Abstract
Population pharmacokinetic (PK) and exposure–response analyses of apixaban were performed using data from phase I–III studies to predict bleeding risks for patients receiving apixaban 2.5 mg b.i.d. after total knee or hip replacement (TKR, THR) surgery (N = 5,510). Renal function, age, gender, and body weight impacted apixaban exposure. Bleeding risk increased as a function of exposure. Predicted bleeding frequencies for TKR and THR populations at risk for high apixaban exposure (female, age > 75 years, calculated creatinine clearance (cCrCL) < 30 ml/min, body weight < 50 kg) (6.85 and 10.3%, respectively) were comparable to the reference population (male/female, age 65−75 years, cCrCL ≥ 80 ml/min, body weight 65−85 kg) (6.18 and 9.32%, respectively). A 100% increase in apixaban exposure is expected to raise bleeding frequencies to 7.25% (TKR) and 10.9% (THR), whereas a 200% increase would raise them to 8.49 and 12.7%. Coexistence of combined patient risk factors or doubling of exposure is not likely to result in a substantial, clinically relevant increase in bleeding risk with 2.5 mg b.i.d. apixaban.
Venous thromboembolism (VTE) is a serious and potentially fatal complication after total knee or hip replacement (TKR or THR) surgery. Therefore, routine thromboprophylaxis with unfractionated heparin, low-molecular-weight heparin, or a vitamin K antagonist is generally recommended for subjects undergoing orthopedic surgery.1 These agents have a long history of efficacy in the hospital setting, although drawbacks have limited their use in the outpatient setting. For example, unfractionated/low-molecular-weight heparins need to be administered as subcutaneous daily (or more frequent) injections and carry a risk of thrombocytopenia.2,3,4 Vitamin K antagonists have high PK variability, significant food–drug interactions, and a narrow therapeutic window requiring frequent visits for laboratory monitoring and dose adjustment.5,6,7,8 Thus, new anticoagulants with improved efficacy, lower bleeding risk, and more convenient formulations are needed to overcome the shortcomings of traditional agents and improve patient care.
Apixaban is an orally bioavailable, highly selective, reversible factor Xa inhibitor that exerts antithrombotic and anticoagulant effects by decreasing the generation of thrombin from prothrombin.9,10,11 Apixaban has an oral bioavailability of ~50% and reaches a peak plasma concentration ~3 h after oral administration.9,12,13,14,15 It is eliminated via multiple pathways, including hepatic metabolism, biliary and intestinal excretion, and renal elimination.14,16,17 The bioavailability of apixaban is not significantly affected by food.9 The potential for co-medications to impact the exposure of apixaban is limited. Studies conducted in healthy subjects observed only a twofold increase in exposure (area under the concentration–time curve) after coadministration with ketoconazole, a strong inhibitor of both cytochrome P3A4 and P-glycoprotein, and a 50% decrease in exposure after coadministration with rifampin, a strong inducer of both cytochrome P3A4 and P-glycoprotein.12
In clinical studies of apixaban in the patient population, apixaban 2.5 mg twice daily (b.i.d.) was superior to enoxaparin 40 mg once daily (q.d.) for VTE prevention in subjects after TKR and THR, without an increase in the risk of bleeding.18,19 Compared with enoxaparin 30 mg b.i.d., apixaban was similar in efficacy with reduced bleeding.20 Therefore, apixaban provides a therapeutic advantage relative to current standards of care and is approved in several countries for the prevention of VTE after elective TKR or THR.
The objectives of the present analysis were to use a model-based approach to (i) characterize the relationship between apixaban dose and exposure (i.e., population PK) in subjects after TKR (12 days of treatment) and THR (35 days of treatment), (ii) identify covariates that may significantly impact exposure, and (iii) quantify the relationship between apixaban exposure and bleeding risk in the target population. This allowed for an evaluation of the potential need for dose adjustment in subpopulations that might be expected to have an increased risk for bleeding due to an increase in apixaban exposure.
Results
Population pharmacokinetic model development
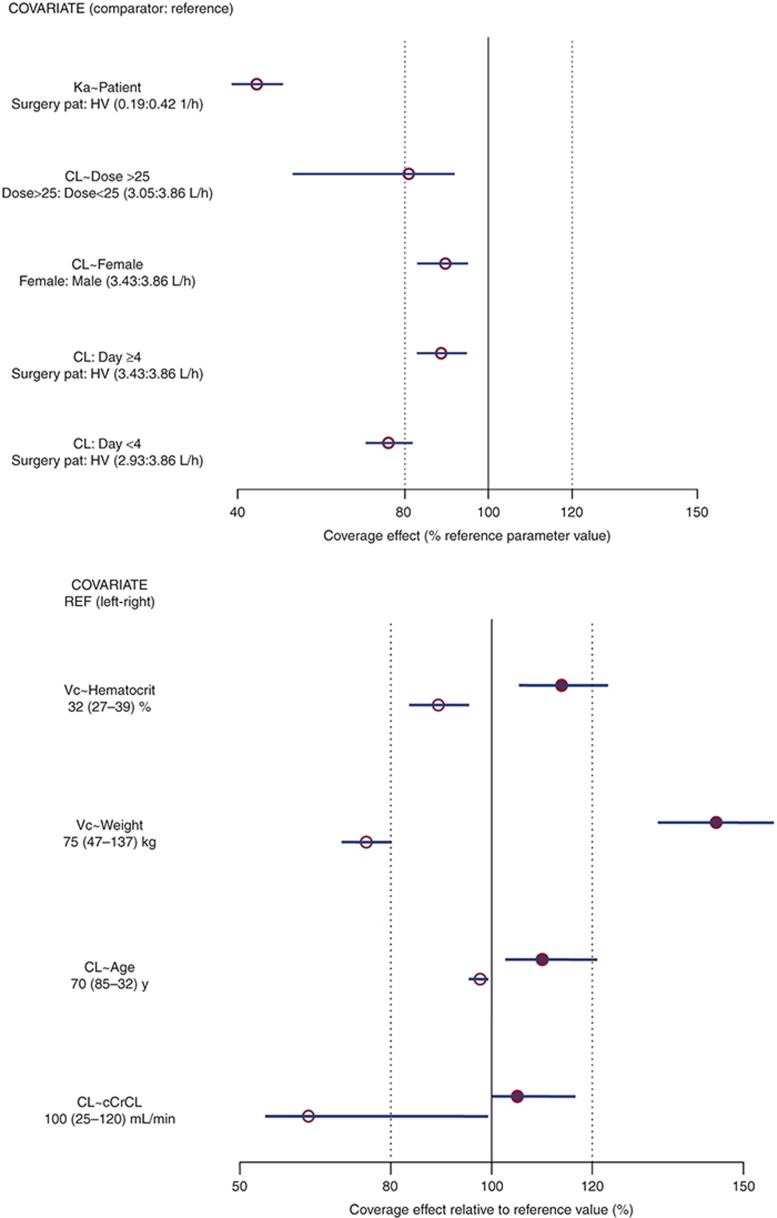
The apixaban population pharmacokinetic (PK) was described by a two-compartment disposition model with first-order absorption and elimination. Several covariate effects were identified as statistically significant in the population PK model (Figure 1). Apixaban clearance seemed to decrease in elderly and female subjects, and immediately after surgery. Apixaban clearance seemed to return to within 10% of pretreatment by the fourth day after surgery. The central volume of distribution of apixaban seemed to increase with increasing body weight and decrease with decreasing hematocrit.
Figure 1.
Effects of covariates in the population pharmacokinetic model. (a) The effect of categorical covariates on CL and Ka; (b) the effect of continuous covariates on CL and Vc. Open circles represent point estimates of the parameter estimate for the comparator relative to the parameter estimate for the reference on the percentage scale, and error bars represent 95% confidence intervals of effect obtained from 500 bootstrap replications; solid vertical line represents the covariate effect at the reference value of the covariate; dotted vertical lines represent the indicators of ±20% covariate effect. For continuous covariate effects, the parameter estimates for the comparator at the lower and upper bounds of the covariate are compared with the parameter estimate at the reference value for the covariate. For example, CL in a subject with cCrCL of 25 ml/min is ~35% lower than CL at the reference cCrCL (100 ml/min). cCrCL, calculated creatinine clearance; CL, clearance; HV, healthy volunteer; Ka, absorption rate constant; pat, patient; Vc, volume of distribution for central compartment.
The population PK model was evaluated using both visual and quantitative predictive checks. The quantitative predictive check was conducted by comparing the 10th, 50th, and 90th percentiles of observed trough plasma concentration with corresponding statistics calculated from simulated data using the final model. Results for both TKR and THR subjects demonstrated that the model was able to predict the range of exposures in both of these populations. More detailed information on population PK model development and diagnostic evaluations can be found in the Supplementary Material.
Exposure–bleeding analysis
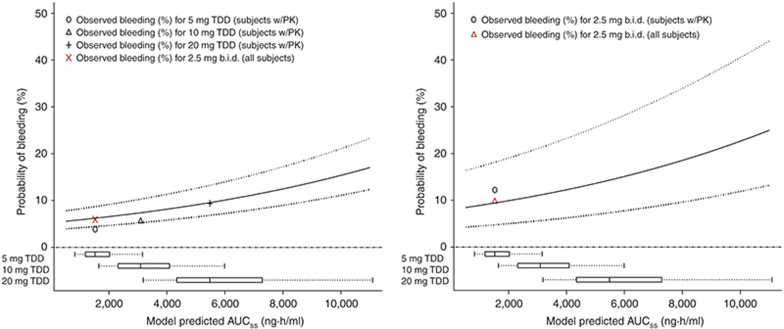
Figure 2 is a Kaplan–Meier plot that shows the observed relationship between apixaban exposure and the probability of a bleeding event as a function of time, after 12 days of treatment for TKR subjects and after 35 days of treatment for THR subjects. A Cox proportional hazards model was used to characterize the relationship of apixaban exposure level and duration with bleeding risk. Of the covariates evaluated in the model (gender, dosing regimen, surgery type, and area under the concentration–time curve (AUC) at steady state (AUCss)), only apixaban daily AUCss was found to be a significant predictor of bleeding risk. Bleeding risk was stratified by surgery type (TKR vs. THR). The interaction of surgery type with apixaban AUCss was evaluated, but not found to be statistically significant. This indicates that bleeding risk will be similar between the two populations for a similar level and duration of apixaban exposure. More detailed information on development of the exposure–bleeding model can be found in the Supplementary Material. The point estimates (and 95% confidence intervals) for predicted bleeding after 12 days of treatment in TKR subjects as a function of apixaban exposure were 6.2% (4.4–8.7%), 7.4% (5.3–10%), and 10% (7.1–14%) for the 2.5-, 5-, and 10-mg b.i.d. dosages, respectively. The point estimates (and 95% confidence intervals) for predicted bleeding at day 35 in THR subjects as a function of apixaban exposure were 9.5% (4.8–18%), 11% (5.8–22%), and 15% (7.8–28%) for the 2.5-, 5-, and 10-mg b.i.d. dosages, respectively. The exposure–bleeding relationship seems to be relatively shallow (Figure 3). The observed rates of bleeding for each of the total daily doses (5, 10, and 20 mg/d) are shown in Figure 3, as are the observed rates for the 2.5-mg b.i.d. dosage in the phase III TKR and THR studies. The 95% confidence intervals for the model predictions seem to capture the observed bleeding rates for the 2.5-mg b.i.d. dosage in those studies (Figure 3).
Figure 2.
Exposure–response relationship vs. time for bleeding (including major bleeding, minor bleeding, potentially significant nonovert bleeding, clinically relevant nonmajor bleeding, and fatal bleeding) in TKR (ADVANCE 2, upper panel) and THR (ADVANCE 3, lower panel) populations. The different curves in the plot represent the probability of a bleed as a function of exposure over time for the 2.5-, 5-, and 20-mg b.i.d. dosages of apixaban. The observed bleeding event data are depicted at the bottom of each figure as the number of subjects at risk on days 0, 4, 8, and 12 for the range of exposures covered by the different dosages of apixaban in TKR subjects (upper panel) and on days 0, 10, 20, and 30 for the range of exposures covered by the 2.5-mg b.i.d. dosage in THR subjects (lower panel). AUCss, area under plasma concentration–time curve at steady state; b.i.d., twice daily; THR, total hip replacement; TKR, total knee replacement.
Figure 3.
Exposure–response relationship for bleeding (including major bleeding, minor bleeding, potentially significant nonovert bleeding, clinically relevant nonmajor bleeding, and fatal bleeding) in TKR (ADVANCE 2, left panel) and THR (ADVANCE 3, right panel) subjects. Solid line represents the median bleeding probability and dotted lines represent the 95% confidence intervals. Observed bleeding (%) in “subjects w/PK” indicates the subjects in the E-R analysis dataset, whereas “all subjects” indicates the observed rate from the overall ADVANCE 2 and 3 populations, respectively. AUCss, area under plasma concentration–time curve at steady state; b.i.d., twice daily; TDD, total daily dose; THR, total hip replacement; TKR, total knee replacement.
Model-based simulations
Simulations from the population PK and exposure–bleeding models were used to predict changes in exposure and bleeding risk in the TKR and THR subpopulations potentially at risk for higher apixaban exposures and bleeding (Table 1). The model predicted that subjects with mild, moderate, and severe renal impairment would be expected to have median exposures that are ~19, 43, and 62% higher, respectively, than for subjects with normal renal function. The effects of other covariates were smaller when compared with calculated creatinine clearance (cCrCL, calculated using Cockroft-Gault equation), but would be expected to have a combined (although not additive) effect on apixaban exposure. For example, the model predicted that older age (>75 years) alone, lower weight (<50 kg) alone, and female gender alone would increase apixaban total exposure by ~22, 33, and 9%, respectively, relative to a reference population (defined as age 65–75 years, cCrCL ≥ 80 ml/min, weight 65–85 kg). For a subject with all these factors combined (female, age > 75 years, cCrCL < 30 ml/min, weight < 50 kg), the exposure was expected to be only ~64% higher than for a reference population (Table 1).
Table 1. Predicted bleeding probability (%) for apixaban 2.5-mg b.i.d. dosage in TKR (ADVANCE 2) and THR (ADVANCE 3) subpopulations.
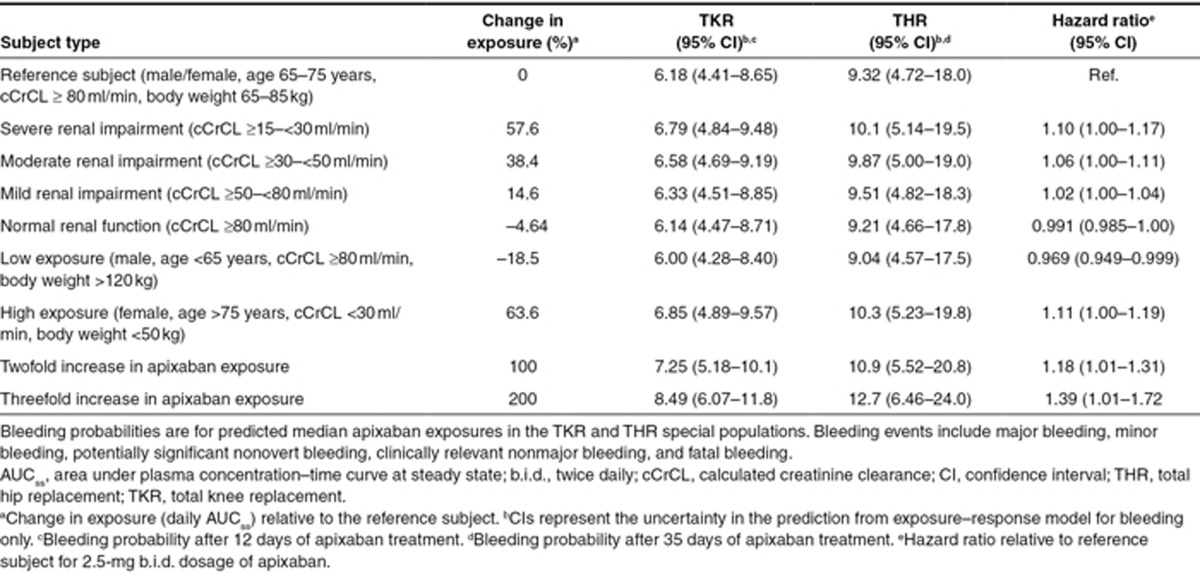
Bleeding risk over the treatment period is expected to increase with an increase in apixaban exposure, which is affected by several covariates, as described above. The effect of covariate combinations that increase apixaban exposure (female gender, age > 75 years, cCrCL < 30 ml/min, and body weight < 50 kg) would be expected to increase bleeding risk by 11% (Table 1). Increases in apixaban exposure of 100% would be expected to increase bleeding risk by 18% relative to a reference population and represents the change in exposure that might be encountered in the presence of a strong inhibitor of cytochrome P3A4 and P-glycoprotein. A 200% increase in apixaban exposure would be expected to increase bleeding risk by 39%; however, such increased exposures were not seen during any clinical trials.
Discussion
A population PK model was developed to characterize the relationship between apixaban dose and plasma exposure in TKR and THR subjects. Covariates were identified that explain some of the variability in the PK of apixaban. For a 30-year-old man not having surgery and with a cCrCL of 80 ml/min, the predicted renal and nonrenal component of apparent plasma clearance of apixaban (CLR/F) and CLNR/F would be 1.19 and 3.10 l/h, respectively, such that renal clearance would account for ~28% of total plasma apixaban CL/F. This prediction is consistent with estimates in healthy subjects, based on noncompartmental analysis.13 The population PK model suggests there is a reduction in total plasma CL/F of 24% in the 3 days immediately after wound closure in TKR and THR subjects. The reduced clearance in the days after surgery may arise from the reduced blood flow to the liver, kidney, and gastrointestinal tract after surgery.21 Apixaban volume of distribution was found to correlate with mean postsurgery hematocrit in TKR and THR subjects. This association may be linked to changes in hemodynamics after surgery22; however, subjects with a low mean hematocrit (27%) would not be expected to have a reduction in volume of distribution (<20% decrease) that would be associated with a clinically meaningful change in peak or trough plasma apixaban concentrations.
A previous exploratory exposure–response analysis based on a phase II clinical study in TKR subjects identified gender and AUCss as significant predictors of bleeding23; however, of the variables tested in the current exposure–response analysis, only daily AUCss was a significant predictor of bleeding. In phase III studies, the observed frequency of postsurgery bleeding events for all apixaban-treated TKR and THR subjects was 5.9 and 9.8%,24 respectively, which is similar to the model-predicted frequency of any bleeding (6.2 and 9.3%, respectively) in the reference population (Table 1). This suggests that, even though only a subset of the TKR and THR subjects was available for development of the exposure–bleeding model, it is representative of the overall target population.
Bleeding risk was also evaluated in subpopulations in which apixaban exposure could be altered by renal function, age, gender, and body weight as well as the combination of these factors. Although severe renal impairment alone would be expected to increase apixaban exposure by 58%, because of the shallow exposure–response relationship, this increase in exposure would not be expected to markedly increase the risk of bleeding in the TKR or THR target population. The combination of other intrinsic factors with renal impairment (e.g., age, body weight) does not seem to significantly increase apixaban exposure for the subpopulation. This is likely because of the high correlation of age and body weight with cCrCL. Results of the simulations suggest that for the apixaban 2.5-mg b.i.d. dosage, bleeding risk would be 6.85 and 10.3% in patients with combined risk factors (female gender, age > 75 years, cCrCL < 30 ml/min, body weight < 50 kg) after TKR and THR, respectively. Although higher than in typical subjects treated with this apixaban dose, this bleeding risk is similar to that observed in an overall population of subjects treated with enoxaparin 40 mg q.d. (6.8% (TKR) and 11.0% (THR)).24 Furthermore, a pooled statistical analysis of major venous thromboembolism (VTE) and bleeding using multivariate logistic regression yielded no convincing evidence that age, weight, gender, or creatinine clearance individually influenced the balance of benefit to risk for apixaban vs. enoxaparin, which is consistent with the findings of the current analysis.25
The relationship between apixaban exposure and efficacy was explored, but a statistically significant relationship could not be detected. This is likely because of a shallow exposure–response relationship (The slope of the exposure–response relationship for VTE was estimated to be 0.0499 ± 0.0578 ml/μg/h) and the low rate of VTE events; however, a recently published, model-based meta-analysis of different anticoagulant treatments for VTE prevention demonstrated a reduction in VTE events and an increase in bleeding events as a function of the dose of factor Xa inhibitors.26 The bleeding frequency reported in this meta-analysis for apixaban was similar to that reported for the current study.
In conclusion, the use of a model-based approach facilitated integration of PK and bleeding data from clinical trials of apixaban in a manner that permitted prediction of bleeding risk in clinically relevant scenarios and subpopulations of TKR and THR subjects. The bleeding probability for apixaban 2.5 mg b.i.d. is expected to increase by less than 1% increase even in subjects whose apixaban exposure is potentially increased because of impaired renal function, low body weight, old age, and/or gender.27
Methods
Study populations and data
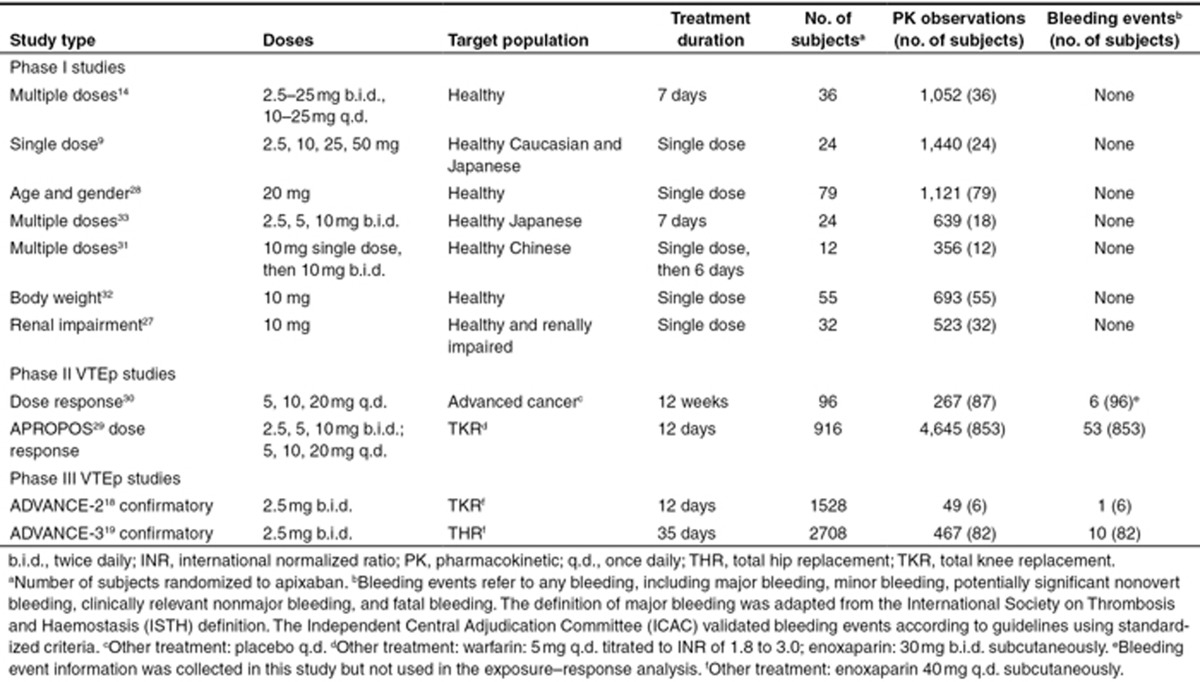
Data for apixaban population PK and exposure–response analyses were obtained from 11 clinical studies (Table 2).9,14,18,19,28,29,30,31,32,33,34 All available PK data from 11 studies have been used for population PK analysis. Exposure–bleeding analysis was conducted using data from one phase II study and two phase III studies. Because no VTE events occurred in the subset of subjects (n = 88) from the phase III studies contributing data to the population PK analysis, an exposure–efficacy analysis was not performed. An exploratory exposure–response analysis based on phase II data was previously reported.23 Intensive PK samples were collected for up to 72 h postdose for the phase I studies, and sparse samples were collected for the phase II and III studies. Plasma apixaban concentration was quantified using a validated liquid chromatography–tandem mass spectrometry method, with a lower limit of quantification of 1 ng/ml and calibration curves ranging from 1 to 1,000 ng/ml.17 Bleeding events included major bleeding, minor bleeding, potentially significant nonovert bleeding, clinically relevant nonmajor bleeding, and fatal bleeding (i.e., collectively called any bleeding, and hereafter, referred to as bleeding). Only events that occurred after the first dose of apixaban were included. For individuals with more than one bleeding event, only the first recorded event was used.
Table 2. Summary of studies and data used for population pharmacokinetic and exposure–bleeding analyses.
Population PK analysis
The relationship of apixaban dose with exposure was characterized by a nonlinear mixed-effects (“population”) compartmental model using NONMEM (version VI, level 1.1; GloboMax LLC, Hanover, MD). Before the evaluation of covariate effects, a parsimonious, base population PK model was selected using the Schwartz Bayesian Criterion to facilitate selection between hierarchical and nonhierarchical submodels35:
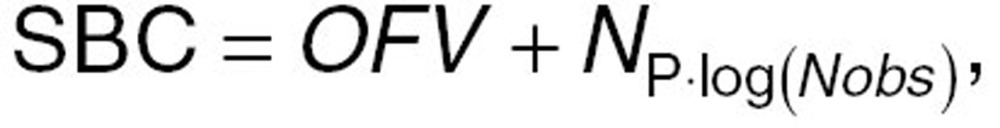 |
where OFV, objective function value, is equivalent to –2 log-likelihood of the data given in the model, NP is the number of parameters in the model, and Nobs is the number of observations in the dataset. In addition, diagnostic plots were evaluated to identify any trends in the base model predictions or residuals that indicate poor fit of the model to the data.
Alternative structural models were assessed to identify the one that could most appropriately describe the PKs of apixaban. Because renal elimination is known to account for approximately one third of apixaban clearance,13,15 the effect of cCrCL on the CL/F of apixaban was incorporated into the base model. cCrCL was derived from Cockroft-Gault equation.36 An EMAX model was used to describe the relationship between cCrCL and apixaban CL/F:
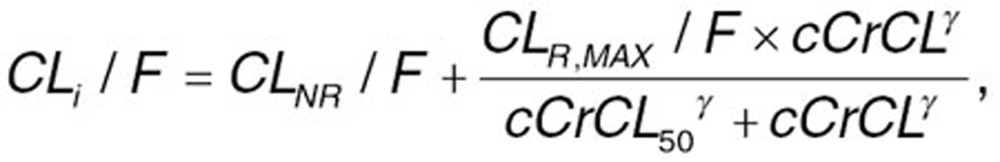 |
 |
where CLNR/F is the nonrenal component of apparent plasma clearance of apixaban, CLR,MAX/F is the maximal renal component of apparent plasma clearance of apixaban, cCrCL50 is the value of cCrCL at which 50% of the maximum renal clearance of apixaban occurs, and ã is the shape parameter that describes the steepness of the relationship between cCrCL and renal clearance. Incorporation of this function to separate the renal and nonrenal components of total apparent plasma clearance of apixaban allows the estimation of the effects of covariates that may impact the nonrenal clearance of apixaban, but are highly correlated with cCrCL (e.g., age, body weight, and gender).
Given the dissolution rate-limited absorption of apixaban at doses >10 mg,37 the loss of dose-proportional exposure as a function of dose was incorporated into the base model as a reduction in bioavailability of higher doses of apixaban relative to the 2.5 mg dose (Frel) as a continuous function of dose using the following equation:
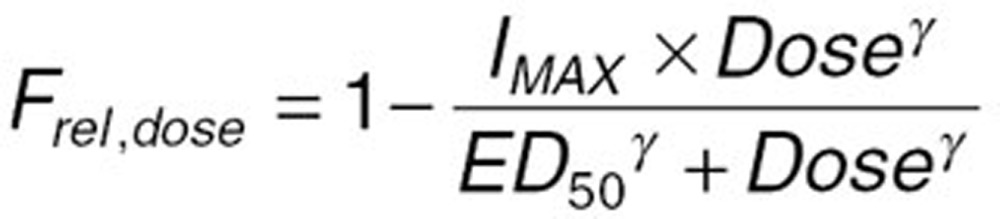 |
where Frel,dose is the relative bioavailability of a given dose (Dose), IMAX is the maximum reduction in relative bioavailability, ED50 is the dose at which half of the maximal reduction in Frel,dose is achieved, and ã is the shape parameter that controls the shape of the relationship between dose and Frel,dose.
Once the base model was established, the full-covariate model was developed by incorporating all potentially significant covariates. The covariate–parameter relationships were screened both visually and statistically. The relationship between the typical value of a parameter (PTV) and a continuous covariate (R) was tested using the following relationship:
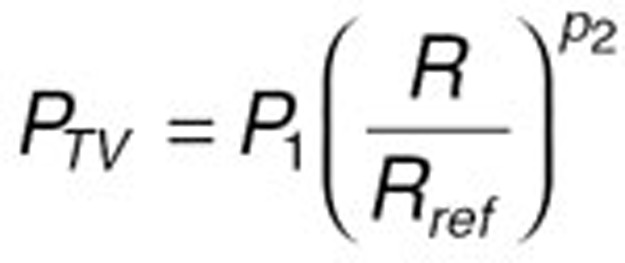 |
where P1 and P2 are fixed-effect parameters and Rref is a reference value of the covariate. The median value for the TKR and THR populations combined was used as the reference value for continuous covariates.
The proportional relationship between the typical value of a parameter (PTV) and a categorical covariate was tested using the following relationship:
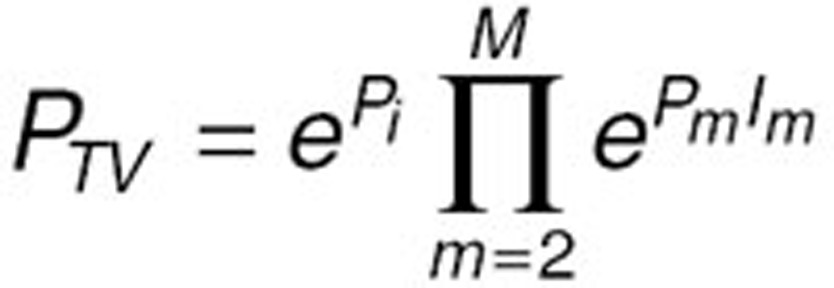 |
where Pm (m=1, ..., M) are fixed-effect parameters and Im is an indicator variable where Im=1 for the mth category, and 0 otherwise.
The population PK model was developed from the full-covariate model by excluding nonsignificant covariate–parameter relationships to provide the most parsimonious model. A stepwise backward elimination procedure was used to retain only significant covariates as determined via Schwartz Bayesian Criterion in the model. Five hundred bootstrap replications of the original dataset and model estimation were generated to obtain robust confidence intervals for the final model parameter estimates. The final model was evaluated using both visual and quantitative predictive checks by comparing model-predicted values with observed data. Predictive checks were performed with 500 Monte Carlo-simulated datasets obtained from the population PK model, as described previously.38
Exposure–bleeding analysis
To model the relationship between apixaban exposure and bleeding events, a Cox proportional hazards time-to-event analysis was performed. This approach allows incorporation of the timing of the bleeding event, in addition to the degree of apixaban exposure, when characterizing the exposure–response relationship. Cox proportional hazards modeling was performed in Spotfire S-Plus (version 8.1 for Linux, Insightful Corporation, Seattle, WA).
A complete model development procedure to investigate all available covariate effects was not performed in the current analysis because this had been conducted in previously reported work.23 As with the previously published analysis, daily AUCss was used as the exposure measure to predict bleeding risk. A full model approach was used in which all covariates were incorporated into the model simultaneously, as well as all potential interactions between the covariates and the exposure parameter. The equation for the full model, stratified by surgery type (THR vs. TKR), is shown below:
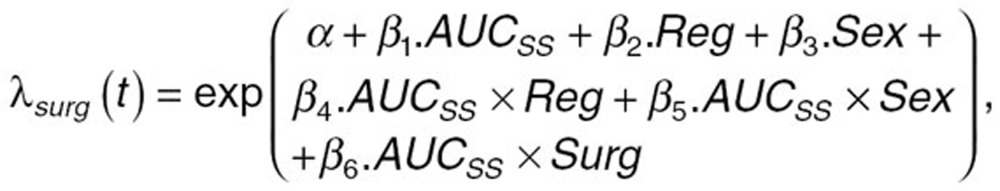 |
where λSurg is the frequency of the bleeding events as a function of time, apixaban exposure, and surgery type; á is the baseline hazard function; â1 is the estimate of the effect of apixaban exposure (AUCss) on the rate of bleeding; â2 is the estimate of the effect of dosing regimen (Reg) on the rate of bleeding; and â3 is the estimate of the effect of gender (Sex) on the rate of bleeding. The interactions between AUCss and the other predictor variables were also included in the full model and are represented by the coefficients â4 (AUCss × Reg) and â5 (AUCss × Sex). Surgery type (THR vs. TKR) was used as a stratification variable to account for any potential differences between the two populations that are not related to the covariate effects of interest. Therefore, the interaction between the stratification variable and AUCss was also tested [â6 (AUCss × Surg)].
A stepwise backward elimination was performed to obtain a final parsimonious model. Because hierarchical models were evaluated, model selection was based on the likelihood ratio test, with a critical P value of 0.01, which translates to a change in −2 times the log-likelihood of 6.63 for 1 degree of freedom based on a chi-squared test. After arriving at the final model, alternate functional forms for incorporation of the effect of apixaban daily AUCss were tested; these included the log AUCss, the restricted cubic spline of AUCss, and the second-order polynomial transformation of AUCss.
Model evaluation was performed with a predictive check. The final model was evaluated by comparing the observed proportion of subjects achieving a safety event with the 95% prediction interval of the exposure–safety relationship. The 95% prediction interval was obtained from the variance–covariance matrix defined by 1,000 bootstrap replications of the final model.
Model-based simulations
To determine the range of apixaban exposure that may be expected in the TKR and THR populations, 500 trials of 10,000 subjects each treated with the 2.5-mg b.i.d. regimen were simulated using the population PK model. Each individual trial simulation was conducted with a new set of population parameters that were sampled from the variance–covariance matrix of the 500 bootstrap parameter estimates from the final model. In addition, a new set of covariates were produced for each trial by sampling from the means and variance–covariance matrix of the covariates in the phase III TKR and THR population samples.
To determine the exposures that may be expected in subpopulations of the TKR and THR population, 500 trials of 50,000 subjects each treated with the apixaban 2.5-mg b.i.d. regimen were simulated from the population PK model, as described above. Daily AUCss was calculated for each individual in each simulation using the individual plasma CL/F estimates.
To determine the bleeding risk in specific subpopulations relative to the reference population, the simulated daily AUCss (median, 5th and 95th percentiles) described above was used as input to the exposure–bleeding model to predict the range of bleeding probabilities to be expected in these populations. The reference population for clinical trial simulations was defined as male and female subjects 65–75 years of age with a cCrCL ≥80 ml/min and body weight of 65–85 kg. Confidence intervals were calculated for each estimated bleeding probability using the variance–covariance matrix derived from 1,000 bootstrap replications of the exposure–bleeding model.
Conflict of interest
T.A.L., C.F., X.W., and F.L. are employees of Bristol-Myers Squibb. M.P. was an employee of Bristol-Myers Squibb at the time of research.
Author contributions
T.A.L., C.F., X.W., M.P., and F.L. wrote the manuscript and analyzed the data.
Study Highlights
Acknowledgments
This study was sponsored by Bristol-Myers Squibb Company and Pfizer. Editorial assistance was provided by Dana Fox, PhD, CMPP (Caudex Medical, New York, NY), and funded by Bristol-Myers Squibb and Pfizer.
Supplementary Material
References
- Geerts W.H., et al. American College of Chest Physicians Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- Hirsh J., Bauer K.A., Donati M.B., Gould M., Samama M.M., Weitz J.I. American College of Chest Physicians Parenteral anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:141S–159S. doi: 10.1378/chest.08-0689. [DOI] [PubMed] [Google Scholar]
- Martel N., Lee J., Wells P.S. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106:2710–2715. doi: 10.1182/blood-2005-04-1546. [DOI] [PubMed] [Google Scholar]
- Morris T.A., Castrejon S., Devendra G., Gamst A.C. No difference in risk for thrombocytopenia during treatment of pulmonary embolism and deep venous thrombosis with either low-molecular-weight heparin or unfractionated heparin: a metaanalysis. Chest. 2007;132:1131–1139. doi: 10.1378/chest.06-2518. [DOI] [PubMed] [Google Scholar]
- Ansell J., Hirsh J., Hylek E., Jacobson A., Crowther M., Palareti G. American College of Chest Physicians Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- Harris J.E. Interaction of dietary factors with oral anticoagulants: review and applications. J. Am. Diet. Assoc. 1995;95:580–584. doi: 10.1016/S0002-8223(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Hawkins D. Limitations of traditional anticoagulants. Pharmacotherapy. 2004;24:62S–65S. doi: 10.1592/phco.24.10.62s.36120. [DOI] [PubMed] [Google Scholar]
- Rewinkel J.B., Adang A.E. Strategies and progress towards the ideal orally active thrombin inhibitor. Curr. Pharm. Des. 1999;5:1043–1075. [PubMed] [Google Scholar]
- Frost C., et al. Apixaban, an oral, direct factor Xa inhibitor: single dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br. J. Clin. Pharmacol. 2013;75:476–487. doi: 10.1111/j.1365-2125.2012.04369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D.J., et al. Discovery of 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide (apixaban, BMS-562247), a highly potent, selective, efficacious, and orally bioavailable inhibitor of blood coagulation factor Xa. J. Med. Chem. 2007;50:5339–5356. doi: 10.1021/jm070245n. [DOI] [PubMed] [Google Scholar]
- Wong P.C., et al. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J. Thromb. Haemost. 2008;6:820–829. doi: 10.1111/j.1538-7836.2008.02939.x. [DOI] [PubMed] [Google Scholar]
- Bristol-Myers Squibb.Eliquis® (apixaban tablets) Prescribing information . < http://packageinserts.bms.com/pi/pi_eliquis.pdf > ( 2013
- Frost C., et al. Apixaban, a direct factor Xa inhibitor: single-dose pharmacokinetics and pharmacodynamics of an intravenous formulation [abstract 148] J. Clin. Pharmacol. 2008;48:1132. [Google Scholar]
- Frost C., et al. Safety, pharmacokinetics and pharmacodynamics of multiple oral doses of apixaban, a factor Xa inhibitor, in healthy subjects. Br. J. Clin. Pharmacol. 2013;76:776–786. doi: 10.1111/bcp.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakkalagadda B., et al. Effect of rifampin on the pharmacokinetics of apixaban, an oral direct inhibitor of factor Xa [abstract] J. Clin. Pharmacol. 2009;49:1091–1130. doi: 10.1007/s40256-015-0157-9. [DOI] [PubMed] [Google Scholar]
- Zhang D., et al. Investigating the enteroenteric recirculation of apixaban, a factor Xa inhibitor: administration of activated charcoal to bile duct-cannulated rats and dogs receiving an intravenous dose and use of drug transporter knockout rats. Drug Metab. Dispos. 2013;41:906–915. doi: 10.1124/dmd.112.050575. [DOI] [PubMed] [Google Scholar]
- Raghavan N., et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab. Dispos. 2009;37:74–81. doi: 10.1124/dmd.108.023143. [DOI] [PubMed] [Google Scholar]
- Lassen M.R., Raskob G.E., Gallus A., Pineo G., Chen D., Hornick P. ADVANCE-2 investigators Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375:807–815. doi: 10.1016/S0140-6736(09)62125-5. [DOI] [PubMed] [Google Scholar]
- Lassen M.R., Gallus A., Raskob G.E., Pineo G., Chen D., Ramirez L.M. ADVANCE-3 Investigators Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N. Engl. J. Med. 2010;363:2487–2498. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- Lassen M.R., Raskob G.E., Gallus A., Pineo G., Chen D., Portman R.J. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N. Engl. J. Med. 2009;361:594–604. doi: 10.1056/NEJMoa0810773. [DOI] [PubMed] [Google Scholar]
- Kennedy J.M., Riji A.M. Effects of surgery on the pharmacokinetic parameters of drugs. Clin. Pharmacokinet. 1998;35:293–312. doi: 10.2165/00003088-199835040-00003. [DOI] [PubMed] [Google Scholar]
- Grosflam J.M., Wright E.A., Cleary P.D., Katz J.N. Predictors of blood loss during total hip replacement surgery. Arthritis Care Res. 1995;8:167–173. doi: 10.1002/art.1790080309. [DOI] [PubMed] [Google Scholar]
- Leil T.A., Feng Y., Zhang L., Paccaly A., Mohan P., Pfister M. Quantification of apixaban's therapeutic utility in prevention of venous thromboembolism: selection of phase III trial dose. Clin. Pharmacol. Ther. 2010;88:375–382. doi: 10.1038/clpt.2010.106. [DOI] [PubMed] [Google Scholar]
- Bristol-Myers Squibb and Pfizer EEIG. Eliquis® (apixaban tablets) Summary of product characteristics . < http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002148/WC500107728.pdf >.
- Pineo G.F., et al. Apixaban after hip or knee arthroplasty versus enoxaparin: efficacy and safety in key clinical subgroups. J. Thromb. Haemost. 2013;11:444–451. doi: 10.1111/jth.12109. [DOI] [PubMed] [Google Scholar]
- Mandema J.W., Boyd R.A., DiCarlo L.A. Therapeutic index of anticoagulants for prevention of venous thromboembolism following orthopedic surgery: a dose-response meta-analysis. Clin. Pharmacol. Ther. 2011;90:820–827. doi: 10.1038/clpt.2011.232. [DOI] [PubMed] [Google Scholar]
- Pineo G.F., et al. Apixaban after hip or knee arthroplasty versus enoxaparin: efficacy and safety in key clinical subgroups. J. Thromb. Haemost. 2013;11:444–451. doi: 10.1111/jth.12109. [DOI] [PubMed] [Google Scholar]
- Chang M., et al. Apixaban pharmacokinetics and pharmacodynamics in subjects with renal impairment. 2012 ACCP Annual Meeting (Abstract 1381212) Clin Pharmacol Drug Dev. 2012;1:185–186. [Google Scholar]
- Frost C., Nepal S., Barrett Y., LaCreta F. Effects of age and gender on the single-dose pharmacokinetics (PK) and pharmacodynamics (PD) of apixaban. J. Thromb. Haemost. 2009;7:PP-MO-407. [Google Scholar]
- Lassen M.R., Davidson B.L., Gallus A., Pineo G., Ansell J., Deitchman D. The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement. J. Thromb. Haemost. 2007;5:2368–2375. doi: 10.1111/j.1538-7836.2007.02764.x. [DOI] [PubMed] [Google Scholar]
- Levine M.N., et al. A randomized phase II trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer. J. Thromb. Haemost. 2012;10:807–814. doi: 10.1111/j.1538-7836.2012.04693.x. [DOI] [PubMed] [Google Scholar]
- Cui Y., et al. Single- and multiple-dose pharmacokinetics, pharmacodynamics, and safety of apixaban in healthy Chinese subjects. Clin. Pharmacol. 2013;5:177–184. doi: 10.2147/CPAA.S51981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upreti V.V., et al. Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br. J. Clin. Pharmacol. 2013;76:908–916. doi: 10.1111/bcp.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamahira N., et al. A placebo-controlled, ascending multiple-dose study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of apixaban in healthy Japanese subjects. (A637) Can. J. Clin. Pharmacol. 2008;15:e420–e781. [Google Scholar]
- Lavielle M., Mentré F. Estimation of population pharmacokinetic parameters of saquinavir in HIV patients with the MONOLIX software. J. Pharmacokinet. Pharmacodyn. 2007;34:229–249. doi: 10.1007/s10928-006-9043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft D.W., Gault M.H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Yu Z., Nepal S., Bragat A., Shenker A., Frost C. Single dose apixaban pharmacokinetics and pharmacodynamics in healthy male Japanese and Caucasian subjects (A647) Can. J. Clin. Pharmacol. 2008;15:e420–e781. doi: 10.2147/CPAA.S169505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibiansky L., Gastonguay M.R.R/NONMEM Toolbox for simulation from posterior parameter (uncertainty) distributions (Abstract 958) PAGE . < http://www.page-meeting.org/?abstract=958 > ( 2006
- Barrett Y.C., et al. A randomised assessment of the pharmacokinetic, pharmacodynamic and safety interaction between apixaban and enoxaparin in healthy subjects. Thromb. Haemost. 2012;107:916–924. doi: 10.1160/TH11-09-0634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.