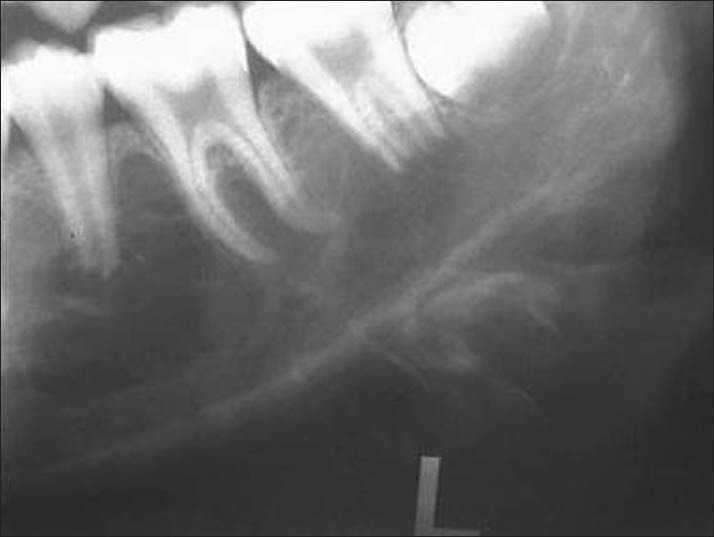
Radiograph showing radiopaque lines resembling whips of cotton adjacent to the lower border of the mandible suggesting sun ray appearance
Figure 3.
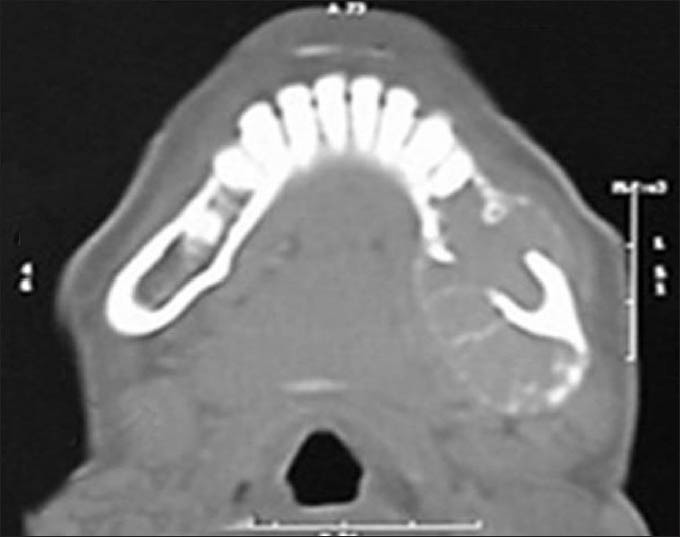
Axial CT section showing extension of the lesion
Figure 4.
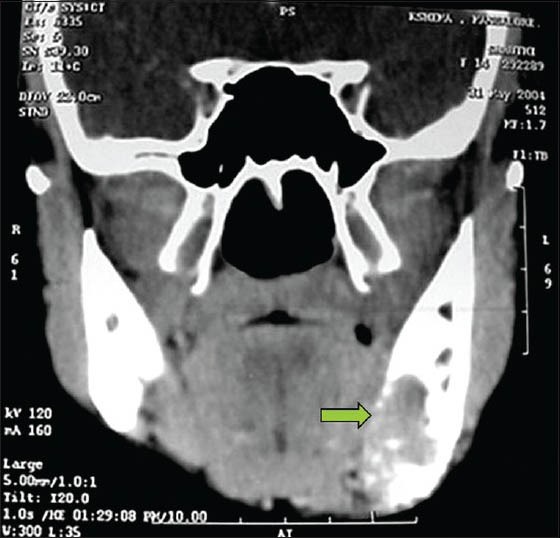
Coronal CT section showing expansile and destructive lesion in the mandible
Incisional biopsy was received for histopathological examination. It showed large and small blood filled spaces separated by strands of neoplastic malignant mononuclear cells intermingled with benign appearing multinucleated osteoclastic giant cells [Figure 5a and b]. These neoplastic cells showed cellular and nuclear pleomorphism, increased nuclear and cytoplasmic ratio, enlarged hyperchromatic nuclei and good number of cells showed prominent nucleoli. Abnormal mitosis was frequently seen [Figure 6]. In some areas, streaks and strands of tumor osteoid were seen surrounding the tumor cells [Figure 7a and b]. On immunohistochemistry, the neoplastic cells were negative for CD31 [Figure 8]. Diagnosis of osteogenic sarcoma telangiectatic type was made.
Figure 5.
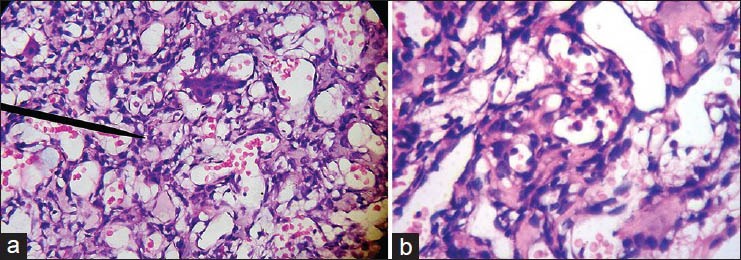
(a) Photomicrograph of vascular spaces separated by benign appearing osteoclastic multinucleated giant cells and strands of neoplastic malignant mononuclear cells (H&E stain ×100). (b) Photomicrograph of vascular spaces separated by strands of neoplastic malignant mononuclear cells with tumor osteoid (H&E stain ×400)
Figure 6.

Photomicrograph of tumor cells showing cellular and nuclear pleomorphism, abnormal and increased mitotic figures with benign appearing multinucleated giant cell lining the vascular space (H&E stain ×400)
Figure 7.
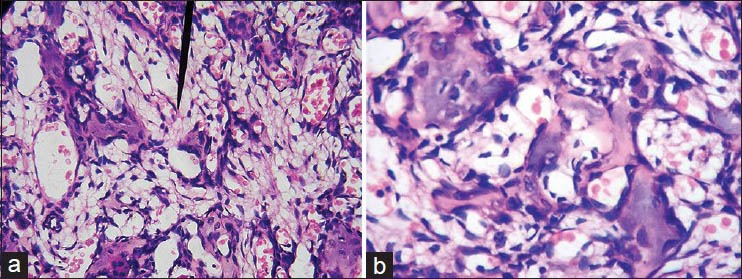
(a) Photomicrograph of tumor cells showing areas of tumor osteoid with vascular spaces and benign appearing multinucleated giant cells (H&E stain ×100) (b) Photomicrograph of pleomorphic tumor cells showing areas of tumor osteoid with vascular spaces (H&E stain ×400)
Figure 8.
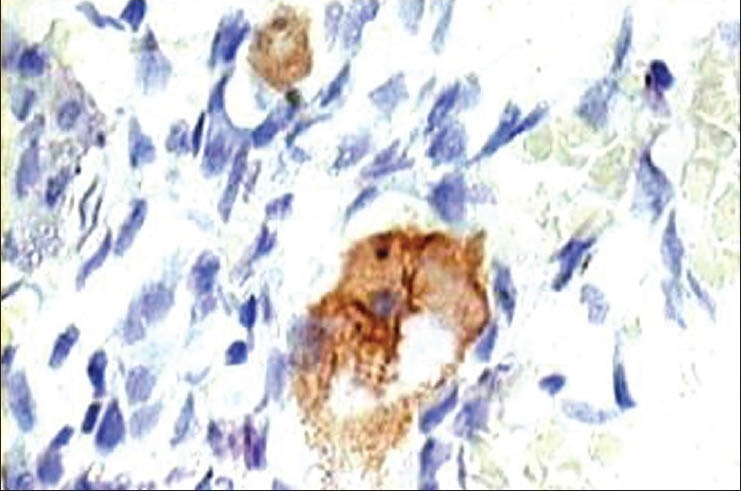
Photomicrograph of immunohistochemical staining revealing negativity of neoplastic cells for CD31 (IHC stain ×400)
Patient was treated by hemi-mandibulectomy. Specimen received consisted of a resected mandible with a soft tissue mass arising in the marrow space. The tumor was breaching the cortex and protruding into the adjoining soft tissue [Figure 9]. Cut section along the tumor was gray-white with cystic areas containing clotted blood. Histopathological features were similar to those as was seen in incisional biopsy. Iliac bone graft reconstruction was done after five years. No radiation or chemotherapy was given. The patient is free of disease since seven years after surgery.
Figure 9.
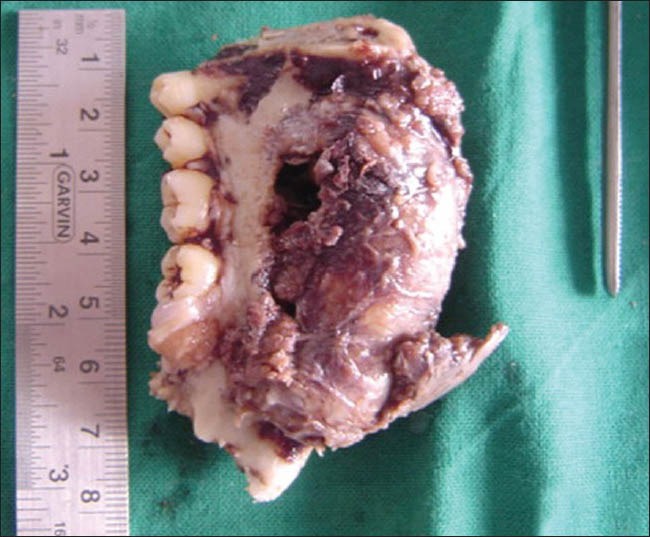
Photograph of gross specimen (resected mandible)
DISCUSSION
Osteosarcoma of the jaw bones are quite rare with an incidence of 1.5-7% of all head and neck primary tumors.[9] TOS was first described by Paget in the year 1854.[7] Originally it was considered to be a malignant bone aneurysm. It was Ewing, who considered this entity as a variant of OS.[5] In pediatric patients, OS accounts for less than 1% of all head and neck tumors.[9] and while TOS alone is considered it is still very rare. Few reports suggest that OS in jaw affects more males than females and few other reports suggest that there is no sex predilection.[10] Mandible involvement was more than that of maxilla.[10]
OS usually occurs as a secondary tumor, following radiation therapy to other tumors.[10] Some OS are syndrome associated and few develop from a pre-existing benign tumor.[9] In present case, there was no associated syndrome and it is unlikely that there was any pre-existing lesion for a short period of history. The patient was only 13 years unusually young for usual OS of jaw bones but this age is expected age for TOS occurring elsewhere in the body.[6] The most common presenting symptoms of OS in head and neck are swelling, pain, mucosal ulceration, loosening of teeth and parasthesia of lip.[10] Swelling was the only complaint in the present case. The time gap between onset of symptoms and the first treatment generally ranged from 3-6 months.[10]
Diagnosis of TOS was based on the criteria given by Matsuno and colleges.[5,6] These criteria are 1) radiographically lyticdestructive lesion with no appreciable area of sclerosis, 2) the lesion presented grossly as a cavity with little solid tumor tissue and no areas of sclerosis and 3) the tumor histologically consisted of single or multiple cystic cavities that contained blood or necrotic tumor and are often traversed by septa composed of anaplastic cells. Osteoid production was minimal, appearing as a fine lace like material between tumor cells. Present case radiographically showed poorly defined irregular destructive bone lesion with blown-out pattern similar to that of other reports.[5] There was presence of sun burst or sun ray appearance in the lower border of mandible in the form of thin radio opaque whips seen in orthopantamograph, which were reported to be seen in OS and central hemangioma. But this feature was not prominent in CT, in the present case. This appearance was due to periosteal reaction and indicates that the neoplasm has penetrated the cortex and extended into soft tissue.[10] There was no lesional sclerosis on CT. On gross examination, there was destruction and perforation of both buccal and lingual cortices by the tumor with extension into the soft tissue suggesting the malignant potential of the tumor. Most areas of this tumor were filled with clotted blood. These findings were similar to the previous reports.[5,7]
TOS is a distinct entity and there are many other lesions that simulate TOS histopathologically. In the present case, the tumor was differentiated from aneurysmal bone cyst (ABC), hemangioendothelioma, giant cell tumour and giant cell granuloma. ABC is an expansive destructive, hemorrhagic, tumor like condition, thought to represent a benign reactive vascular process.[11] Histopathologically, it shows similar picture of TOS, but the mesenchymal stromal cells are benign in nature, unlike in TOS they are malignant in nature. Areas of ABC may also occur as a secondary component in a variety of benign tumors, tumor like bony lesions and conventional OS. Some authors postulate that all ABC represent secondary lesions, in which hemorrhagic process has destroyed all traces of the original primary lesion.[11] Although, malignant transformation of ABC has been reported, virtually all cases represent either radiation induced sarcomas or TOS that were initially misdiagnosed as ABC.[11] Central giant cell granuloma and central giant cell tumor does not show large sinusoidal spaces. In the present case, features of high vascularity and mononuclear malignant cells were seen that are similar to many of the vascular tumors. These lesions were differentiated by presence of tumor osteoid and immunohistochemically, the neoplastic cells were negative for CD31 suggesting that the lesions were not arising from the endothelial cells.
Surgical treatment of resection with adequate margin is the accepted treatment for osteosarcoma. Improved results were claimed with adjuvant chemotherapy for OS of extremities.[6] However, radiotherapy and chemotherapy remain as unsatisfying modes of treatment as far as OS of jaws were concerned.[6,10] They are recommended for the patients, if the surgical margins of the lesion are questionable or positive.[10] It is well known that jaw OS of various histological types show local recurrences more frequently than distant metastasis.[6] In the study conducted by Matsuno on TOS, most patients had metastatic disease (92%) and died with an average of 10 months.[5] This outcome, according to the argument of Huvos might not signify the poorer prognosis of this tumor variant, because the patient had not been treated in the initial stages. However, none of the cases were considered in the jaw.[7] There was no metastasis of TOS reported in study as by Chan et al., though the size of the tumor was large with local invasion.[6] ++In the present case, the early diagnosis was made and complete tumor was excised. There was no evidence of recurrence with a seven years post-operative follow-up.
CONCLUSION
Jaw OS can arise as a primary non-radiation and non-syndrome associated OS. Jaw OS usually occur in later ages, when compared to other sites whereas TOS of jaw can occur in the early age as in the present case. Patient usually presents with symptoms of swelling of short duration. This case report suggests that OS in general including TOS (as in the present case) behave differently when they occur in the jaw bones, when compared to elsewhere in the body. These tumors tend to behave as low grade neoplasms and have a long-term survival. Chemotherapy and radiotherapy are recommended in doubt of complete tumor excision. Long term follow-up of patient is mandatory. Further studies and large samples are required for establishing the reasons for this distinct behavior, when it occurs in the jaw bones.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Ogunlewe MO, Ajayi OF, Adeyemo WL, Ladeinde AL, James O. Osteogenic sarcoma of the jaw bones: A single institution experience over a 21-year period. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:76–81. doi: 10.1016/j.tripleo.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 2.Bennet JH, Thomas G, Evans AW, Speight PM. Osteosarcoma of the jaws: A 30-year retrospective review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:323–32. doi: 10.1067/moe.2000.108274. [DOI] [PubMed] [Google Scholar]
- 3.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–43. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slootweg PJ, Muller H. Osteosarcoma of jaw bones. Analysis of 18 cases. J Maxillofac Surg. 1985;13:158–66. doi: 10.1016/s0301-0503(85)80040-0. [DOI] [PubMed] [Google Scholar]
- 5.Matsuno T, Unni KK, McLeod RA, Dahlin DC. Telangiectatic osteogenic sarcoma. Cancer. 1976;38:2538–47. doi: 10.1002/1097-0142(197612)38:6<2538::aid-cncr2820380643>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Chan CW, Kung TM, Ma L. Telangiectatic osteosarcoma of the mandible. Cancer. 1986;58:2110–5. doi: 10.1002/1097-0142(19861101)58:9<2110::aid-cncr2820580924>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 7.Huvo AG, Rosen G, Bretsky SS, Butler A. Telangiectaticosteogenic sarcoma: A Clinicopathologic study of 124 patients. Cancer. 1982;49:1679–89. doi: 10.1002/1097-0142(19820415)49:8<1679::aid-cncr2820490824>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Alves FA, Lopes MA, Ikeda MK, Kowalski LP, Almeida OP. Oral metastasis of Telangiectatic osteosarcoma. Oral Dis. 2003;9:104–6. doi: 10.1034/j.1601-0825.2003.00890.x. [DOI] [PubMed] [Google Scholar]
- 9.Gadwal SR, Gannon FH, Fanburg-Smith JC, Becoskie EM, Thompson LD. Primary osteosarcoma of the head and neck in pediatric patients: A clinicopathologic study of 22 cases with a review of the literature. Cancer. 2001;91:598–605. [PubMed] [Google Scholar]
- 10.Donaldson ME, Geist JR, Daley TD. Osteosarcoma of the jaws in children. Int J Peadiatr Dent. 2004;14:54–60. doi: 10.1111/j.1365-263x.2004.00519.x. [DOI] [PubMed] [Google Scholar]
- 11.Kyriakos M, Hardy D. Malignant transformation of aneurismal bone cyst, with an analysis of the literature. Cancer. 1991;68:1770–80. doi: 10.1002/1097-0142(19911015)68:8<1770::aid-cncr2820680821>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]


