Abstract
Objective
Coronary heart disease (CHD) is the leading cause of death in the United States, yet assessing risk of its development remains challenging. The present study evaluates a new automated assay of small dense low-density lipoprotein cholesterol content (sdLDL-C) and whether sdLDL-C is a risk factor for CHD compared with LDL-C or small LDL particle concentrations derived from nuclear magnetic resonance spectroscopy.
Approach and Results
sdLDL-C was measured using a new automated enzymatic method, and small LDL concentrations were obtained by nuclear magnetic resonance in 4387 Multi-Ethnic Study of Atherosclerosis participants. Cox regression analysis estimated hazard ratios for developing CHD for 8.5 years after adjustments for age, race, sex, systolic blood pressure, hypertension medication use, high-density lipoprotein cholesterol, and triglycerides. Elevated sdLDL-C was a risk factor for CHD in normoglycemic individuals. Those in the top sdLDL-C quartile showed higher risk of incident CHD (hazard ratio, 2.41; P=0.0037) compared with those in the bottom quartile and indicated greater CHD risk than the corresponding quartile of LDL-C (hazard ratio, 1.75; P=0.019). The association of sdLDL-C with CHD risk remained significant when LDL-C (<2.57 mmol/L) was included in a multivariate model (hazard ratio, 2.37; P=0.012). Nuclear magnetic resonance–derived small LDL concentrations did not convey a significant risk of CHD. Those with impaired fasting glucose or diabetes mellitus showed higher sdLDL-C and small LDL concentrations but neither was associated with higher CHD risk in these individuals.
Conclusions
This new automated method for sdLDL-C identifies risk for CHD that would remain undetected using standard lipid measures, but only in normoglycemic, nondiabetic individuals.
Keywords: coronary, disease
Coronary heart disease (CHD) is the leading cause of death in the United States, and yet disease risk assessment remains inadequate. The current practice of using lipid levels to evaluate the likelihood of future CHD has proven moderately effective in determining patient risk; however, tens of thousands of individuals with normal cholesterol experience CHD events every year.1 To improve risk assessment, measurements of lipid and nonlipid biomarkers have been suggested, including lipoprotein (a), C-reactive protein, homocysteine, and lipoprotein subfraction analysis. Among these assays, low-density lipoprotein subfraction analysis has shown notable potential, particularly the quantification of small dense low-density lipoprotein (sdLDL) particles.
Previous studies have shown that sdLDL particle concentrations are higher in cases of incident coronary artery disease,2 myocardial infarction,3 stroke,4 and overall cardiovascular disease (CVD).5 Moreover, sdLDL levels have been shown to correlate with CHD more strongly than LDL-C and large LDL particle concentrations across multiple prospective and case–control studies5–8 although not all.9–11 Until recently, practical considerations made routine measurement of sdLDL in a clinical laboratory setting unfeasible. Methods such as ultracentrifugation, nuclear magnetic resonance (NMR) spectroscopy, and gradient gel electrophoresis require the use of laboratory equipment that may be unavailable or cost prohibitive. In contrast, a newly developed assay that measures a surrogate of sdLDL particles, sdLDL cholesterol content (sdLDL-C), uses automated and readily available clinical laboratory instrumentation.12
Thus far, only a limited number of studies have evaluated the efficacy of sdLDL-C measurement, and no studies have examined its use in predicting CHD risk in a multi-ethnic pro-spective study population. The present study was conducted in 4387 Multi-Ethnic Study of Atherosclerosis (MESA) participants for an 8.5-year period to determine whether sdLDL-C levels (1) are independently associated with greater risk of incident CHD and (2) identify risk of CHD in those with normal LDL-C levels. Because diabetes mellitus and pre–diabetes mellitus are known to influence lipids and the small LDL subclass,12–15 subgroup analyses of those with normal fasting glucose (NFG), impaired fasting glucose (IFG), and type II diabetes mellitus (T2D) were conducted, and appropriate statistical adjustments for triglycerides and high-density lipoprotein cholesterol (HDL-C) levels were included.
Materials and Methods
Materials and methods are available in the online-only Supplement.
Results
Demographic and clinical characteristics of MESA participants (n=4387), as well as subgroups of those with NFG (n=3334) and IFG or T2D (n=1048), are shown in Table 1.
Table 1.
Demographic, Lifestyle, and Clinical Characteristics of a Subcohort of Multi-Ethnic Study of Atherosclerosis Participants
| All (n=4387) |
NFG (n=3334) |
IFG or Diabetes Mellitus (n=1048*) |
|
|---|---|---|---|
| Age, mean (SD), y | 61.5 (10.3) | 60.7 (10.3) | 64.1 (9.8) |
| Sex, n (%) female | 2329 (53.1) | 1861 (55.8) | 466 (44.5) |
| Race | |||
| Black, n (%) | 1257 (28.7) | 900 (27.0) | 355 (33.9) |
| White, n (%) | 1588 (36.2) | 1342 (40.3) | 245 (23.4) |
| Chinese, n (%) | 546 (12.4) | 401 (12.0) | 144 (13.7) |
| Hispanic, n (%) | 996 (22.7) | 691 (20.7) | 304 (29.0) |
| Current smoker, n (%) | 557 (12.8) | 424 (12.8) | 133 (12.8) |
| Alcohol current use, n (%) | 2399 (68.6) | 1898 (70.6) | 499 (62.1) |
| Hypertension, n (%) | 1765 (40.2) | 1174 (35.2) | 588 (56.1) |
| BMI (SD) | 28.1 (5.5) | 27.5 (5.2) | 30.2 (5.7) |
| Total cholesterol, mmol/L (SD) | 5.08 (0.92) | 5.08 (0.90) | 5.05 (0.96) |
| LDL-C, mmol/L (SD) | 3.10 (0.81) | 3.10 (0.80) | 3.08 (0.83) |
| HDL-C, mmol/L SD) | 1.32 (0.39) | 1.36 (0.40) | 1.20 (0.32) |
| TG levels, mmol/L (SD) | 1.45 (0.99) | 1.36 (0.83) | 1.71 (1.32) |
| Small LDL-P, mmol/L (SD) | 527.8 (388.3) | 488.1 (376.3) | 654.5 (399.2) |
| sdLDL-C, mmol/L (SD) | 0.97 (0.44) | 0.94 (0.43) | 1.06 (0.45) |
| CHD events (all) | 234 | 150 | 84 |
HDL-C indicates high-density lipoprotein cholesterol; IFG, impaired fasting glucose; LDL-C, low-density lipoprotein cholesterol; LDL-P, low-density lipoprotein particle; NFG, normal fasting glucose; sdLDL-C, small dense low-density lipoprotein cholesterol; and TG, triglyceride.
Five individuals with missing diabetes mellitus data.
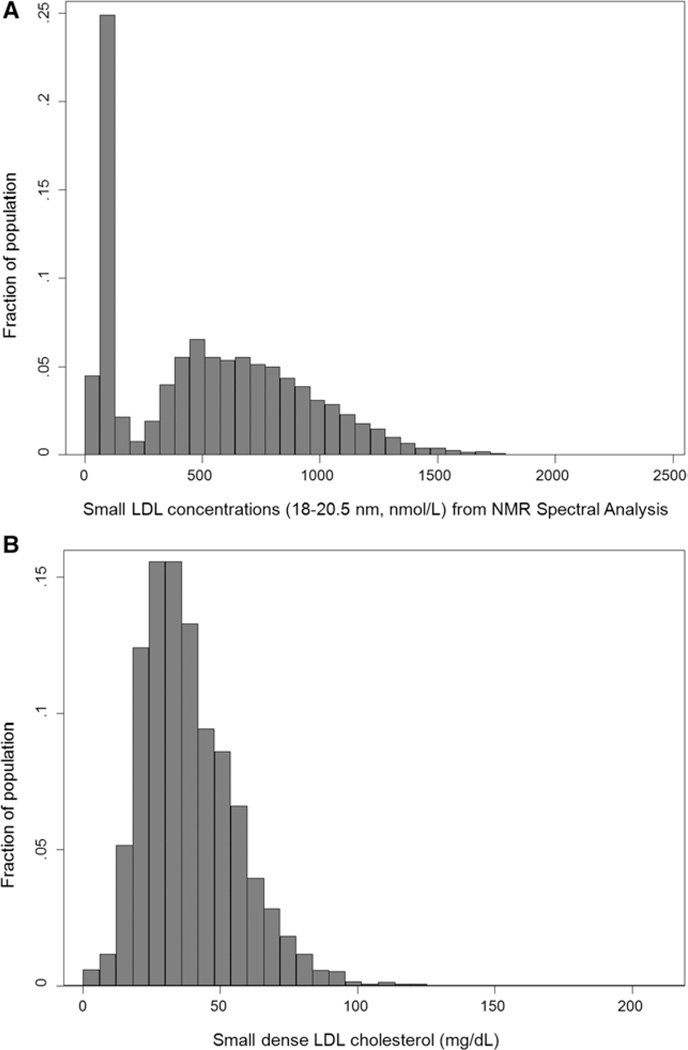
Distributions of NMR-derived small LDL concentrations and sdLDL-C levels are shown in the Figure. NMR-derived small LDL concentrations showed a bimodal distribution skewed to the right, in which ≈30% samples showed low concentrations (≤200 nmol/L) and ≈70% showed higher concentrations (>200 nmol/L; Figure [A]). The distribution of sdLDL-C was approximately normal (Figure [B]). The mean sdLDL-C value was 0.97 mmol/L, and thus ≈32.4% of total LDL-C on average was sdLDL-C in this population. A modest correlation (r=0.59) was found between sdLDL-C values and NMR-derived small LDL concentrations.
Figure.
Distributions of (A) small low-density lipoprotein (LDL) particle concentrations derived from nuclear magnetic resonance (NMR) and (B) small dense low-density lipoprotein cholesterol content (sdLDL-C) among Multi-Ethnic Study of Atherosclerosis participants (n=4387).
Quartile ranges for each lipid and lipoprotein target for normoglycemic, nondiabetic individuals are as follows: sdLDL-C, mmol/L: first (0.0025–0.66), second (0.66–0.89), third (0.89–1.23), and fourth (1.19–5.37); small LDL particle (LDL-P), nmol/L: first (0–101), second (101–467), third (467–805), and fourth (757–2020); and LDL-C, mmol/L: first (0.52–2.56), second (2.56–3.08), third (3.08–3.59), and fourth (3.60–7.34). For those with impaired fasting glucose or diabetes mellitus, quartile ranges for each lipid and lipoprotein target individuals are as follows: sdLDL-C, mmol/L: first (0.0025–0.74), second (0.74–1.01), third (1.01–1.32), and fourth (1.32–4.31); small LDL-P, nmol/L: first (0–361), second (361–659), third (659–921), and fourth (921–2300); and LDL-C, mmol/L: first (0.31–2.53), second (2.53–3.05), third (3.05–3.59), and fourth (3.59–8.15).
Hazard ratios (HRs) of incident CHD are presented by quartiles of LDL-C, sdLDL-C, and small LDL particle concentrations adjusted for sex, systolic blood pressure, hypertension medication use, age, ethnicity, HDL-C, and log triglycerides (Tables 2 and 3). Normoglycemic individuals in the top quartiles of LDL-C and sdLDL-C showed 1.75-fold (P=0.019) and 2.41-fold (P=0.0037) higher risks of future CHD, respectively (Table 2) when compared with those in the bottom quartiles. In contrast, higher NMR-derived small LDL particle concentrations did not convey a significantly higher risk of CHD (HR, 1.37; P=0.35). For those with IFG or T2D, higher levels of LDL-C, small LDL particle concentrations, or sdLDL-C were not found to increase the risk of CHD (Table 3).
Table 2.
Estimated Cox Proportional Hazard Ratios (95% Confidence Interval) of Incident Coronary Heart Disease by Quartiles of sdLDL-C, Nuclear Magnetic Resonance–Derived Small LDL Particle Concentrations, and LDL-C in Normoglycemic, Nondiabetic Participants (n=3334, 150 events) With Normal Fasting Glucose for an 8.5-Year Follow-Up Period
| Quartile | LDL-C (0.52–7.3 mmol/L) |
Small LDL-P (0–2018 nmol/L) |
sdLDL-C (0.003–5.4 mmol/L) |
|---|---|---|---|
| 1 | Ref | Ref | Ref |
| 2 | 0.85 (0.51–1.43) | 0.78 (0.45–1.37) | 1.45 (0.86–2.43) |
| 3 | 1.50 (0.94–2.40) | 0.90 (0.50–1.64) | 1.56 (0.90–2.68) |
| 4 | 1.75 (1.10–2.80) | 1.37 (0.71–2.64) | 2.41 (1.33–4.35) |
| P=0.019 | P=0.35 | P=0.0037 |
Ranges for each analyte are specified. Analyses were adjusted for sex, systolic blood pressure, hypertension medication use, ethnicity, age (category), high-density lipoprotein cholesterol, and log triglycerides. Quartiles in which associations reached significance (P≤0.05) are shown in the table. LDL-C indicates low-density lipoprotein cholesterol; LDL-P, low-density lipoprotein particle; and sdLDL-C, small dense low-density lipoprotein cholesterol.
Table 3.
Estimated Cox Proportional Hazard Ratios (95% Confidence Interval) of Incident Coronary Heart Disease by Quartiles of sdLDL-C, Nuclear Magnetic Resonance–Derived Small LDL Particle Concentrations, and LDL-C in Multi-Ethnic Study of Atherosclerosis Participants With Impaired Fasting Glucose or Diabetes Mellitus (n=1048*, 84 events) for an 8.5-Year Follow-Up Period
| Quartile | LDL-C (0.31–8.15 mmol/L) |
Small LDL-P (0–2299 nmol/L) |
sdLDL-C (0.003–4.3 mmol/L) |
|---|---|---|---|
| 1 | Ref | Ref | Ref |
| 2 | 0.91 (0.49–1.70) | 0.89 (0.44–1.83) | 1.06 (0.56–2.01) |
| 3 | 1.15 (0.62–2.12) | 0.66 (0.30–1.48) | 0.75 (0.37–1.57) |
| 4 | 1.15 (0.61–2.19) | 0.90 (0.40–2.02) | 1.06 (0.50–2.23) |
Ranges for each analyte are specified. Analyses were adjusted for sex, systolic blood pressure, hypertension medication use, ethnicity, age (category), high-density lipoprotein cholesterol, and log triglycerides. Quartiles in which associations reached significance (P≤0.05) are shown in the table. LDL-C indicates low-density lipoprotein cholesterol; LDL-P, low-density lipoprotein particle; and sdLDL-C, small dense low-density lipoprotein cholesterol.
Five individuals with missing diabetes mellitus data.
Using the same model criteria as above, we conducted a secondary analysis in which outcomes were restricted to myocardial infarction, resuscitated cardiac arrest, and CHD death. LDL-C, small LDL-P, and sdLDL-C were not found to be significant risk factors for these outcomes in either NFG or IFG+T2D groups (Table I in the online-only Data Supplement); however, those with NFG and in the top quartile of sdLDL-C showed a 1.92-fold higher risk of a hard CHD event compared with those in the bottom quartile, although this finding did not reach statistical significance (P=0.084).
HRs of incident CHD were further determined by including both quartiles of sdLDL-C and dichotomized LDL-C (<2.59 versus ≥2.59 mmol/L) for normoglycemic individuals. The results are presented in Table 4 and adjusted for sex, systolic blood pressure, hypertension medication use, ethnicity, age, HDL-C, and log triglycerides. With LDL-C being adjusted for, individuals in the fourth quartile of sdLDL-C incurred a 2.37-fold higher risk of CHD (P=0.012) compared to those in the reference quartile. In this multivariate model, elevated (≥2.59 mmol/L) LDL-C only showed a nonsignificant HR of 1.02 (P=0.93) compared to those with optimal LDL-C (<2.59 mmol/L). In contrast, a multivariate model with dichotomized LDL-C and NMR-derived small LDL concentrations showed no significant associations with CHD risk in any quartile of small LDL (Table 5). An interaction model between quartiles of sdLDL-C and dichotomized LDL-C was also fitted, but no significant interaction was found (P>0.1), possibly because of limited number of events and high correlation between sdLDL-C and LDL-C (data not shown).
Table 4.
Estimated Cox Proportional Hazard Ratios (95% Confidence Interval) of Incident Coronary Heart Disease by Quartiles of sdLDL-C, Dichotomized by LDL-C (2.59 mmol/L) in 3334 Multi-Ethnic Study of Atherosclerosis Participants With Normal Fasting Glucose for an 8.5-Year Follow-Up Period
| sdLDL-C Quartile |
LDL-C<2.59 | LDL-C>2.59 |
|---|---|---|
| 1 | Ref | 1.02 (0.64–1.63) |
| 2 | 1.43 (0.82–2.50) | 1.46 (0.82–2.60) |
| 3 | 1.54 (0.85–2.80) | 1.57 (0.87–2.82) |
| 4 | 2.37 (1.21–4.67) | 2.42 (1.32–4.44) |
| P=0.012 | P=0.004 |
Analyses were adjusted for sex, systolic blood pressure, hypertension medication use, ethnicity, age (category), high-density lipoprotein cholesterol, and log triglycerides. Quartiles in which associations reached significance (P≤0.05) are shown in the table. LDL-C indicates low-density lipoprotein cholesterol; and sdLDL-C, small-dense low-density lipoprotein cholesterol.
Table 5.
Estimated Cox Proportional Hazard Ratios (95% Confidence Interval) of Incident Coronary Heart Disease by Quartiles of Small LDL Concentrations (NMR), Dichotomized by LDL-C (2.59 mmol/L) in 3334 Multi-Ethnic Study of Atherosclerosis Participants With Normal Fasting Glucose for an 8.5-Year Follow-Up Period
| Small LDL (NMR) Quartile |
LDL-C<2.59 | LDL-C>2.59 |
|---|---|---|
| 1 | 1 | 1.28 (0.84–1.95) |
| 2 | 0.75 (0.43–1.32) | 0.963 (0.50–1.86) |
| 3 | 0.841 (0.46–1.55) | 1.078 (0.55–2.11) |
| 4 | 1.24 (0.63–2.43) | 1.58 (0.78–3.20) |
Analyses were adjusted for sex, systolic blood pressure, hypertension medication use, ethnicity, age (category), high-density lipoprotein cholesterol, and log triglycerides. Quartiles in which associations reached significance (P≤0.05) are shown in the table. LDL-C indicates low-density lipoprotein cholesterol; NMR, nuclear magnetic resonance; and sdLDL-C, small dense low-density lipoprotein cholesterol.
Discussion
In this multi-ethnic prospective study of 4387 MESA participants, we examined whether an automated enzymatic assay of sdLDL-C may identify CHD risk beyond standard lipid measures. The highest quartile of sdLDL-C conveyed an approximate 2-fold higher CHD risk, regardless of LDL-C levels, but only in normoglycemic, nondiabetic participants. The finding remained significant after adjustments for demographic characteristics and other related clinical laboratory measurements, such as triglycerides and HDL-C.
The traditional clinical measure of LDL-C quantifies the cholesterol content of LDL particles but does not address the vast heterogeneity of LDL particles among individuals. This limitation is crucial as smaller LDL particles have been shown to be more atherogenic because of their higher susceptibility to oxidation.16 In addition, sdLDL particles have a higher kd for the hepatic LDL receptor, resulting in a longer circulating half-life.17 Combined with their smaller size and propensity for receptor-independent binding to the endothelium,17 smaller LDL particles have a greater opportunity for infiltration into the subendothelial space.18 Finally, compared with large LDL particles, sdLDL particles show an ≈3-fold higher enzymatic activity of lipoprotein-associated phospholipase A2, an independent risk factor for CVD and vascular inflammatory marker.19 Thus, an individual with a predominance of small LDL particles will have a larger atherogenic burden but may have optimal LDL-C levels (<2.59 mmol/L) because of the lower cholesterol carrying capacity of smaller lipoprotein particles. Taken together, using LDL-C to evaluate cholesterol- related CHD risk will underestimate actual risk in individuals who have optimal LDL-C but high levels of sdLDL.
Although measurement of sdLDL may improve risk prediction models, an effective and readily available assay has remained elusive. The well-studied and commercialized method of NMR analysis has allowed for the quantification of LDL-P sizes and subclass concentrations. Studies have shown that LDL-P concentrations more strongly associate with CVD20 and CHD cases5 than LDL-C. In contrast, the prospective Women’s Health Study21 and a case–control study in Cardiovascular Health Study participants11 were unable to demonstrate that small or total LDL-P concentrations outperform LDL-C in predicting CVD or CHD—particularly after adjustments for other lipids. In the present study, we also found that NMR-derived small LDL particle concentrations were not associated with future CHD events in a multivariate model.
Because the automated homogenous sdLDL-C assay can be performed with existing equipment in all clinical laboratories, it may have more widespread applicability as a marker for CHD risk. It was first demonstrated to have the potential for clinical use in an observational study of subjects with combined hyperlipidemia, diabetes mellitus, or CHD, all of which showed increased levels of sdLDL-C.6 These findings were subsequently confirmed in a case–control study of 871 Japanese men, which further demonstrated that sdLDL-C concentrations were more strongly associated with the presence of severe stable CHD (odds ratio, 1.022; 95% confidence interval, 1.005–1.039) compared with LDL-C (odds ratio, 1.009; 95% confidence interval, 0.994–1.024).7 Likewise, a case–control study in Framingham Offspring study participants also reported higher sdLDL-C, but only in female patients with CHD compared with controls (0.83 versus 0.68 mmol/L, respectively; P=0.0015) although notably, both male and female patients with CHD showed a higher percentage of sdLDL-C/LDL-C compared with controls.8 Finally, a pro-spective study of 2098 Japanese men and women without a history of CVD found that a 10-mg/dL increment in sdLDL-C results in a 21% increase in risk of future incident CVD22; however, sdLDL-C was not a significant predictor of coronary artery disease after multivariable adjustments (HR, 1.05; 95% confidence interval, 0.0.81–1.36). Overall, it has been demonstrated that sdLDL-C levels are higher in patients with CHD and increase the risk of CVD. We extend these findings and show that elevated sdLDL-C levels are a significant risk marker for future CHD in participants with NFG with multivariate adjustments including HDL-C and triglycerides.
Apart from the above findings, we confirm earlier studies that individuals with IFG or T2D have higher levels of both NMR-derived small LDL13–15,23 and sdLDL-C12,24 compared with those with NFG. Nonetheless, elevated sdLDL-C levels were not associated with future CHD in the present study population. The basis for the disparity between the NFG and IFG/T2D groups is unclear, but several possibilities may account for the result. First, the number of events in participants with IFG or T2D (n=84) limits the statistical power of this analysis and may have contributed to the null finding. A larger study may find an association with individuals with IFG, and still more power may be needed for individuals with diabetes mellitus. In addition, individuals with diabetes mellitus are known to have a considerably higher risk of CHD, and Adult Treatment Panel III guidelines designate diabetics as having equivalent risk to those who have had a previous cardiac event; thus, the risk conveyed by higher levels of sdLDL-C may be attenuated in those with IFG or diabetes mellitus, and other risk factors may be the primary drivers of disease development and progression. Although our null finding does not exclude the possibility that sdLDL-C contributes to CHD in those with IFG or T2D, our data indicate that sdLDL-C is most useful for assessing CHD risk in normoglycemic individuals.
We were unable to demonstrate that elevated NMR-derived small LDL concentrations are associated with higher CHD risk in participants with NFG. The discordant results between NMR and the sdLDL-C assay were unexpected, as both methods putatively quantify the small LDL subfraction. Yet, the correlation between small LDL from NMR and sdLDL-C was relatively modest (Spearman correlation, r=0.59) and distribution patterns were distinct. We speculate that the discrepancy may be because of the different particle subpopulations measured by these 2 methods: sdLDL-C is composed of the cholesterol content of LDL particles measuring 15 to 20 nm; NMR measures particles 18 to 20.5 nm in diameter. In addition, it must also be recognized that these methods are based on different analytic principles. Whereas the sdLDL-C assay measures aggregate cholesterol in small LDL particles, NMR determines lipoprotein particle concentrations by the amplitudes of their corresponding lipid methyl group signals. The coefficients of variation of these assays are also different, with 3.2% for sdLDL-C and 8% for NMR. Whether the observed differences in CHD risk are because of the distinctions in LDL subpopulations of NMR versus the sdLDL-C assay, the fundamental difference in analytic techniques, the relative differences in assay coefficients of variation, or some combination of the above is unclear, but further studies may reveal the root cause(s) for the disparate results between these methodologies.
It must be acknowledged that lipid-lowering medication may be a potential confounder when examining lipids and CHD events in the MESA prospective study population. In the present analysis, those using lipid-lowering medication at baseline were excluded; however, several individuals in the remaining subcohort began using lipid-lowering medication at later dates. We therefore combined MESA follow-up data from visits 2 to 5 and evaluated lipid-lowering medication use as a discrete time-dependent variable. The associations among sdLDL-C, LDL-C, and small LDL particles with CHD events remained similar to our current findings (Table II in the online-only Data Supplement).
The present analysis contains several strengths and limitations. First, particle size of LDL is known to be influenced by serum triglyceride levels. As such, adjustments for triglyceride and HDL-C levels were made in the present Cox regression models, and the highest quartile of sdLDL-C levels was still found to confer >2-fold increased risk of CHD. In terms of limitations, we were limited by the number of events in our analysis for myocardial infarction and CHD death. Although we adjusted for demographic and other CHD factors within the statistical model, the presence of a residual confounder(s) cannot be excluded.
Conclusions
In the present study, we demonstrated that sdLDL-C levels generated from the newly developed automated assay of sdLDL-C (Denka Seiken) are associated with CHD events independent of LDL-C levels. Clinically, sdLDL-C assessment may be most beneficial in patients with intermediate CHD risk, where those with higher sdLDL-C levels may be designated for more aggressive treatment protocols.
Supplementary Material
Significance.
The present analysis represents the largest study to examine prospectively whether small dense low-density lipoprotein cholesterol (LDLC) associates with future risk of coronary heart disease. We show that elevated levels convey a significant risk for incident coronary heart disease independent of traditional risk factors. Most significantly, in individuals with normal healthy levels of LDL-C (<2.59 mmol/L or <100 mg/dL), we found that those with small dense LDL-C levels in the 75th percentile have an ≈2.4-fold greater risk of developing coronary heart disease compared with those with lower small dense LDL-C levels. Overall, this study provides evidence that small dense LDL-C identifies risk of heart disease development in individuals that would otherwise be undetected using current guidelines.
Acknowledgments
We thank the other investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating Multi-Ethnic Study of Atherosclerosis (MESA) investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding
This research was supported by contracts, N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute.
Nonstandard Abbreviations and Acronyms
- CHD
coronary heart disease
- CVD
cardiovascular disease
- HDL-C
high-density lipoprotein cholesterol
- HR
hazard ratio
- IFG
impaired fasting glucose
- LDL-P
small low-density lipoprotein particle
- MESA
Multi-Ethnic Study of Atherosclerosis
- NFG
normal fasting glucose
- NMR
nuclear magnetic resonance
- sdLDL-C
small dense low-density lipoprotein cholesterol
- T2D
type II diabetes mellitus
Footnotes
Disclosures
M.Y. Tsai received consulting fees from the manufacturer of the small dense low-density lipoprotein cholesterol (sdLDL-C) assay (Denka Seiken). R. Warnick, D.M. Hoefner, and J. McConnell are associated with Health Diagnostic Laboratory, Inc, the clinical laboratory responsible for obtaining sdLDL-C data in the present study. J. McConnell serves as the chief medical officer, R. Warnick serves as the chief scientific officer, and D.M. Hoefner serves as the director of Technology and Development. The other authors report no conflicts.
References
- 1.Sachdeva A, Cannon CP, Deedwania PC, Labresh KA, Smith SC, Jr, Dai D, Hernandez A, Fonarow GC. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J. 2009;157:111–117. e2. doi: 10.1016/j.ahj.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 2.El Harchaoui K, van der Steeg WA, Stroes ES, Kuivenhoven JA, Otvos JD, Wareham NJ, Hutten BA, Kastelein JJ, Khaw KT, Boekholdt SM. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;49:547–553. doi: 10.1016/j.jacc.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 3.Kamigaki AS, Siscovick DS, Schwartz SM, Psaty BM, Edwards KL, Raghunathan TE, Austin MA. Low density lipoprotein particle size and risk of early-onset myocardial infarction in women. Am J Epidemiol. 2001;153:939–945. doi: 10.1093/aje/153.10.939. [DOI] [PubMed] [Google Scholar]
- 4.Zeljkovic A, Vekic J, Spasojevic-Kalimanovska V, Jelic-Ivanovic Z, Bogavac-Stanojevic N, Gulan B, Spasic S. LDL and HDL subclasses in acute ischemic stroke: prediction of risk and short-term mortality. Atherosclerosis. 2010;210:548–554. doi: 10.1016/j.atherosclerosis.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 5.Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106:1930–1937. doi: 10.1161/01.cir.0000033222.75187.b9. [DOI] [PubMed] [Google Scholar]
- 6.Hirano T, Ito Y, Koba S, Toyoda M, Ikejiri A, Saegusa H, Yamazaki J, Yoshino G. Clinical significance of small dense low-density lipoprotein cholesterol levels determined by the simple precipitation method. Arterioscler Thromb Vasc Biol. 2004;24:558–563. doi: 10.1161/01.ATV.0000117179.92263.08. [DOI] [PubMed] [Google Scholar]
- 7.Koba S, Yokota Y, Hirano T, Ito Y, Ban Y, Tsunoda F, Sato T, Shoji M, Suzuki H, Geshi E, Kobayashi Y, Katagiri T. Small LDL-cholesterol is superior to LDL-cholesterol for determining severe coronary atherosclerosis. J Atheroscler Thromb. 2008;15:250–260. doi: 10.5551/jat.e572. [DOI] [PubMed] [Google Scholar]
- 8.Ai M, Otokozawa S, Asztalos BF, Ito Y, Nakajima K, White CC, Cupples LA, Wilson PW, Schaefer EJ. Small dense LDL cholesterol and coronary heart disease: results from the Framingham Offspring Study. Clin Chem. 2010;56:967–976. doi: 10.1373/clinchem.2009.137489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman DS, Otvos JD, Jeyarajah EJ, Barboriak JJ, Anderson AJ, Walker JA. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler Thromb Vasc Biol. 1998;18:1046–1053. doi: 10.1161/01.atv.18.7.1046. [DOI] [PubMed] [Google Scholar]
- 10.Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, Bloomfield HE, Robins SJ. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113:1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 11.Kuller L, Arnold A, Tracy R, Otvos J, Burke G, Psaty B, Siscovick D, Freedman DS, Kronmal R. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arterioscler Thromb Vasc Biol. 2002;22:1175–1180. doi: 10.1161/01.atv.0000022015.97341.3a. [DOI] [PubMed] [Google Scholar]
- 12.Ito Y, Fujimura M, Ohta M, Hirano T. Development of a homogeneous assay for measurement of small dense LDL cholesterol. Clin Chem. 2011;57:57–65. doi: 10.1373/clinchem.2010.149559. [DOI] [PubMed] [Google Scholar]
- 13.Festa A, Williams K, Hanley AJ, Otvos JD, Goff DC, Wagenknecht LE, Haffner SM. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation. 2005;111:3465–3472. doi: 10.1161/CIRCULATIONAHA.104.512079. [DOI] [PubMed] [Google Scholar]
- 14.Mora S, Otvos JD, Rosenson RS, Pradhan A, Buring JE, Ridker PM. Lipoprotein particle size and concentration by nuclear magnetic resonance and incident type 2 diabetes in women. Diabetes. 2010;59:1153–1160. doi: 10.2337/db09-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julius U, Dittrich M, Pietzsch J. Factors influencing the formation of small dense low-density lipoprotein particles in dependence on the presence of the metabolic syndrome and on the degree of glucose intolerance. Int J Clin Pract. 2007;61:1798–1804. doi: 10.1111/j.1742-1241.2007.01507.x. [DOI] [PubMed] [Google Scholar]
- 16.Chait A, Brazg RL, Tribble DL, Krauss RM. Susceptibility of small, dense, low-density lipoproteins to oxidative modification in subjects with the atherogenic lipoprotein phenotype, pattern B. Am J Med. 1993;94:350–356. doi: 10.1016/0002-9343(93)90144-e. [DOI] [PubMed] [Google Scholar]
- 17.Galeano NF, Al-Haideri M, Keyserman F, Rumsey SC, Deckelbaum RJ. Small dense low density lipoprotein has increased affinity for LDL receptor-independent cell surface binding sites: a potential mechanism for increased atherogenicity. J Lipid Res. 1998;39:1263–1273. [PubMed] [Google Scholar]
- 18.Nordestgaard BG, Nielsen LB. Atherosclerosis and arterial influx of lipoproteins. Curr Opin Lipidol. 1994;5:252–257. doi: 10.1097/00041433-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Karabina SA, Liapikos TA, Grekas G, Goudevenos J, Tselepis AD. Distribution of PAF-acetylhydrolase activity in human plasma low-density lipoprotein subfractions. Biochim Biophys Acta. 1994;1213:34–38. doi: 10.1016/0005-2760(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 20.Musunuru K, Orho-Melander M, Caulfield MP, Li S, Salameh WA, Reitz RE, Berglund G, Hedblad B, Engström G, Williams PT, Kathiresan S, Melander O, Krauss RM. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler Thromb Vasc Biol. 2009;29:1975–1980. doi: 10.1161/ATVBAHA.109.190405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–939. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arai H, Kokubo Y, Watanabe M, Sawamura T, Ito Y, Minagawa A, Okamura T, Miyamato Y. Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the Suita study. J Atheroscler Thromb. 2013;20:195–203. doi: 10.5551/jat.14936. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins AJ, Lyons TJ, Zheng D, Otvos JD, Lackland DT, McGee D, Garvey WT, Klein RL DCC/EDIC Research Group. Serum lipoproteins in the diabetes control and complications trial/epidemiology of diabetes intervention and complications cohort: associations with gender and glycemia. Diabetes Care. 2003;26:810–818. doi: 10.2337/diacare.26.3.810. [DOI] [PubMed] [Google Scholar]
- 24.Maeda S, Nakanishi S, Yoneda M, Awaya T, Yamane K, Hirano T, Kohno N. Associations between small dense LDL, HDL subfractions (HDL2, HDL3) and risk of atherosclerosis in Japanese-Americans. J Atheroscler Thromb. 2012;19:444–452. doi: 10.5551/jat.11445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.