Abstract
Ameloblastic carcinoma is a rare malignant odontogenic neoplasm that can arise either as a de novo lesion or from pre-existing ameloblastoma. Histopathologically, the tumor retains an ameloblastomatous differentiation pattern but shows cytological features of malignancy. Owing to variable biologic behavior and paucity of long-term follow-up cases, there has been no clear consensus on treatment protocol. The present case of ameloblastic carcinoma arose in the mandible of a 24-year-old male. Surgical treatment involved resection of the mandible along with regional lymph nodes. The patient has been on follow up for the past one year without any recurrence or metastases. An update on ameloblastic carcinoma encompassing the histogenesis, immunohistochemical features and treatment aspects are included.
Keywords: Ameloblastic carcinoma, odontogenic carcinoma, odontogenic tumors
INTRODUCTION
Intraosseous carcinomas of the jaw bones are rare occurrences and arise as a consequence of transformation of epithelial remnants of odontogenic or salivary origin. In addition, they can also occur as secondary deposits from primary tumors elsewhere in the body.[1]
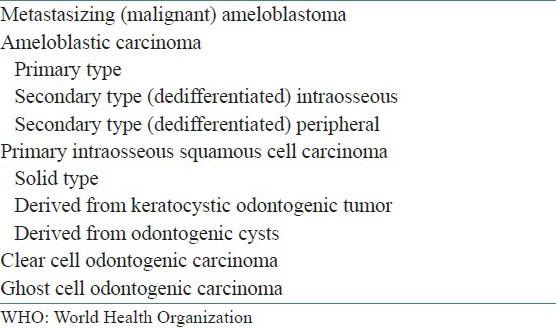
The rarity and the varied clinical course of odontogenic carcinomas have resulted in considerable confusion as evidenced by several changes in terminologies and classification over the years. Carcinomas derived from ameloblastomas have been designated by a variety of terms, including malignant ameloblastoma, ameloblastic carcinoma, metastatic ameloblastoma and primary intra-alveolar carcinoma.[2] The term “Malignant ameloblastoma” is used for ameloblastomas that metastasize without any histological features of malignancy in both the primary and the metastatic foci and the term “Ameloblastic carcinoma” for tumors with ameloblastomatous differentiation showing cytological features of malignancy with or without metastasis.[3] The World Health Organization (WHO) classifications of odontogenic tumors of 1972 and 1992 do not refer to the term ameloblastic carcinoma.[2] It was recognized as a separate entity by Elzay,[4] Slootweg and Miller,[3] Eversole,[5] Reichart and Philipsen,[2] and by the WHO in 2005 in its classification of odontogenic carcinomas [Table 1].[6]
Table 1.
World Health Organization (WHO; 2005) classification of odontogenic carcinomas
Ameloblastic carcinomas are locally aggressive lesions showing rapid growth with or without pain, paresthesia and anesthesia, trismus and dysphonia. Ameloblastic carcinomas have been reported with local recurrences and metastasis to sites like the lungs, brain, liver and bones.[1,7]
CASE REPORT
A 24-year-old male patient reported to our oral and maxillofacial surgical unit with a chief complaint of swelling in the lower jaw since 5 years. The swelling was slow growing and was not associated with any other symptoms like pain, anesthesia, or paresthesia. The patient presented with no significant medical or surgical history.
On extra-oral examination, the swelling was seen extending about 6 cm from the midline of the lower jaw bilaterally and about 5 cm superoinferiorly from the line joining the commissures to the lower border of the chin. It extended about 2 cm posteriorly from the chin. The margins were diffuse. The skin over the swelling appeared stretched, especially in the midline, with no other changes [Figure 1]. There was no local rise of temperature and the swelling was non-tender and hard in consistency. Lymph node examination revealed enlarged bilateral submandibular nodes which were non-tender, soft in consistency and unattached to the overlying skin or underlying structures.
Figure 1.
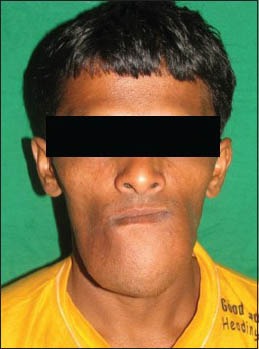
Clinical photograph showing extraoral swelling
Intraoral examination revealed a cauliflower-like sessile exophytic growth involving the lower alveolus and floor of the mouth extending from 36 to 45 with significant bicortical expansion about 5 cm in the anterior region. The surface appeared irregular and proliferative. The buccal vestibule was obliterated till the molar region on both sides. The borders were diffuse [Figure 2]. On palpation, the swelling was non-tender, the base was hard in consistency and the superior exophytic growth was soft to firm. Hard tissue examination revealed 41, 42, 31 and 33 to be displaced anteriorly, 34 was seen floating on the superior surface of the swelling. Tooth 32 was clinically missing.
Figure 2.
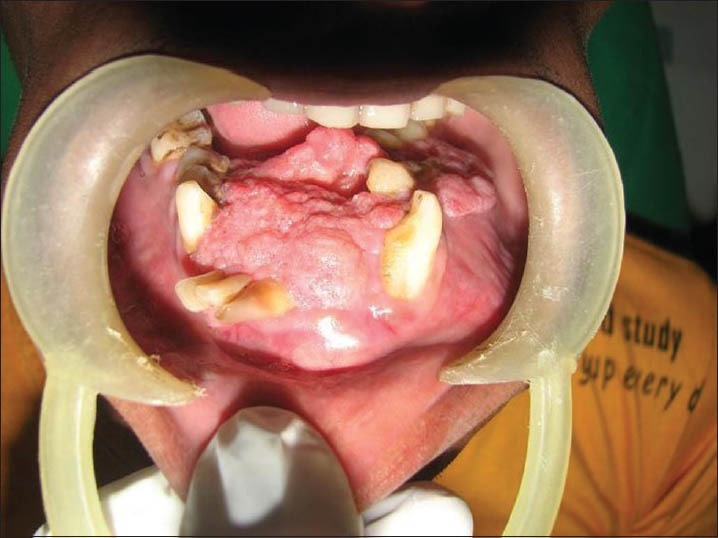
Clinical photograph showing intraoral swelling involving lower alveolar ridge and floor of mouth
Based on the above findings, a working diagnosis of ameloblastoma was considered. Differential diagnosis included both benign aggressive lesions and malignancies of the jaws. odontogenic myxoma, ameloblastic fibroma, ameloblastic carcinoma, primary intraosseous carcinoma and metastatic malignancy were considered.
The patient was subjected to radiographic investigations. The Orthopantomograph (OPG) revealed a multilocular radiolucency with scalloped borders extending from the distal aspect of the root of 37 to mesial aspect of the root of 48. The lower border of mandible showed expansion with thinning of inferior cortex. Multiple teeth exhibited teeth root resorption and loss of lamina dura. 34 and 35 appeared displaced superiorly [Figure 3]. Mandibular occlusal radiograph showed significant buccal and lingual cortical plate expansion with buccal cortex erosion in the left anterior region. 41, 42 and 31 were displaced anteriorly.
Figure 3.
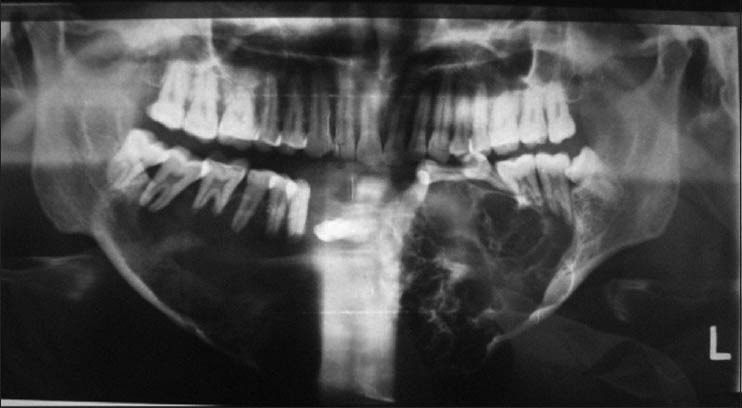
Orthopantomograph showing multilocular radiolucency extending to the third molar region bilaterally
A tentative radiographic diagnosis of ameloblastoma was considered based on the large multilocular appearance, significant buccolingual expansion and the changes seen in the surrounding structures. The incisional biopsy was obtained from the intraoral verrucous surface revealed odontogenic epithelium in the form of interconnecting strands and islands with peripheral bilayered columnar cells. The central regions of the strands were hypercellular with prominent squamous metaplasia and keratin pearl formation. Pleomorphism, hyperchromasia and mitosis, about 2 per high power field (HPF), were evident in few foci. Towards the surface, the epithelium showed severe pleomorphism and increased mitosis. Based on the histopathological features, a diagnosis of ameloblastic carcinoma was arrived upon. Chest radiographs were taken to rule out any metastatic lesion.
Surgical excision of the lesion by segmental resection of the mandible along with level I lymph node was planned. General anesthesia was secured using an endotracheal tube. An incision was placed from right to left angle of mandible and lip was split with a midline incision. The entire tumor was exposed with careful dissection. Mandible was sectioned using Giggly saw from right to the left angle region. Defect area was reconstructed using a double angled reconstruction plate and covered with pectoralis major myocutaneous flap.
Mandibular resection specimen with posterior extension upto the distal aspect of third molar bilaterally along with level I lymph nodes were received for histopathologic examination. Gross examination of the specimen revealed brownish white swelling with whitish cauliflower like exophytic growth in the anterosuperior surface. The gross specimen measured 10 × 8× 5 cm and was hard in consistency. Cortical perforation was noted on the lingual aspect of the left body of mandible. Cut surface revealed grayish-white solid lesion with three macrocystic spaces [Figure 4]. Tissues for histopathologic examination were taken from multiple areas including all the surfaces, area of perforation, solid and the cystic areas. The surgical margins were obtained for marginal clearance. Level I lymph nodes including sub-mental and right and left submandibular lymph nodes numbering ten that were soft to firm in consistency were harvested.
Figure 4.
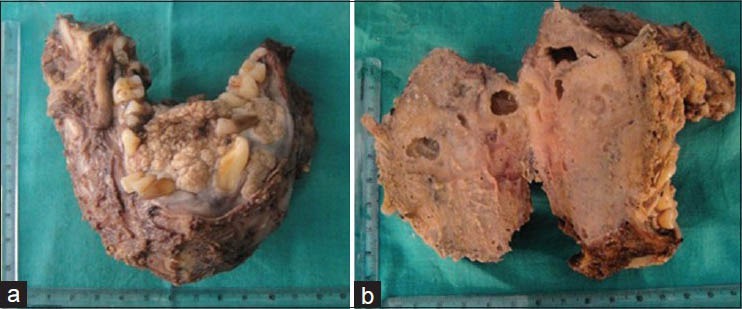
(a) Photograph showing the resected specimen.(b) Cut surface of the specimen showing macrocystic spaces
The findings of the excisional biopsy from the tumor proper were similar to the incisional biopsy. In addition, tissues from the area of perforation and the exophytic superior surface showed irregular papillary surfaced parakeratinized stratified squamous epithelium continuous with underlying interconnecting strands of odontogenic epithelium. The cells within the strands in these regions showed prominent pleomorphism, hyperchromasia, mitosis about 5-6/HPF, individual cell keratinization and keratin pearl formation [Figures 5–8].
Figure 5.
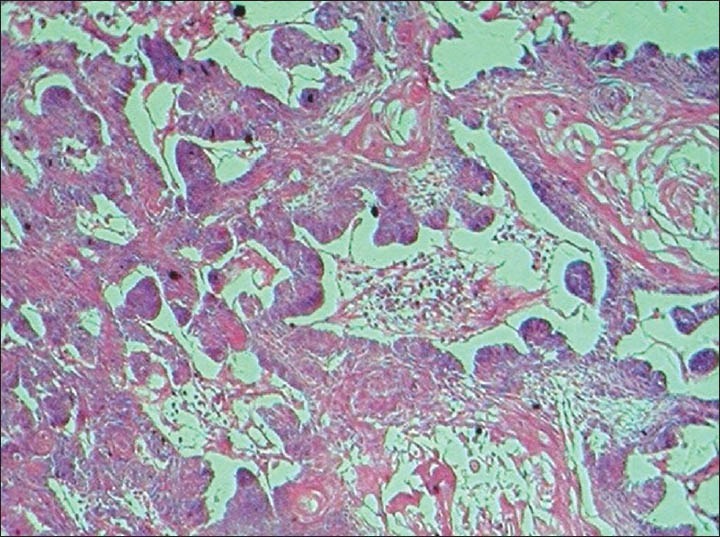
Photomicrograph showing interconnecting odontogenic epithelium with prominent hyperchromasia and keratin pearl formation. (H&E ×40)
Figure 8.
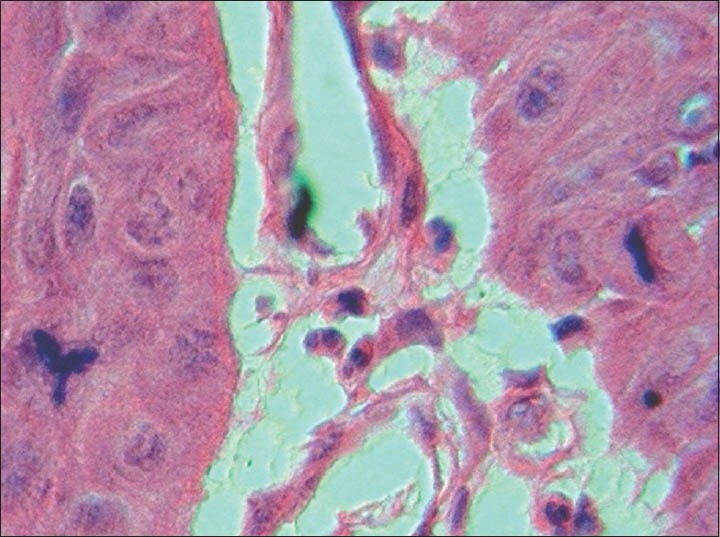
Photomicrograph showing increased and abnormal mitotic figures (H&E ×1000)
Figure 6.
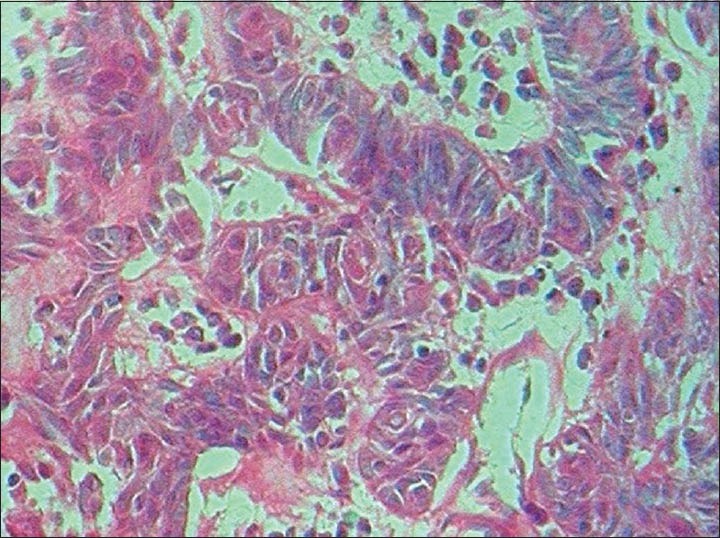
Photomicrograph depicting pleomorphism and hyperchromasia in the ameloblastomatous foci (H&E ×400)
Figure 7.
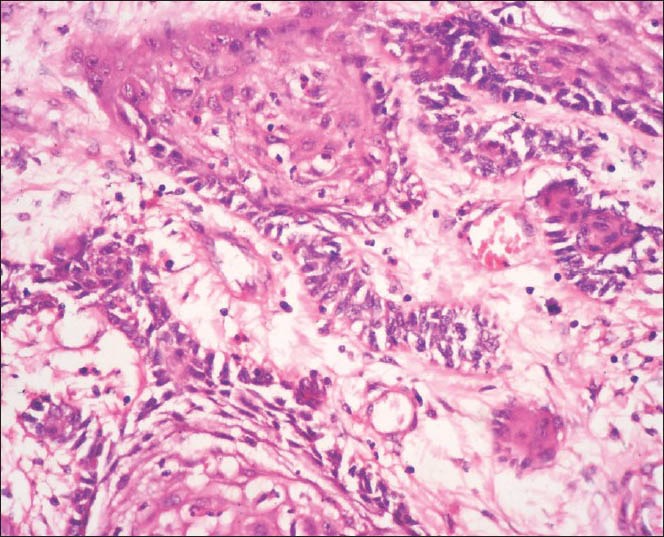
Photomicrograph depicting pleomorphism and nuclear atypia and mitotic activity in the ameloblastomatous foci (H&E ×400)
The surgical margins were negative for tumor infiltration and all the lymph nodes were negative for tumor metastasis and showed reactive hyperplasia.
REVIEW
Malignant odontogenic tumors constitute a small proportion of all odontogenic tumors. One of the earliest comprehensive reviews on ameloblastic carcinomas was performed by Slootweg and Muller in 1984, who reviewed 42 cases and reported two of their own.[3] In a review of 319 odontogenic tumors in Nigerian hospital by Ladiende et al.,[8] malignant tumors constituted only 3.4%. In an analysis of 1,642 cases of odontogenic tumors in the Chinese population, 4.7% cases were malignant tumors, of which ameloblastic carcinomas constituted 1.6%.[9] The incidence of ameloblastic carcinoma is highest in Africa followed by China and with lesser number of cases in Europe and the America's.[9] Retrospective analysis of ameloblastic carcinoma in the English literature was performed on PubMed and a total of 138 cases including the present case were considered for review.[7,8,9,10,11,12,13,14,15,16,17,18,19,20] Only cases with adequate clinicopathological information were included. Data were computed using a personal computer (PC) and Statistical Package for the Social Sciences (SPSS) 10.0 software program.
A total of 91 cases occurred in the males and 47 in females with a male:female ratio of 1.9:1. Age of presentation ranged from 4 to 91 years with most cases occurring in the 5th and 7th decades followed by the 3rd decade. Spindle cell variants occur in age range of 20-75 years with a male:female ratio of 5:3.[19] The present case is on the relatively younger side at 24 years.
The tumor occurred in the mandible with 90 cases followed by 45 cases in the maxilla with a ratio of 2:1. In both the maxilla and the mandible, the posterior regions were favored than the anterior regions. Data available from 100 cases regarding age of occurrence in the jaws revealed an average age of 42.9 for the mandible and a higher average age of 50 for the maxilla. Cases have been reported to involve the anterior skull base, maxillary sinus, gingiva and the temporomandibular joint.[7,14,20] Swelling and pain are most commonly seen followed by ulceration, rapid growth and trismus.[7] Other features seen in ameloblastic carcinoma include teeth mobility asymmetry, cortical plate perforation and paresthesia. A total of 80 cases including the present case had a history of follow-up, of which the 27 cases that exhibited metastasis, 19 arose from the mandible and 8 from the maxilla. Metastases were to the lungs (8 cases), lymph node (5 cases), bone (4 cases), brain (2 cases) and multiple bone involvement (8 cases). Metastases can occur in a range of 4-47 months postoperatively.[7]
Radiographically, it is similar to solid/multicystic ameloblastoma and appears usually as an ill-defined radiolucent lesion, either unilocular or multilocular. Slight marginal scleroses without periosteal new bone formation, loss of lamina dura, resorption of the tooth apex and tooth displacement are also noted. Foci of radiopacities representative of dystrophic calcification may be present. Advanced diagnostic imaging methods like the computed tomography (CT) and magnetic resonance imaging (MRI) have been used and demonstrate extraosteal extension, perineural invasion and cortical and root resorption. Pre and postoperative positron emission tomography (PET)-CT and fludeoxyglucose (FDG)-PET have been suggested for staging, diagnosis of metastasis and treatment surveillance.[21]
Ameloblastic carcinoma metastasises most commonly to the lungs.[22] Other sites for distant metastasis include the lymph node, bone and brain. Multiple sites too can be involved.[1] Evaluation of these regions is mandatory to rule out metastasis if any.
A basic subtype of ameloblastoma, most commonly the follicular type is usually evident.[7] The central regions, unlike in ameloblastoma, is less orderly, being condensed and hypercellular with stellate reticulum-like structures being absent in most cases.[7] A spindled cell variant has also been described wherein the central cells are spindle shaped simulating a low grade sarcoma.[23] Hallmarks of malignancy seen in ameloblastic carcinoma included nuclear pleomorphism, hyperchromatism, abnormal nuclear-cytoplasmic ratio, atypical mitosis and an increase in the number of mitosis. The number of mitosis can range from 2-8/High-Power Field (HPF). Other features seen reflecting the malignant process are tumor necrosis, vascular and neural invasion. Ghost cells, clear cells, calcifications, individual cell keratinisation, keratin pearl formation and melanin have also been reported in ameloblastic carcinomas. The stroma is usually fibrous, inflamed with areas of hemorrhage and hemosiderin.[2] In our case, cellular and nuclear pleomorphisms were increased and atypical mitosis was evident. In the fine needle aspiration cytology (FNAC), presence of stellate cells, palisaded basaloid cells, along with cellular and nuclear atypia are indicative ameloblastic carcinoma.[24]
Malignancies of odontogenic origin arise from dental embryonic residues, either involving dental epithelium-odontogenic carcinomas or dental connective tissue-odontogenic sarcomas and occur in the jaws or gingiva.[23] Fujita et al. reported a case of peripheral ameloblastic carcinoma in a 71-year-old male and considered it to the product of proliferation and transformation basal cells of gingival epithelium.[20]
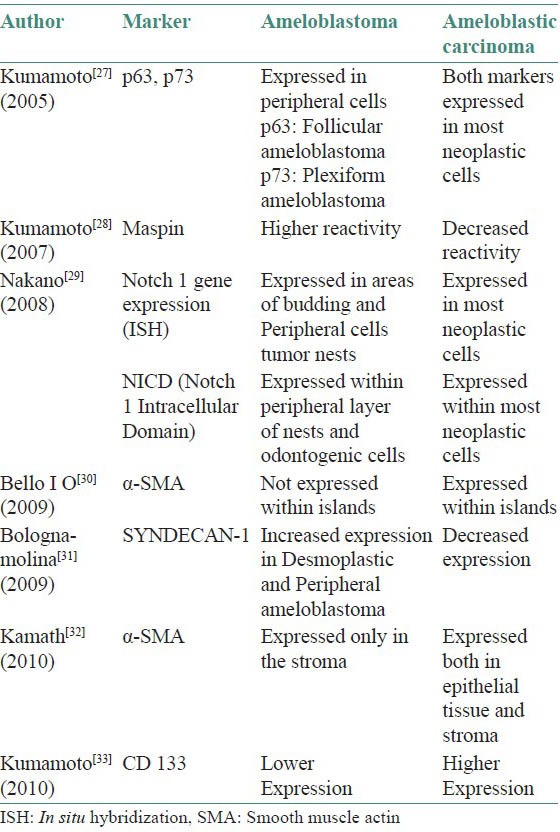
Most ameloblastic carcinomas occur de novo as lesions with histologic features of ameloblastomas with less-differentiated areas, however they can also arise from pre-existing ameloblastomas with subsequent de-differentiation secondary to surgical and ionising therapies.[22] Such cases may be distinguished by presence of transitional areas of malignancy adjoining benign ameloblastomatous areas.[3] A case has also been reported arising from an unicystic ameloblastoma.[19] Malignant odontogenic tumors show up-regulation of genes coronin, MYD 88 (Myeloid Differentiation Primary Response) and downregulation of STK19 (Serine/Threonine Kinase), RFP (Red Fluorescent Protein) and TXK (Tyrosine Kinase).[25] Nodit L[26] et al. reported that the rate of allelic loss in a study involving chromosomes 1p, 3p, 9p, 10q and 17p in ameloblastic carcinomas are similar to those in benign tumors indicating that different genetic mechanisms may be responsible in malignant behavior. Immunohistochemical studies have indicated differential expression of markers between ameloblastoma and ameloblastic carcinoma [Table 2].[27,28,29,30,31,32,33]
Table 2.
Immunohistochemical profiles of ameloblastoma and ameloblastic carcinoma
Fewer apoptotic reactions have been found to occur in malignant ameloblastic tumors as compared to ameloblastomas. Mitochondrial apoptosis signalling molecules AIF (Apotosis Inducing Factor), which has been implicated in lung carcinoma, has been considered to play a role in malignant transformation in ameloblastomas.[34,35] Changes in the BH3-only proteins, which are responsible for apoptosis in odontogenic tissues can lead to formation of tumors.[36] Malignant transformation in ameloblastic tumors may also be due to altered PD-ECGF TP (platelet-derived endothelial cell growth factor/thymidine phosphorylase) expression.[37] Abiko Y et al. concluded that hyper methylation of p16 gene was involved in the malignant transformation of pre-existing benign ameloblastoma to an ameloblastic carcinoma.[11]
Increased cellular proliferation is seen in ameloblastic carcinomas. Ameloblastic carcinomas also have revealed strong positivity for Bcl-2, cytokeratin's 5, 14, 18 and negativity for cytokeratin 7.[7] Strong expression of CAM 5, 6, AE1 and AE3 and Ki-67/MIB-1 has also been seen in ameloblastic carcinoma.[13,14]
Histopathological differential diagnosis includes primary intraosseous carcinoma, basaloid squamous cell carcinoma, mucoepidermoid carcinoma, acanthomatous ameloblastoma, keratoameloblastoma, calcifying epithelial odontogenic tumor, squamous odontogenic tumor and carcinomas and tumors metastasising to the jaws from sites such as the lung, breast or the Gastrointestinal tract.[7]
With a common progenitor in the odontogenic remnants, primary intraosseous carcinoma (PIOC) has been considered to be a closely related, less differentiated, usually non-keratinising form of ameloblastic carcinoma. Giving credence to the squamous differentiation and lack of ameloblastomatous components, the WHO in 2005 separated PIOC from ameloblastic carcinoma and renamed it primary intraosseous squamous cell carcinoma (PIOSCC).[6]
Squamous cell carcinoma arising in odontogenic cysts can also pose diagnostic difficulties, but histologically resembles oral squamous cell carcinoma more than ameloblastic carcinoma.
Basaloid squamous cell carcinoma (BSC) histopathologically has features similar to ameloblastic carcinoma, including tumor cells with peripheral palisading, central lobular necrosis and microcystic spaces with deposition of basement membrane material. PAS positivity in the microcystic spaces of BSC help differentiate it from ameloblastic carcinoma among other tumors.[7]
Clear cell differentiation in ameloblastic carcinoma can mimic a mucoepidermoid carcinoma.[1] The low as well as high grade mucoepidermoid carcinomas have a destructive growth pattern and may exhibit metastasis. The squamous differentiation and mucicarmine positivity help to differentiate the mucoepidermoid carcinoma from the ameloblastic carcinoma.[23]
Though the presence of ameloblastic structures and keratinisation in acanthomatous ameloblastoma and keratoameloblastomas is similar to ameloblastic carcinoma, these lesions however do not present with the cytologic features of malignancy that is characteristic of ameloblastic carcinoma.
Squamous odontogenic tumor must be considered in the differential diagnosis due to similarities with ameloblastic carcinoma. The features seen in both include tumor islands with lack of stellate reticulum and peripheral palisading, occasional presence of microcystic spaces and calcification. However, squamous odontogenic tumor lacks cytological features of malignancy.
A differential diagnosis for spindle cell ameloblastic carcinomas should include sarcomas, odontogenic sarcoma and ameloblastic carcinosarcoma. Spindle cell areas, in the spindle cell variant are negative for vimentin, desmin, actin, factor VIII and positive for cytokeratin.[19]
In view of the aggressive clinical behavior and local recurrences wide surgical resection with 2-3 cm of bony margins is the treatment of choice. Recurrence rates can be as high as 92.3% when conservative line of treatment is instituted.[7] Resection in cases occurring in the maxilla may be complicated due to rapid growth and extension into sinuses, orbit and other vital areas nearby.[17] An exhaustive assessment of the resected specimen is indicated to rule out ameloblastic carcinoma arising in a prexisting ameloblastoma. Clear cell ameloblastic carcinomas have higher recurrence rates (62.5%) than nonclear cell groups (33%) and therefore require aggressive treatment protocols.[1] Unlike squamous cell carcinoma, hematogenic spread is seen with ameloblastic carcinoma.[13] However, assessment of lymph node metastasis is mandatory and neck dissection indicated for both prophylactic and therapeutic considerations.
While surgical resection remains the mainstay, radiotherapy and chemotherapy have been utilised in different treatment protocols, as an adjuvant, as a method for tumor size reduction prior to surgery and also for tumors that are too advanced and not conducive for surgery. The use of radiotherapy in a primary role may not be useful as reported by Hall et al.[1] in an assessment of 14 cases, which all went into recurrence. Recurrence rates decline with surgical treatment than with use of chemotherapy and radiotherapy alone.[7] Extensive lesions that require mutilating surgery, or in patient unfit or unwilling for surgery and in cases of relapse, carbon ion therapy may be considered.[38] Post-operative reconstruction may need to be delayed to account for the extreme rapid rates of recurrence, which in cases may be upto two years. Maxillary ameloblastic carcinomas have poorer prognosis as do tumors in which both primary and metastatic foci are dedifferentiated. The present case has had a follow up for the past one year and no recurrences have been reported. Periodic reassessment with a follow-up period of at least 10 years is mandatory.[2,7,13]
CONCLUSION
Due to the paucity of cases of ameloblastic carcinoma, there is a need for reporting and long term evaluation of cases with detailed clinico-pathological correlation to differentiate it from other more commonly occurring tumors, especially ameloblastoma and to formulate treatment protocols. An exhaustive histopathologic examination of resected ameloblastomas is mandatory to rule out the presence any malignant transformation. Spindle cell variants have a more aggressive biologic behavior.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Hall JM, Weathers DR, Unni KK. Ameloblastic carcinoma: An analysis of 14 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:799–807. doi: 10.1016/j.tripleo.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 2.Reichart PA, Philipsen HP. Hanover, Germany: 2004. Odontogenic Tumors and Allied Lesions. [Google Scholar]
- 3.Slootweg PJ, Muller H. Malignant ameloblastoma or ameloblastic carcinoma. Oral Surg Oral Med Oral Pathol. 1984;57:168–76. doi: 10.1016/0030-4220(84)90207-x. [DOI] [PubMed] [Google Scholar]
- 4.Elzay RP. Primary intraosseous carcinoma of the jaws. Review and update of odontogenic carcinomas. Oral Surg Oral Med Oral Pathol. 1982;54:299–303. doi: 10.1016/0030-4220(82)90099-8. [DOI] [PubMed] [Google Scholar]
- 5.Eversole LR. Malignant epithelial odontogenic tumors. Semin Diagn Pathol. 1999;16:317–24. [PubMed] [Google Scholar]
- 6.Barnes L, Eveson J, Reichart P, Sidransky D. Lyon: IARC Press; 2005. World Health Organization classification of tumours; pathology and genetics of head and neck tumors. [Google Scholar]
- 7.Yoon HJ, Hong SP, Lee JI, Lee SS, Hong SD. Ameloblastic carcinoma: An analysis of 6 cases with review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:904–13. doi: 10.1016/j.tripleo.2009.06.045. [DOI] [PubMed] [Google Scholar]
- 8.Ladeinde AL, Ajayi OF, Ogunlewe MO, Adeyemo WL, Arotiba GT, Bamgbose BO, et al. Odontogenic tumors: A review of 319 cases in a Nigerian teaching hospital. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:191–5. doi: 10.1016/j.tripleo.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Jing W, Xuan M, Lin Y, Wu L, Liu L, Zheng X, et al. Odontogenic tumors: A retrospective study of 1642 cases in a Chinese population. Int J Oral Maxillofac Surg. 2007;36:20–5. doi: 10.1016/j.ijom.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Kawauchi S, Hayatsu Y, Takahashi M, Furuya T, Oga A, Niwa S, et al. Spindle-cell ameloblastic carcinoma: A case report with immunohistochemical, ultrastructural, and comparative genomic hybridization analyses. Oncol Rep. 2003;10:31–4. [PubMed] [Google Scholar]
- 11.Abiko Y, Nagayasu H, Takeshima M, Yamazaki M, Nishimura M, Kusano K, et al. Ameloblastic carcinoma ex ameloblastoma: Report of a case-possible involvement of CpG island hypermethylation of the p16 gene in malignant transformation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:72–6. doi: 10.1016/j.tripleo.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Naik V, Kale AD. Ameloblastic carcinoma: A case report. Quintessence Int. 2007;38:873–9. [PubMed] [Google Scholar]
- 13.Benlyazid A, Lacroix- Triki M, Aziza R, Gomez- Brouchet A, Guichard M, Sarini J. Ameloblastic carcinoma of the maxilla: Case report and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:e17–24. doi: 10.1016/j.tripleo.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Angiero F, Borloni R, Macchi M, Stefani M. Ameloblastic carcinoma of the maxillary sinus. Anticancer Res. 2008;28(6B):3847–54. [PubMed] [Google Scholar]
- 15.Praveen KB, Vaishali K, Anjana BS, Arvind S, Raghavendra B. Ameloblastic carcinoma. JIAOMR. 2009;21:114–8. [Google Scholar]
- 16.Ismail SB, Zain RB, Yaacob HB, Abraham MT. Ameloblastic carcinoma (spindle cell variant) Pathology. 2009;41:292–5. doi: 10.1080/00313020902756345. [DOI] [PubMed] [Google Scholar]
- 17.Lucca M, D’Innocenzo R, Kraus JA, Gagari E, Hall J, Shastri K. Ameloblastic carcinoma of the maxilla: A report of 2 cases. J Oral Maxillofac Surg. 2010;68):2564–9. doi: 10.1016/j.joms.2009.09.088. [DOI] [PubMed] [Google Scholar]
- 18.Karakida K, Aoki T, Sakamoto H, Takahashi M, Akamatsu T, Ogura G, et al. Ameloblastic carcinoma, secondary type: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:e33–7. doi: 10.1016/j.tripleo.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Jindal C, Palaskar S, Kaur H, Shankari M. Low-grade spindle cell ameloblastic carcinoma: Report of an unusual case with immunohistochemical findings and review of the literature. Curr Oncol. 2010;17:52–7. doi: 10.3747/co.v17i5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujita S, Anami M, Satoh N, Yamashita H, Asahin I, Ikeda T, et al. Cytopathologic features of secondary peripheral ameloblastic carcinoma: A case report. Diagn Cytopathol. 2011;39:354–8. doi: 10.1002/dc.21427. [DOI] [PubMed] [Google Scholar]
- 21.Devenney-Cakir B, Dunfee B, Subramaniam R, Sundararajan D, Mehra P, Spiegel J, et al. Ameloblastic carcinoma of the mandible with metastasis to the skull and lung: Advanced imaging appearance including computed tomography, magnetic resonance imaging and positron emission tomography computed tomography. Dentomaxillofac Radiol. 2010;39:449–53. doi: 10.1259/dmfr/29356719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corio LR, Goldblatt LI, Edwards PA, Hartman KS. Ameloblastic carcinoma: A clinicopathologic study and assessment of eight cases. Oral Surg Oral Med Oral Pathol. 1987;64:570–6. doi: 10.1016/0030-4220(87)90063-6. [DOI] [PubMed] [Google Scholar]
- 23.Slater LJ. Odontogenic malignancies. Oral Maxillofacial Surg Clin N Am. 2004;16:409–24. doi: 10.1016/j.coms.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Nai GA, Grosso RA. Fine-needle aspiration biopsy of ameloblastic carcinoma of the mandible: A case report. Braz Dent J. 2011;22:254–7. doi: 10.1590/s0103-64402011000300013. [DOI] [PubMed] [Google Scholar]
- 25.Carinci F, Palmieri A, Delaiti G, Rubini C, Fioroni M, Martinelli M, et al. Expression profiling of ameloblastic carcinoma. J Craniofac Surg. 2004;15:264–9. doi: 10.1097/00001665-200403000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Nodit L, Barnes L, Childers E, Finkelstein S, Swalsky P, Hunt J. Allelic loss of tumor suppressor genes in ameloblastic tumors. Mod Pathol. 2004;17:1062–7. doi: 10.1038/modpathol.3800147. [DOI] [PubMed] [Google Scholar]
- 27.Kumamoto H, Ohki K, Ooya K. Expression of p63 and p73 in ameloblastomas. J Oral Pathol Med. 2005;34:220–6. doi: 10.1111/j.1600-0714.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 28.Kumamoto H. Ooya Immunohistochemical detection of uPA, uPAR, PAI-1, and maspin in ameloblastic tumors. J Oral Pathol Med. 2007;36:488–94. doi: 10.1111/j.1600-0714.2007.00554.x. [DOI] [PubMed] [Google Scholar]
- 29.Nakano K, Siar CH, Tsujigiwa H, Nagatsuka H, Nagai N, Kawakami T. Notch signalling in benign and malignant ameloblastic neoplasms. Eur J Med Res. 2008;13:476–80. [PubMed] [Google Scholar]
- 30.Bello IO, Alanen K, Slootweg PJ, Salo T. Alpha-smooth muscle actin within epithelial islands is predictive of ameloblastic carcinoma. Oral Oncol. 2009;45:760–5. doi: 10.1016/j.oraloncology.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Bologna-Molina R, Mosqueda-Taylor A, Lopez-Corella E, de Almeida OP, Carrasco-Daza D, Farfán-Morales JE, et al. Comparative expression of syndecan-1 and Ki-67 in peripheral and desmoplastic ameloblastomas and ameloblastic carcinoma. Pathol Int. 2009;59:229–33. doi: 10.1111/j.1440-1827.2009.02355.x. [DOI] [PubMed] [Google Scholar]
- 32.Kamath KP, Vidya M, Shetty N, Karkera BV, Jogi H. Nucleolar organizing regions and alpha-smooth muscle actin expression in a case of ameloblastic carcinoma. Head Neck Pathol. 2010;4:157–62. doi: 10.1007/s12105-010-0173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumamoto H, Ohki K. Detection of CD133, Bmi-1, and ABCG2 in ameloblastic tumors. J Oral Pathol Med. 2010;39:87–93. doi: 10.1111/j.1600-0714.2009.00807.x. [DOI] [PubMed] [Google Scholar]
- 34.Joseph B, Marchetti P, Formstecher P, Kroemer G, Lewensohn R, Zhivotovsky B. Mitochondrial dysfunction is an essential step for killing of non-small cell lung carcinomas resistant to conventional treatment. Oncogene. 2002;21:65–77. doi: 10.1038/sj.onc.1205018. [DOI] [PubMed] [Google Scholar]
- 35.Kumamoto H, Ooya K. Detection of mitochondria-mediated apoptosis signalling molecules in ameloblastomas. J Oral Pathol Med. 2005;34:565–72. doi: 10.1111/j.1600-0714.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 36.Kumamoto H, Ooya K. Immunohistochemical detection of BH3-only proteins in ameloblastic tumors. Oral Dis. 2008;14:550–5. doi: 10.1111/j.1601-0825.2007.01417.x. [DOI] [PubMed] [Google Scholar]
- 37.Kumamoto H, Ooya K. Immunohistochemical detection of platelet-derived endothelial cell growth factor/thymidine phosphorylase and angiopoietins in ameloblastic tumors. J Oral Pathol Med. 2006;35:606–12. doi: 10.1111/j.1600-0714.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 38.Jensen AD, Ecker S, Ellerbrock M, Nikoghosyan A, Debus J, Munter MW. Carbon ion therapy for ameloblastic carcinoma. Radiat Oncol. 2011;6:13. doi: 10.1186/1748-717X-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


