Abstract
Background:
The commonly used clearing agent, xylene is supposed to be highly toxic and carcinogenic. As previous research studies have shown the effectiveness of different vegetable oils as clearants, this study was designed to evaluate the efficacy of coconut oil.
Materials and Methods:
Two equal halves of 60 soft tissue specimens were processed simultaneously in xylene and coconut oil as clearing agents. The Xylene-treated specimens (XY-S) and Coconut oil–treated specimens (CO-S) were checked for gross and histological features and comparison was done between the two groups.
Results:
Significant shrinkage was noted in XY-S compared to that in CO-S. No difference was found in either of the sections when checked for cellular details and staining quality. Morphometrically, there was significant reduction in the mean cell area in XY-S compared to that in CO-S.
Conclusion:
Coconut oil may be substituted for the highly hazardous xylene as a clearing agent without compromising the quality of histological details.
Keywords: Clearing agent, coconut oil, histopathology laboratory, vegetable oils
INTRODUCTION
Xylene (aromatic hydrocarbon) has been widely used as a de-alcoholization agent of choice, in spite of its toxicity to laboratory personnel and the danger it poses to the environment. The toxic effects of xylene include acute neurotoxicity, cardiac and kidney injury, cancer, blood dyscrasias, skin diseases, gastrointestinal disturbances, musculoskeletal system disorders, fetotoxicity and so on.[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18] On account of the Occupational Safety and Health Administration (OSHA) regulations, various xylene substitutes, such as, limonene reagents, aliphatic hydrocarbons, vegetable oils and mineral oils were tried in the past to avoid xylene in the laboratory.[11,16,19,20,21,22,23] However, these substitutes were found to be less effective and more expensive. Coconut oil is a commonly used vegetable oil, available throughout the tropical world. It is non-toxic, heat stable, slow to oxidize and has highest resistance to rancidity.[24] In the present study, we have tried to compare the efficacy of coconut oil with that of xylene, as a clearant, as it is readily available, less expensive and a safer alternative to xylene.
MATERIALS AND METHODS
Totally 60 tissue specimens were considered for this study. Each of the specimens was cut into two equal halves. The first half of the tissue bit was processed in xylene and the other half simultaneously in coconut oil [Figure 1]. The duration of clearing was constant for both the solutions (one hour each: Two changes). The tissue bits were measured before and after clearing, to check for shrinkage. After de-alcoholization, the specimens were also tested for gross changes after clearing. All the sections were stained with hematoxylin and eosin (H and E) to permit evaluation of the histological details. Few of the sections (salivary gland specimens) were subjected to periodic acid Schiff (PAS) also, in order to see whether coconut oil was interfering in this routinely used special staining procedure. The sophisticated technique of computer-assisted morphometry was performed, to observe the morphological features like the mean cell area, to see if any consistent change existed between the study groups.
Figure 1.

Each equal half of every tissue cleared in parallel solutions, either in xylene / coconut oil
Sample selection
Specimens for this study were selected from the anatomical structures in the head and neck region, such as, skin, buccal mucosa, salivary gland, tendon, muscle and lymph node. The inclusion criteria were as follows: Only soft tissue was considered for this study. The specimen size was 0.5 × 1 cm or greater and a thickness of 3-5 mm was taken for processing (for better penetration of the processing fluids). The tissue was then divided into equal halves: During clearing, one was processed in coconut oil and the other in xylene.
Evaluation
Gross tissue specimen: After clearing in two different solvents, the gross tissue features, such as, translucency (surface translucency when viewed for reflected light), rigidity (palpation with two fingers), change after impregnation (change in the rigidity because of infiltration of wax) and ease in section cutting, were noted down for each specimen separately, for CO-S and XY-S. Scoring was done while comparing the parameters for both the agents: The finding of CO-S that was inferior to XY-S was considered as score 0, similar to XY-S as score 1 and superior to XY-S as score 2 [Table 1]. The tissue bits were measured just after clearing, to compare the gross-shrinkage for the two solvents [Figure 2].
Table 1.
Comparison of gross features of CO-S with respect to XY-S
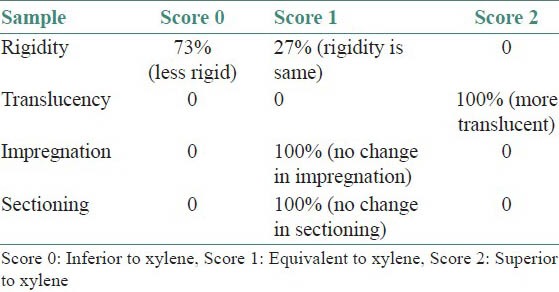
Figure 2.
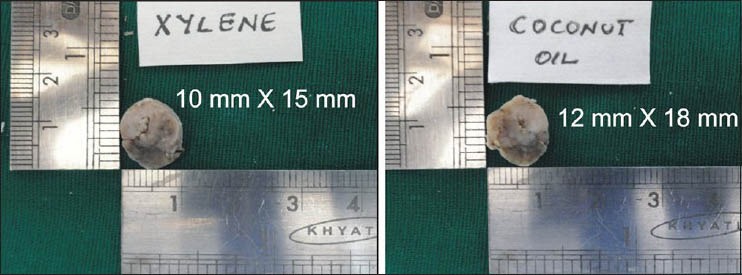
Measurements for gross tissue shrinkage
Cellular architecture: (a) For cellular details, distinct architecture and good nuclear-cytoplasmic contrast is considered as score 1 and indistinct/blurred nuclear-cytoplasmic contrast as score 0. (b) For nuclear details, distinct chromatin condensation, prominent nuclear membrane and crisp staining of the nucleus is considered as score 1 and indistinct smudging and pyknosis of the nuclei as score 0.
Quality of staining: The staining of tissues was evaluated as poor, satisfactory and good. Poor indicated that the tissue failed to take up the stain adequately, stained unevenly (score = 0). ‘Satisfactory’ pointed toward details like not visualized up to the mark (score = 1). ‘Good’ designated good contrast between the nucleus and cytoplasm and visibility of details, along with brilliance of staining (score = 2).
Morphometric analysis: After reviewing, the sections were further subjected to morphometric analysis. The images were captured using a three-chip CCD camera attached to a trinocular research microscope with a 100X objective. The final image captured on the monitor had a magnification of 1000X. For each specimen, five most representative fields were selected. The selected fields included representative cells where distinct cellular and nuclear outlines were seen, avoiding overlapping. A total of 100 cells (20 cells in five different high-power fields) were randomly selected and measured for any difference in the XY-S specimens and CO-S specimens. Histologically identifiable acini, adipocytes and epithelial cells in the para basal layer were subjected for measurement. The images were classified, transferred and stored in the computer. The actual measurements of the morphometric parameters were done using the image analyzer software Image-Proexpress (Media Cybernetics, Silver Spring, MD, USA). The cell area (CA) was measured in square microns when the perimeter was traced; the software automatically calculated the CA (number of pixels detected, converted to micrometers) [Figure 3].
Figure 3.
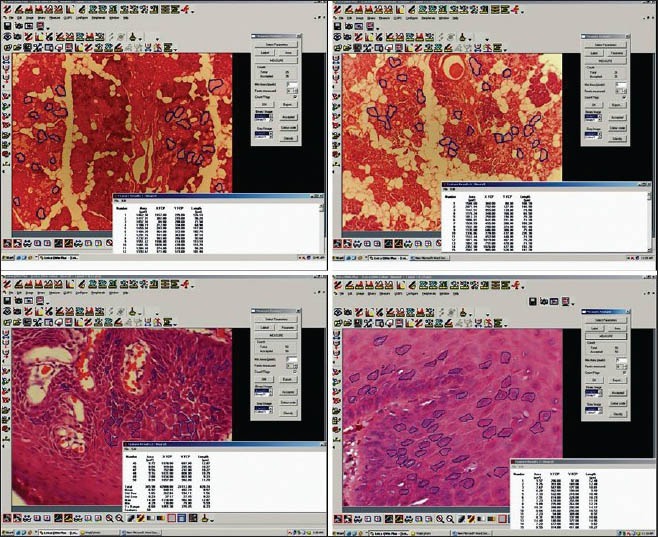
Morphometrical analysis of the mean area of an individual cell to assess shrinkage at the cellular level
As most of the evaluative criteria were subjective, the scoring and assessment was carried out by three different observers and the mean scoring was considered, which would prevent interobserver and intraobserver bias. The obtained data was subjected to statistical analysis using the Wilcoxon matched pair test and the Mann-Whitney U Test.
RESULTS
Most of the specimens (73%) were more rigid in XY-S when compared with CO-S. Although in 16 specimens, the rigidity was same in both the groups [Table 1]. Translucency was visibly better in all CO-S than XY-S [Table 1]. However, there was no difference observed in the tissue bits as far as rigidity after impregnation and ease of sectioning was concerned, in both the groups [Table 1]. There was no significant shrinkage in the tissue bits after clearing in coconut oil (P = 1.000). However, with respect to XY-S, the specimen shrank significantly, when compared with the measurements taken before clearing (P = 0.0117) [Table 2 and Figure 2]. There was no difference in staining quality and tissue architecture in both kinds of specimens [Table 3 and Figure 4 and 5A]. CO-S, when stained with Periodic acid-Schiff (PAS), showed similar details as seen in XY-S [Figure 5B]. Morphometrically, there was a significant decrease in the mean area of the individual cells in XY-S, compared to CO-S (P = 0.0006), [Table 4 and Figure 3].
Table 2.
Comparison of gross shrinkage after clearing
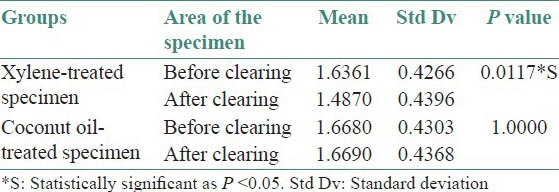
Table 3.
Comparison of staining quality
Figure 4.
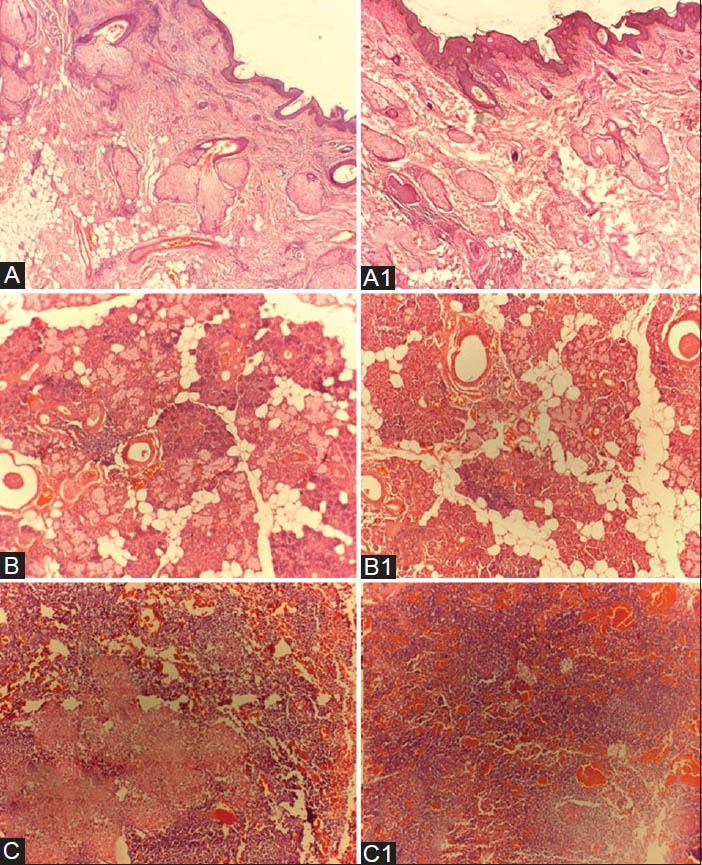
A,B,C — Hematoxylin and Eosin stained tissue sections of Xylene-treated specimen. A1, B1,C1 — Hematoxylin and Eosin stained tissue sections of Coconut oil–treated specimen. [A and A1 — skin tissue, B and B1 — salivary gland tissue, C and C1 — lymph node tissue]
Figure 5.
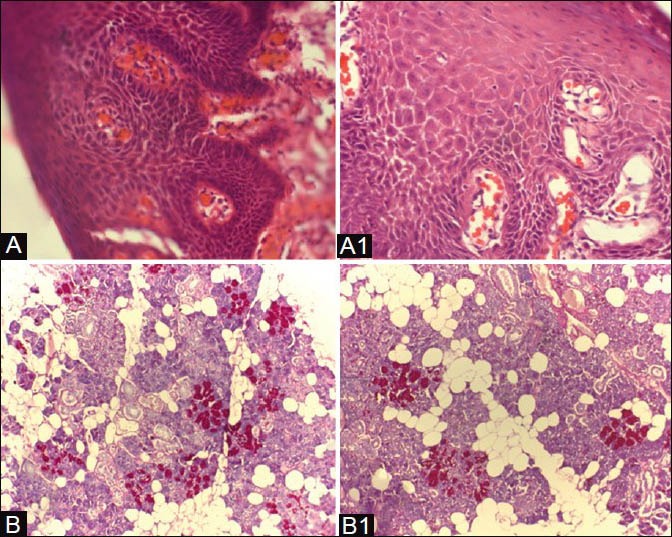
A, B — Xylene treated specimen. A1, B1 — Coconut oil– treated specimen. [A and A1 — oral epithelium; B and B1 — salivary gland stained with PAS]
Table 4.
Morphometric analysis of mean area of individual cell

DISCUSSION
Considering the toxicity of xylene and its hazards, various substitutes, including vegetable oils and mineral oils, have been tried in the past.[11,16,19,20,21,22,23] However, most of them showed an inconsistent outcome, which motivated us to take up this study. Coconut oil was selected, as it is, profusely available in the tropical world, especially in South Asia, it is less expensive and non-hazardous. When compared with xylene, it is not harmful to the environment.[24]
The results of the present study showed that CO-S, after clearing, was apparently more translucent compared to XY-S. Although less rigid in contrast to XY-S, it did not adversely affect impregnation and section cutting.
Morphometrically, the shrinkage was relatively less in CO-S when compared with XY-S, which was noted as a statistically significant difference in the mean cell area of individual cells between sections (P = 0.0006). However, there was no change in cellular, nuclear and cytoplasmic staining, when both groups were compared.
Buesa used a mixture of ethanol, isopropyl alcohol and mineral oil as an alternative for xylene and found the mixture to be as efficient as xylene in de-alcoholization.[19] Instead, we considered the environment-friendly, readily-available alternative, coconut oil, as we wanted to avoid chemicals such as ethanol and isopropyl alcohol, which were also hazardous. A mixture of coconut oil and olive oil was tried by Rasmussen et al. and they noted incomplete impregnation, leading to problems in the cutting sections and therefore, they concluded that this mixture was ineffective as a clearing agent.[20] In contrast to their observation, we found that CO-S, when used alone, was as effective as xylene, without interfering with further impregnation and cutting. This difference could be because of the olive oil in the mixture, which would have adversely affected the procedure, counteracting with the favorable properties of coconut oil. Instead, there was increased translucency and less rigidity.
A study by Andre et al.[23] substituted xylene with a mixture of peanut oil, soyabean oil, coconut oil and cotton oil and concluded that it was a poor alternative, as the quality of sections with respect to XY-S were better. The present study showed sections with similar cellular architecture and better staining quality. Even the special staining procedure showed good results, proving no interference by coconut oil with the tissue composition and it just acted as a transient media. As the result of our study showed less shrinkage in CO-S, compared to XY-S, we would suggest that this would be a preferred procedure, where morphometric studies have to be carried out. The only drawback associated with coconut oil, is its tendency to get solidified at a lower temperature. However, this can be overcome by performing the clearing procedure in an incubator, maintaining the required temperature. This research study is unique, as we have tried to assess the efficacy of two clearing agents at different stages of the histopathological procedure, such as, processing, impregnation, sectioning, staining and microscopic evaluation, including morphometry.
CONCLUSION
The results of the present study infer that coconut oil is an efficient substitute for xylene, as it is non-hazardous, less expensive and causes less shrinkage of the tissue. It can be used as a de-alcoholization agent in the histopathological laboratory, without losing the quality of the histological details. All the xylene-substitutes have to be analyzed thoroughly, before concluding which alternative is better. Further research in this area is expected, where the coconut oil–treated specimen can be subjected to all stains and advanced histological procedures (like immunohistochemistry), in order to consider this agent as a better and safer substitute for xylene.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Kandyala R, Raghavendra SP, Rajasekharan ST. Xylene: An overview of its health hazards and preventive measures. J Oral Max Pathol. 2010;14:1–5. doi: 10.4103/0973-029X.64299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atlanta: Georgia; 1993. Toxicological profile for xylene, U.S Department of Health and Human Services, public health service, Agency for toxic substance and disease registry. [Google Scholar]
- 3.Occupational safety and health administration-2005. Air contaminants, occupational safety and health administration. [Last accessed on 2013 Jan 01]. Available from: http://www.osha.gov .
- 4.Savoleinen H, Pfaffli P. Dose dependant neurochemical changes during short term inhalation exposure to xylene. Arch Toxicol. 1980;45:117–22. doi: 10.1007/BF01270909. [DOI] [PubMed] [Google Scholar]
- 5.Honma T, Sudo A, Miyagawa M, Sato M, Hasegawa H. Significant changes in the amounts of neurotransmitter and related substances in rat brain induced by subacute exposure to low levels of toluene and xylene. Ind Health. 1983;21:143–51. doi: 10.2486/indhealth.21.143. [DOI] [PubMed] [Google Scholar]
- 6.Anderson K, Fuxe K, Nilsen OG. Production of discrete changes in dopamine and noradrenaline levels and turnover in various parts of the rat brain following exposure to xylene. Toxicol Appl Pharmacol. 1981;60:535–48. doi: 10.1016/0041-008x(81)90340-9. [DOI] [PubMed] [Google Scholar]
- 7.Uchida Y, Nakatsuka H, Ukai H, Watanabe T, Liu YT, Huang MY. Symptoms and signs in workers exposed predominantly to xylene. Int Arch Occup Environ Health. 1993;64:597–605. doi: 10.1007/BF00517707. [DOI] [PubMed] [Google Scholar]
- 8.Nylen P, Ebendal T, Eriksdotter-Nilsson M, Hansson T, Henschen A, Johnson AC. Testicular atrophy and loss of nerve growth factor-immunoreactive germ cell line in rats exposed to xylene. Arch Toxicll. 1989;63:296–307. doi: 10.1007/BF00278643. [DOI] [PubMed] [Google Scholar]
- 9.Taskinen H, Anttila A, Lindbohm ML, Sallmen M, Hemminki K. Spontaneous abortions and congenital malformations among the wives of men occupationally exposed to organic solvents. Scand J Work Environ Health. 1989;15:345–5. doi: 10.5271/sjweh.1839. 2. [DOI] [PubMed] [Google Scholar]
- 10.Ankle MR, Joshi PS. A study to evaluate the efficacy of xylene-free hematoxylin and eosin staining procedure as compared to the conventional hematoxylin and eosin staining: An experimental study. J Oral Max Pathol. 2011;15:161–7. doi: 10.4103/0973-029X.84482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buesa RJ, Maxim VP. Histology without xylene. Ann Diagn Pathol. 2009;13:246–56. doi: 10.1016/j.anndiagpath.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Hipolito RN. Xylene poisoning in laboratory workers: Vase reports and discussion. Lab Med. 1980;11:593–5. [Google Scholar]
- 13.Revilla AS, Pestana CR, Pardo-Andreu GL, Santos AC, Uyemura SA, Gonzales ME, et al. Potential toxicity of toluene and xylene evoked by mitochondrial uncoupling. Toxicol in vitro. 2007;21:782–8. doi: 10.1016/j.tiv.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Faust RA. US: Department of Energy; 1994. Toxicity summary for xylene. Oak Ridge Reservation Environmental Restoration Program; p. 9. [Google Scholar]
- 15.Erickson T, Amed V, Leibach SJ, Bushnik P, Saxon A, Hryhorczuk DO, et al. Acute bone marrow toxicity and pancytopenia following exposure to lead chromate, xylene, and ethylbenzene in a degloving injury. Am J Hematol. 1994;47:257–61. doi: 10.1002/ajh.2830470402. [DOI] [PubMed] [Google Scholar]
- 16.Falkeholm L, Grant CA, Magnusson A, Möller E. Xylene-free method for histological preparation: A multicentre evaluation. Lab Invest. 2001;81:1213–21. doi: 10.1038/labinvest.3780335. [DOI] [PubMed] [Google Scholar]
- 17.Kurppa K, Tola S, Hernberg S, Tolonen M. Industrial Sovents and Liver. Lancet. 1983;1:129. [Google Scholar]
- 18.Magnusson G, Majeed SK, Down WH, Sacharin RM, Jorgeson RP. Hepatic effects of xylidine isomers in rats. Toxicology. 1979;12:63–74. doi: 10.1016/0300-483x(79)90033-7. [DOI] [PubMed] [Google Scholar]
- 19.Buesa RJ. Mineral oil: The best xylene substitute for tissue processing yet. J Histotechnol. 2000;23:143–9. [Google Scholar]
- 20.Rasmussen B, Hjort K, Mellerup I, Sether G, Christensen N. Vegetable oils instead of xylene in tissue processing. Acta Pathol Microbio Immunol Scandinavica. 1992;100:827–31. doi: 10.1111/j.1699-0463.1992.tb04006.x. [DOI] [PubMed] [Google Scholar]
- 21.Lyon H, Holm I, Prento P, Balslev E. Non-hazardous organic solvents in the paraffin embedding technique: A rational approach. Histochem Cell Biol. 1995;103:263–9. doi: 10.1007/BF01457410. [DOI] [PubMed] [Google Scholar]
- 22.Reinherdt PA, Leonard KL, Ashbrook PC. Pollution prevention and waste minimization in laboratories. Vol. 3. Florida: CRC press, Lewis Publiseers; 1996. Xylene substitutes; p. 346. [Google Scholar]
- 23.Andre GG, Wenger JB, Rebolloso D, Arrington JB, Mehm WJ. Evaluation of clearing and infiltration mixtures (CIMs) as xylene substitutes for tissue processing. J Histotechnol. 1994;17:137–42. [Google Scholar]
- 24.Fife B. Piccadilly books, Ltd. USA: Colorado Springs; 2005. Coconut Cures: Preventing and treating common health problems with coconut; pp. 184–5. [Google Scholar]


