Abstract
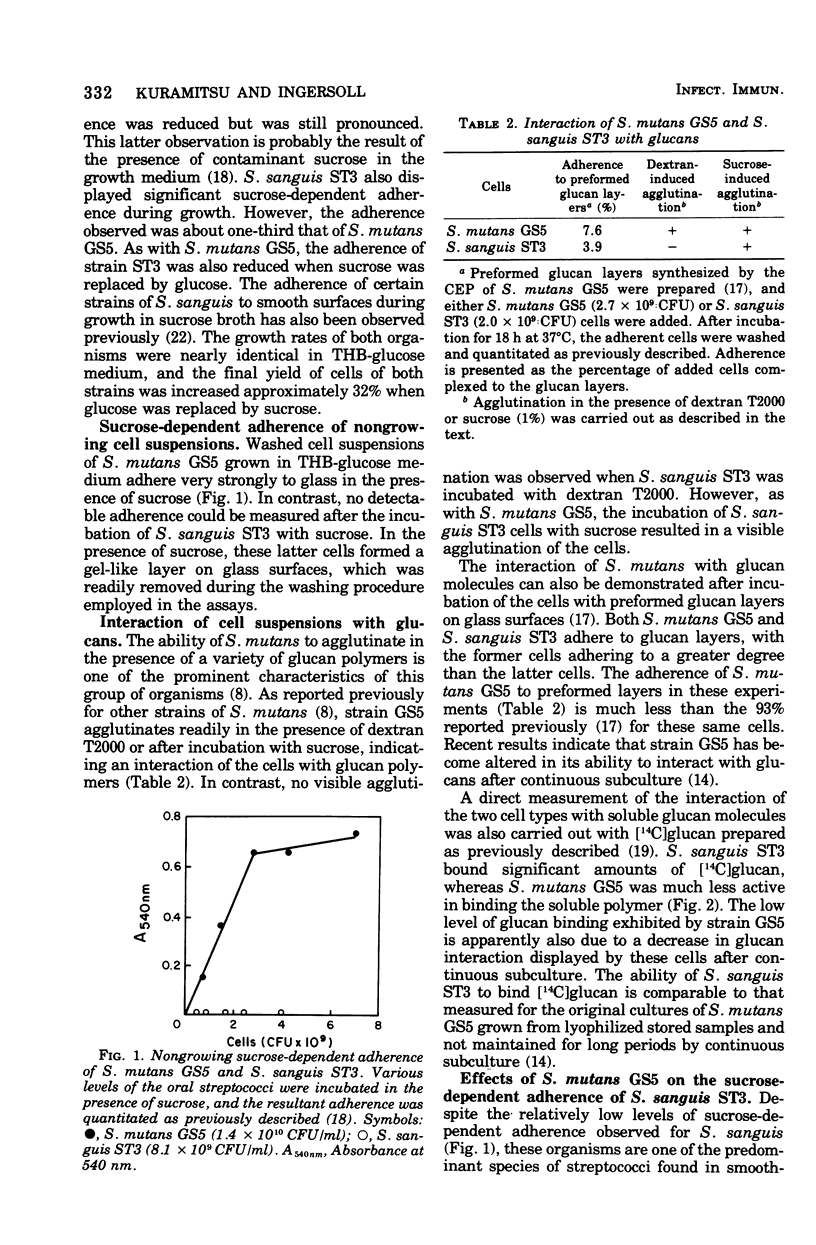
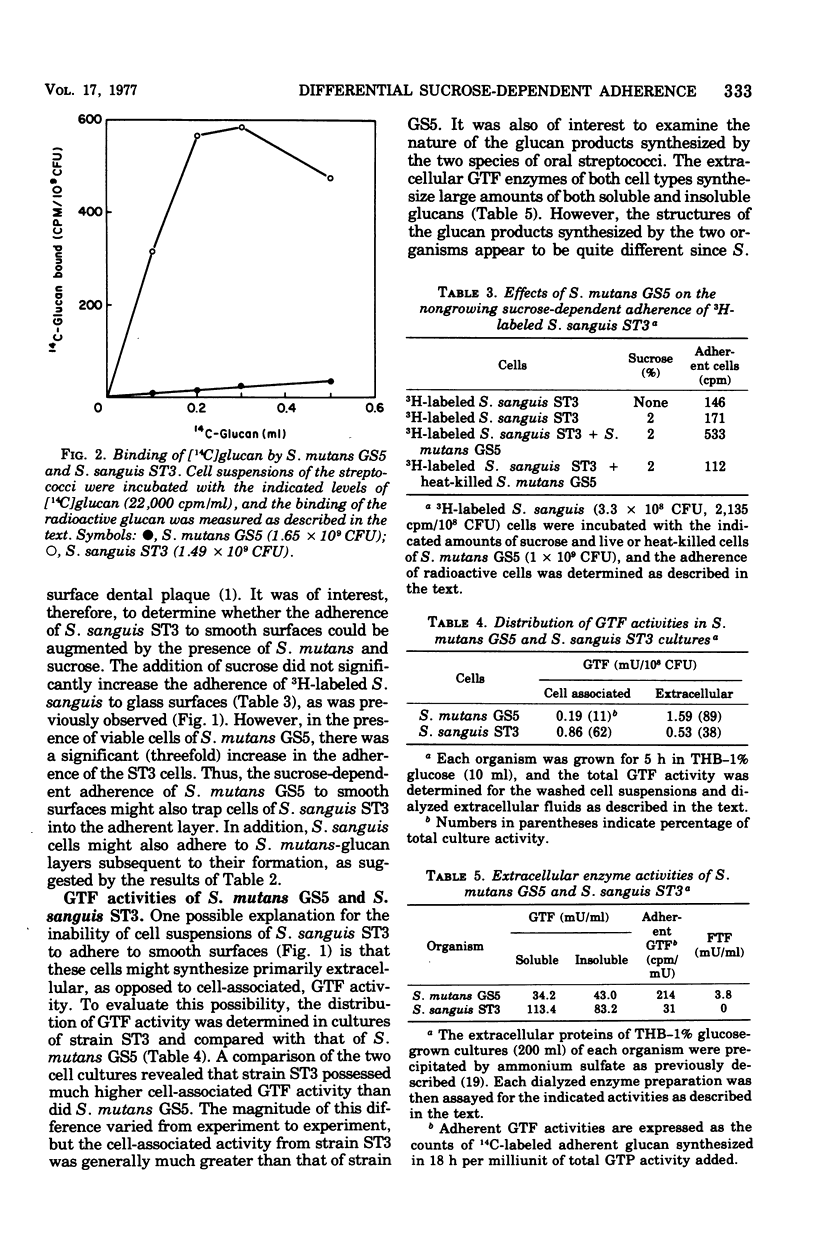
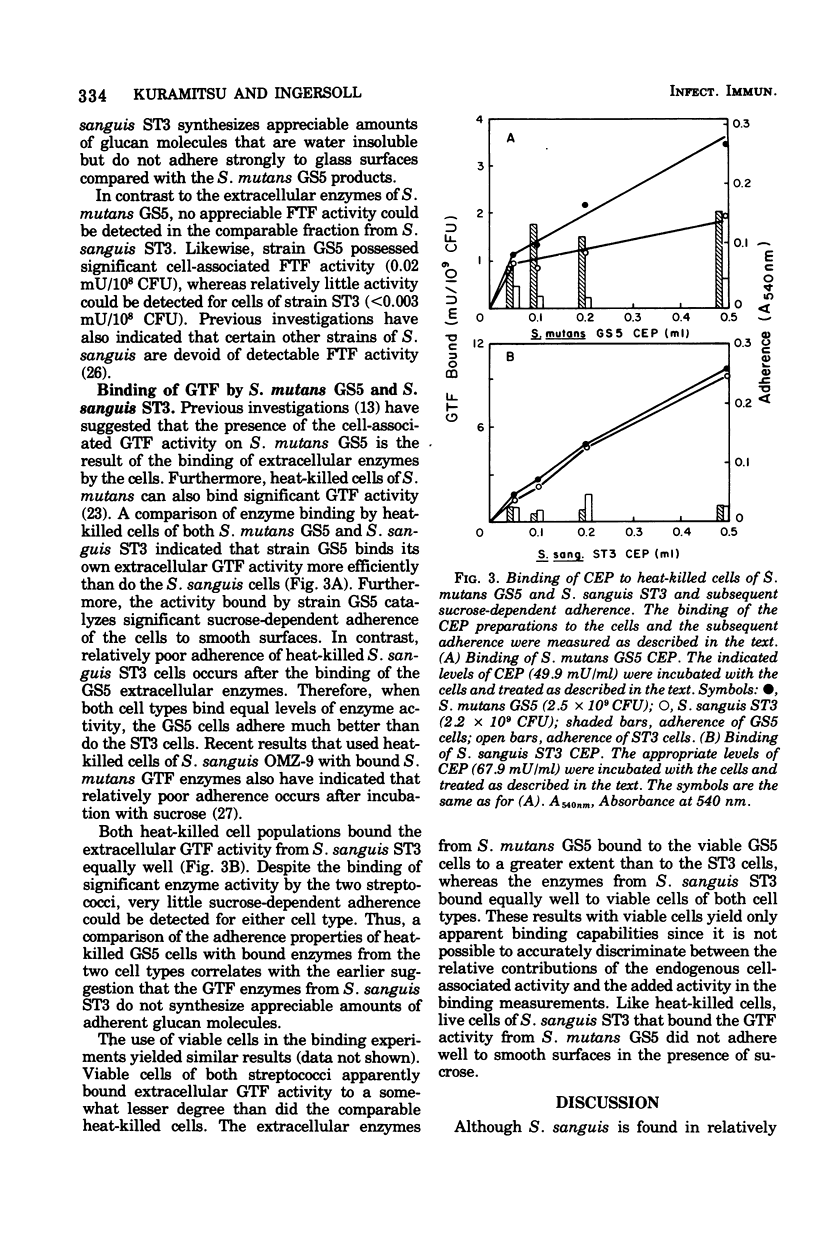
The enzymatic and adherence properties of Streptococcus mutans GS5 and S. sanguis ST3, both isolated from human carious lesions, have been compared. During growth in sucrose media, S. mutans GS5 adheres to smooth surfaces approximately three times more effectively than dose S. sanguis ST3. However, strain ST3 does not display sucrose-dependent adherence under nongrowth conditions, whereas strain GS5 displays significant adherence. Although both organisms synthesize both water-soluble and -insoluble glucans, the glucosyltransferases from S. mutans GS5 synthesize much more adherent glucan molecules than do the comparable enzymes from S. sanguis ST3. Both cell types bind exogenous glucosyltransferases synthesized by strain ST3 equally well, whereas cells of strain GS5 bind the comparable enzyme fraction that it synthesizes to a greater degree than do cell of S. sanguis ST3. However, in contrast to the results with cells of S. mutans GS5, the absorption of the glucosyltransferase activity synthesized by S. mutans GS5 to the surface of S. sanguis ST3 results in low levels of subsequent sucrose-dependent adherence. These results are discussed in terms of the molecular basis for the sucrose-dependent adherence of the oral streptococci to smooth surfaces.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlsson J., Newbrun E., Krasse B. Purification and properties of dextransucrase from Streptococcus sanguis. Arch Oral Biol. 1969 May;14(5):469–478. doi: 10.1016/0003-9969(69)90140-x. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Zooglea-forming streptococci, resembling Streptococcus sanguis, isolated from dental plaque in man. Odontol Revy. 1965;16(4):348–358. [PubMed] [Google Scholar]
- Ceska M., Granath K., Norrman B., Guggenheim B. Structural and enzymatic studies on glucans synthesized with glucosyltransferases of some strains of oral streptococci. Acta Chem Scand. 1972;26(6):2223–2230. doi: 10.3891/acta.chem.scand.26-2223. [DOI] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer DIRKS O. The effect of carbohydrate restriction on the presence of Streptococcus mutans, Streptococcus sanguis and iodophilic polysaccharide-producing bacteria in human dental plaque. Caries Res. 1970;4(2):114–123. doi: 10.1159/000259633. [DOI] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer Dirks O. The relationship between extracellular polysaccharide-producing streptococci and smooth surface caries in 13-year-old children. Caries Res. 1969;3(2):190–199. doi: 10.1159/000259582. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Berman K. S., Knoettner P., Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966 Jun;11(6):549–560. doi: 10.1016/0003-9969(66)90220-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Presence of an invertase-like enzyme and a sucrose permeation system in strains of Streptococcus mutans. Caries Res. 1972;6(2):122–131. doi: 10.1159/000259784. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Dental caries. Annu Rev Med. 1975;26:121–136. doi: 10.1146/annurev.me.26.020175.001005. [DOI] [PubMed] [Google Scholar]
- Guggenheim B. Enzymatic hydrolysis and structure of water-insoluble glucan produced by glucosyltransferases from a strain of streptococcus mutans. Helv Odontol Acta. 1970 Nov;14(Suppl):89+–89+. [PubMed] [Google Scholar]
- Ikeda T., Sandham H. J., Bradley E. L., Jr Changes in Streptococcus mutans and lactobacilli in plaque in relation to the initiation of dental caries in Negro children. Arch Oral Biol. 1973 Apr;18(4):555–566. doi: 10.1016/0003-9969(73)90076-9. [DOI] [PubMed] [Google Scholar]
- Janda W. M., Kuramitsu H. K. Properties of a variant of Streptococcus mutans altered in its ability to interact with glucans. Infect Immun. 1977 May;16(2):575–586. doi: 10.1128/iai.16.2.575-586.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda W. M., Kuramitsu H. K. Regulation and extracellular glucosyltransferase production and the relationship between extracellular and cell-associated activities in Streptococcus mutans. Infect Immun. 1976 Jul;14(1):191–202. doi: 10.1128/iai.14.1.191-202.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket S., Donaldson C. G. Saliva-induced aggregation of oral streptococci. J Bacteriol. 1972 Dec;112(3):1127–1133. doi: 10.1128/jb.112.3.1127-1133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Adherence of Streptococcus mutans to dextran synthesized in the presence of extracellular dextransucrase. Infect Immun. 1974 Apr;9(4):764–765. doi: 10.1128/iai.9.4.764-765.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of cell-associated dextransucrase activity from glucose-grown cells of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):227–235. doi: 10.1128/iai.10.1.227-235.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of invertase activity from cariogenic Streptococcus mutans. J Bacteriol. 1973 Sep;115(3):1003–1010. doi: 10.1128/jb.115.3.1003-1010.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Properties of a mutant of Streptococcus mutans altered in glucosyltransferase activity. Infect Immun. 1976 Feb;13(2):345–353. doi: 10.1128/iai.13.2.345-353.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H., Ingersoll L. Immunological relationships between glucosyltransferases from Streptococcus mutans serotypes. Infect Immun. 1976 Sep;14(3):636–644. doi: 10.1128/iai.14.3.636-644.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. H., Kleinman J. L. Effect of microbial interactions on in vitro plaque formation by Streptococcus mutans. J Dent Res. 1974 Mar-Apr;53(2):427–434. doi: 10.1177/00220345740530024201. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. II. Nature of the binding site and the adsorption of dextran-levan synthetase enzymes on the cell-wall surface of the streptococcus. Infect Immun. 1974 Feb;9(2):419–429. doi: 10.1128/iai.9.2.419-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson G. A., Bleiweis A. S., Small P. A., Jr Adherence inhibition of Streptococcus mutans: an assay reflecting a possible role of antibody in dental caries prophylaxis. Infect Immun. 1972 Apr;5(4):419–427. doi: 10.1128/iai.5.4.419-427.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M., Dhillon A. S., Newbrun E. Cell-bound glucosyltransferase activity of Streptococcus sanguis strain 804. Arch Oral Biol. 1974 Nov;19(11):1063–1072. doi: 10.1016/0003-9969(74)90096-x. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Upeslacis V. N. Studies of the mechanism of sucrose-associated colonization of Streptococcus mutans on teeth of conventional rats. J Dent Res. 1976 Mar-Apr;55(2):216–222. doi: 10.1177/00220345760550020901. [DOI] [PubMed] [Google Scholar]
- van Houte J., de Moor C. E., Jansen H. M. Synthesis of iodophilic polysaccharide by human oral streptococci. Arch Oral Biol. 1970 Mar;15(3):263–266. doi: 10.1016/0003-9969(70)90084-1. [DOI] [PubMed] [Google Scholar]



