Abstract
Background
The results of several studies suggest that there may be common neurocircuits regulating drug-seeking behaviors. Common biological pathways regulating drug-seeking would explain the phenomenon that seeking for one drug can be enhanced by exposure to another drug of abuse. The objective of the current study was to assess the time-course effects of acute cocaine administration on alcohol seeking and relapse.
Methods
Alcohol-Preferring (P) rats were allowed to self-administer 15% ethanol (EtOH) and water. EtOH-seeking was assessed through use of the Pavlovian Spontaneous Recovery (PSR) model, while relapse EtOH drinking was assessed through use of the alcohol deprivation effect.
Results
Cocaine (0, 1 or 10 mg/kg), injected immediately, 30 min, or 4 hr prior to the 1st PSR testing session, dose-dependently increased responding on the EtOH lever compared to extinction responses and responding by saline controls. Under relapse conditions, cocaine given immediately prior to the relapse session had no effect (1 mg/kg) or reduced responding (10 mg/kg). In contrast, cocaine given 4 hr prior to the relapse session markedly enhanced EtOH responding compared to saline.
Conclusion
The enhanced expression of EtOH-seeking and relapse behaviors may be a result of a priming effect of cocaine on neuronal circuits mediating these behaviors. The effect of cocaine on EtOH-relapse drinking is indicative of the complex interactions that can occur between drugs of abuse; production of conflicting behaviors (immediate) and priming of relapse/seeking (4 hour delay).
Keywords: Alcohol Preferring P rats, Cocaine, EtOH relapse, EtOH-seeking, Pavlovian Spontaneous Recovery
INTRODUCTION
The ability of drugs of abuse to elicit seeking for another drug of abuse is commonly observed in human research. For example, access to one drug (cocaine or alcohol) during abstinence in polydrug abusers increased self-reported craving for both cocaine and alcohol (Fox et al., 2005). Marijuana exposure can increase self-reported craving for alcohol, cocaine, tobacco, and other drugs of abuse (Fox et al., 2013; Filbery and DeWitt, 2012). Alcohol consumption can increase the urge to smoke, and smoking or exposure to smoking cues can increase the urge to consume alcohol (Sayette et al., 2005).
The cross-reactivity between drugs of abuse to elicit craving suggests there are common mechanisms/pathways for the behavior. A quantitative meta-analysis of the response to drug cues indicated that there was a convergence of activity within the anterior cingulate cortex, right pallidum, and the mesolimbic dopamine system (ventral tegmental area and nucleus accumbens) for alcohol, cocaine, and nicotine craving (Kuhn and Gallinat, 2011). The areas determined to mediate craving may overlap with some areas considered to regulate drug reward.
The hypothesis that there are common biological pathways promoting drug craving includes an assertion that any compound that activates these neurocircuits should enhance drug-seeking behaviors. The overlap between the neurocircuitries regulating drug reward and drug-seeking has led to the postulation that exposure to a reinforcer that acts within the drug reward system could stimulate learned drug-seeking behaviors which would increase the likelihood of relapse drug use.
There are several reports indicating a common genetic predisposition to use cocaine and alcohol, cross-sensitivity between the two drugs, an interaction between drug use, and cross-reactivity for the expression of drug seeking. For example, there is a high prevalence rate for alcoholics to have co-dependency for both alcohol and cocaine (Miller et al., 1989; Stinson et al., 2005) and cocaine users are more likely to be diagnosed with alcoholism (Rounsaville et al., 1991). Between 50% and 90% of cocaine users reported co-administering EtOH during cocaine binges (Brookoff et al., 1996; Magura and Rosenblum, 2000). Co-administration of alcohol during cocaine binges allows the user to prolong the euphoric effects and diminish the anxiogenic effects of cocaine (Williamson et al., 1997). Mikkola et al. (2001) reported that the mesolimbic dopamine (DA) system of alcohol preferring Alko Alcohol (AA) rats is more readily sensitized to the effects of cocaine compared to the Alko Non-Alcohol (ANA) rats. Selective breeding for high alcohol preference resulted in a greater sensitivity to the reinforcing properties of cocaine within the nucleus accumbens shell (AcbSh) of alcohol-preferring (P) rats compared to Wistar rats (Katner et al., 2011).
Intravenous (i.v.) self- administration of cocaine can increase EtOH intake (Knackstedt et al., 2006). Pre-exposure to combined i.v. EtOH + cocaine solution enhanced responding for EtOH (Ikegami et al., 2002). Systemic administration of cocaine resulted in decreases (Uemura et al., 1998) or had no effect (Cailhol and Mormede, 2000) on EtOH intake. Collectively, these studies show that cocaine effects on EtOH intake are complex and may vary depending on the experimental protocol. Furthermore, these studies did not examine the effects of cocaine on EtOH-seeking or EtOH intake under relapse drinking conditions.
To better understand the complex interactions between drugs of abuse on seeking and relapse behaviors a temporal analysis of any effect should occur. The ability of priming exposure to drugs of abuse to have persistent effects on drug-seeking has not been adequately examined. The effects of nicotine on alcohol-seeking and relapse in P rats were dependent on the interval between nicotine administration and testing (Hauser et al., 2012). Therefore, it would be important to determine if the effects of cocaine on alcohol seeking and relapse are limited to a short time frame or can persist for hours.
The objective of the current experiments was to determine the effects of cocaine on EtOH-seeking and –relapse drinking in rats that readily self-administer pharmacologically relevant levels of alcohol. EtOH-seeking was assessed with the Pavlovian Spontaneous Recovery (PSR) model, a context-induced drug-seeking paradigm (c.f., Rodd-Henricks et al., 2002a, b). EtOH-relapse drinking was assessed through the use of the alcohol-deprivation effect paradigm; increase in alcohol consumption following a period of deprivation (c.f., McKinzie et al., 1998; Rodd-Henricks et al., 2000; Rodd et al., 2003). In addition, a temporal analysis for the effects of cocaine on EtOH-related behaviors was included in the experimental design.
MATERIALS AND METHODS
Animals
Adult female P rats from the 55th – 56th generations weighing 250–325g at the start of the experiment were used. Rats were maintained on a 12-hr reversed light-dark cycle (lights off at 0900 hr). Food and water were available ad libitum throughout the experiment, except during operant testing. The animals used in these experiments were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All research protocols were approved by the institutional animal care and use committee and are in accordance with the guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council 1996).
Operant Training
P rats were placed in the standard two-lever operant chamber, as previously described (Rodd-Henricks et al., 2002a, b). Operant sessions were 60 min in duration and occurred daily for 10 weeks (Rodd et al., 2006). The EtOH concentration used during self-administration was 15% (vol/vol). During the initial 4 weeks of daily operant access, both solutions (EtOH and water) were reinforced on a fixed ratio 1 (FR1) schedule. The response requirement for EtOH was increased to an FR3 schedule for 3 weeks, and then to FR5 schedule for 3 weeks. After the P rats had established stable levels of responding on the FR5 schedule for EtOH and FR1 for water, they underwent 7 sessions of extinction (60 min/session), when neither water nor EtOH was available (Hauser et al., 2012; Rodd et al., 2006).
Pavlovian Spontaneous Recovery (PSR) testing
After extinction training, all rats were maintained in the home cages for 14 days, before being returned to the operant chambers for PSR testing . The FR5-FR1 schedule, lever contingencies and dipper functioning were maintained, but EtOH and water were absent during the 60 min/session, which were conducted for 4 consecutive sessions (Hauser et al., 2012; Rodd et al., 2006). Four PSR sessions were conducted because previous studies showed that exposure to EtOH odor cues or EtOH priming (Rodd-Henricks et al., 2002a, b) and some drugs (Dhaher et al., 2010) may enhance PSR responding for more than one session.
2.4 Relapse testing
Following the PSR phase of the experiment, all rats were maintained in the home cages for 7 days. Rats were then transferred to the operant chambers with both 15% EtOH and water available for the 60 min sessions and the FR5-FR1 schedule lever contingencies and dipper functioning were maintained, as previously described (Hauser et al., 2012; Rodd et al., 2006).
Cocaine Effects on EtOH-Seeking and Relapse Drinking
Cocaine HCl was provided by the NIDA Drug Supply Program (Research Triangle Park, NC). Cocaine HCl was dissolved in saline. The i.p. doses of cocaine used were 0, 1 and 10 mg/kg. Following extinction training, P rats were randomly assigned to groups that received injections of cocaine (1 or 10 mg/kg) immediately, 30 min, or 4 hr prior to only the 1st PSR test session (n = 7–8/dose/time point). To reduce the number of rats used in the experiment, the saline group consisted of 12 rats equally distributed at each time point (n = 4/time point).
The same rats were also used to test the effects of cocaine during relapse responding, using a counterbalanced design (i.e., rats that were administered 1 mg/kg cocaine immediately prior to PSR test sessions were randomly assigned to separate groups that received vehicle or one of the 2 doses of cocaine for all time points during relapse testing). For relapse testing, rats received 1 or 10 mg/kg cocaine (n = 7–8/group/time point) immediately, 30 min or 4 hr prior to the first relapse session. Similarly, to reduce the number of rats used in the experiment, the saline group consisted of 12 rats equally distributed at each time point (n = 4/time point).
2.6 Statistical Analyses
Overall operant responses (60 min) on the EtOH and water levers were analyzed with a mixed factorial ANOVA with a between subject factors of dose and time point and a repeated measure of ‘session’. For the PSR experiments, the baseline measure for the factor of ‘session’ was the average number of responses on the EtOH (or water) lever for the last 3 extinction sessions. For the relapse studies, the baseline measure for the factor of ‘session’ was the average number of responses on the EtOH (or water) lever for the 3 sessions immediately prior to extinction training. Operant EtOH responding data were also analyzed in 10-min blocks, which required the additional repeated measure of time. Post-hoc Tukey’s b tests were performed to determine individual differences.
RESULTS
Cocaine Effects on EtOH-Seeking
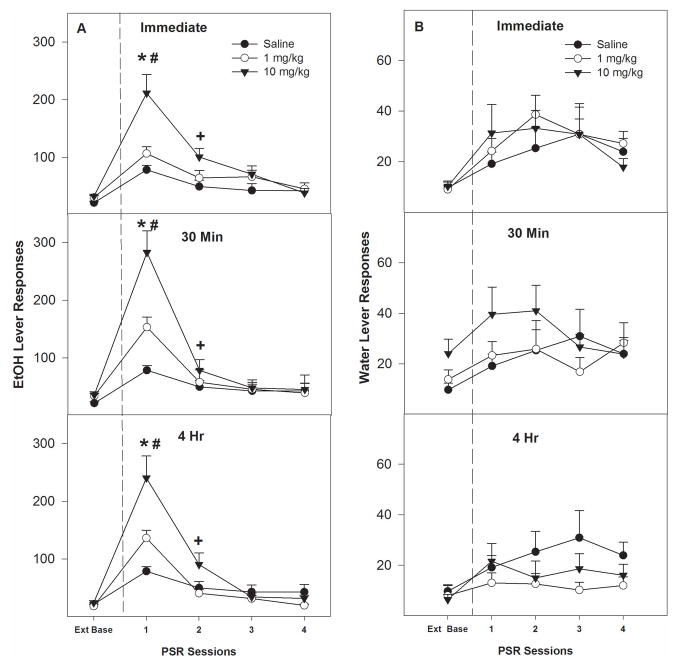
In general, i.p. cocaine increased EtOH-seeking behavior during the 1st PSR session in a dose-dependent manner at all 3 time points (Fig. 1A). All groups responded comparably during the last 3 extinction sessions (p = 0.74). During PSR testing, examining the number of responses on the lever previously associated with the delivery of EtOH (Fig. 1A) indicated a significant effect of ‘session’ (F4,43 = 44.6; p < 0.001), ‘dose’ (F2,46 = 12.3; p < 0.001), and a ‘session’ by ‘dose’ interaction (F8, 88 = 4.5; p < 0.001), but no effect of ‘time of injection’ (p > 0.096). Rats treated with saline (all time points collapsed) displayed the typical increase in responding on the lever previously associated with the delivery of EtOH during the 1st PSR test session (p < 0.001). Regardless of the time that cocaine was administered prior to PSR testing, cocaine increased EtOH-seeking (Fig. 1A). Collapsing over all ‘time of injection’ groups, there was a significant effect of ‘dose’ for the 1st and 2nd PSR test session (F 2, 52 = 30.1; p < 0.001; F 2, 52 = 8.2; p < 0.001). Post-hoc comparisons (Tukey’s b) indicated that, during the 1st PSR session, all groups were different from each other (Fig. 1A; p < 0.001). During the 2nd PSR session, the group administered 10 mg/kg cocaine before the 1st PSR session was different from saline treated group (Fig. 1A; p < 0.001).
Fig. 1.
(A) Mean (±S.E.M.) responses per session on the lever previously associated with the delivery of EtOH in P rats given i.p. saline (n = 4/time point) or 1 or 10 mg/kg cocaine (n = 7–8/dose/time point) immediately, 30 minute or 4 hour prior to only the 1st PSR session. (*) indicates that rats administered saline or cocaine immediately, 30 minute or 4 hour prior to the 1st PSR responded significantly (p < 0.05) more on the EtOH lever during the 1st PSR session compared to extinction baseline levels. (#) indicates higher responding by the 10 mg/kg cocaine group during the1st PSR session compared to the saline and 1 mg/kg cocaine groups (p<0.05), and responding by the 1 mg/kg cocaine group was higher than the saline group. Plus (+) indicates that the 10 mg/kg group had higher EtOH responding during the 2nd PSR session compared to extinction baseline levels. (B) Mean (±S.E.M.) responses per session on the lever previously associated with the delivery of water in P rats given i.p. saline (n = 4/time point) or 1 or 10 mg/kg cocaine (n = 7–8/dose/time point) immediately, 30 minute or 4 hour prior to only the 1st PSR session.
Analysis of responses on the lever previously associated with water revealed that there was a significant effect of ‘session’ (F4, 32 = 14.0; p < 0.01), but there was no significant ‘dose’ (F 2, 35 = 0.27; p = 0.77), or ‘dose’ x ‘session time’ interaction (F 8, 66 = 1.19; p = 0.32). There were no significant differences between the saline group compared to either cocaine groups at any time point (Fig. 1B).
Time Course Effects of Cocaine on EtOH-Seeking
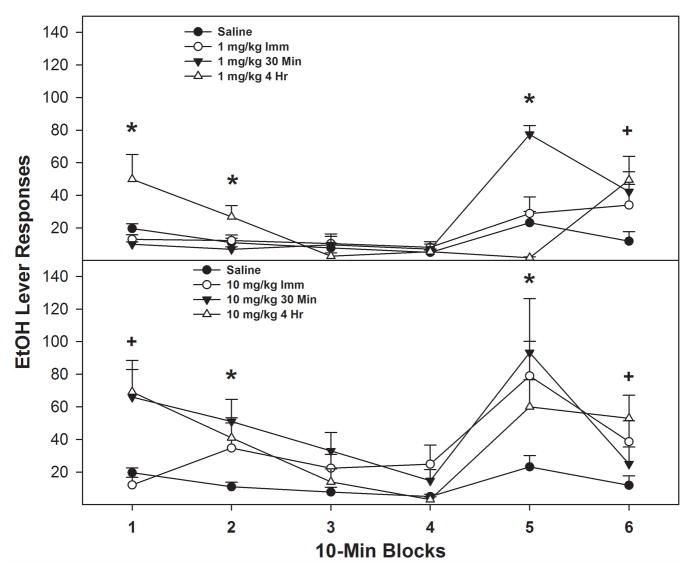
Examining the time course effects of cocaine within the 1st PSR session (Fig. 2) by holding ‘doses’ constant indicated a significant ‘time of injection’ x ‘session time’ interaction (F 10,32 = 4.6; p < 0.001) in rats administered either 1 or 10 mg/kg cocaine (saline group was not different, p = 0.56). For both cocaine groups, the administration of cocaine 4 hr prior to PSR testing stimulated EtOH lever responding within the first 20-min period (Fig. 2; top and bottom panels). Cocaine administered immediately or 30 min prior to PSR testing increased EtOH lever responding later in the operant session (40–60-min time period). Individual ANOVAs performed for each time block indicated that in rats administered 1 mg/kg: a) the 4-hr group responded more than all other groups during the 0–20-min time period; b) the 30-min group increased EtOH lever responding during the 40–50-min time period compared to all other groups; and c) during the 50–60-min time period, all cocaine temporal groups responded more than saline controls. In rats administered 10 mg/kg cocaine, the time block analysis indicated that: a) the 30-min or 4-hr group displayed increased EtOH lever presses during the 0–10-min period, b) all temporal groups displayed increased responding during the 10–20 and 40–50-min time periods compared to saline; and c) the 4-hr group displayed elevated EtOH lever responding during the 50–60-min time period compared to the saline group.
Fig. 2.
Mean (±S.E.M.) responses per 10-minute block during the 1st PSR test session on the lever previously associated with the delivery of EtOH in P rats given i.p. saline (n = 4/time point) or 1 or 10 mg/kg cocaine (n = 7–8/dose/time point) immediately, 30 minute or 4 hour prior to session. Upper Panel: (*) indicates that rats administered 1 mg/kg cocaine 4 hour prior to the session responded significantly (p < 0.05) more on the EtOH lever during 0–20 minute of PSR testing compared to all the other group, whereas 1 mg/kg cocaine administered 30 minute prior to PSR testing increased EtOH lever responses during 40–50-minute time period compared to all other groups. (+) indicates that, during the 6th 10-minute block, all temporal groups that received 1 mg/kg responded more than saline group. Lower Panel: (*) indicates that rats administered 10 mg/kg cocaine displayed elevated EtOH responding during the 2nd and 5th 10-minute block compared to saline. (+) indicates that 10 mg/kg cocaine given 30 minute or 4 hour prior to PSR testing displayed increased responding during the 1st 10-minute block and 10 mg/kg cocaine given 4 hour prior to PSR testing elevated EtOH lever presses during the 6th 10-minute block.
Cocaine Effects on EtOH Relapse Drinking
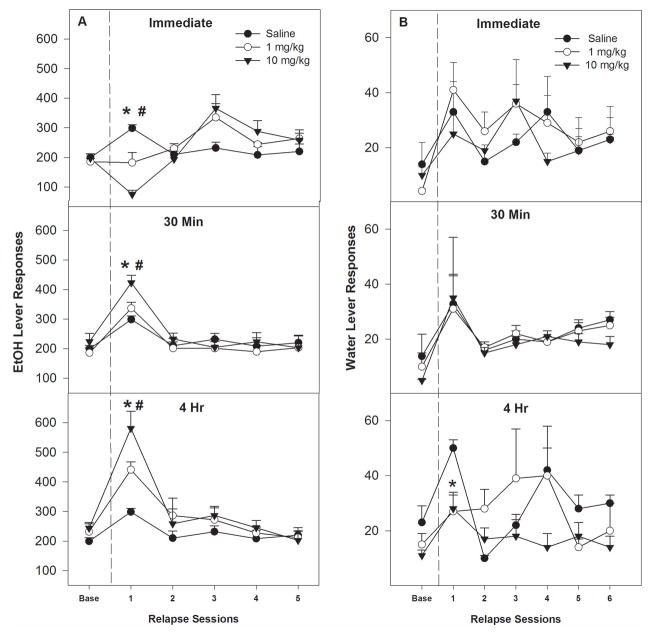
The effects of cocaine on EtOH responding under relapse conditions were dependent upon the dose and time of injection (Fig. 3A). Cocaine administered immediately prior to the 1st relapse test session reduced EtOH responding compared to the saline group, whereas cocaine administered 30 min or 4 hr prior to EtOH relapse testing increased EtOH lever responding compared to the saline group. There was a significant ‘session’ by ‘dose’ by ‘time of injection’ interaction (F20, 180 = 2.0; p = 0.006) and ‘session’ effect (F5, 42 = 23.9; p < 0.001). Decomposing the interaction term by holding ‘time of injection’ constant indicated that, for rats injected immediately prior to the EtOH relapse test session (top panel, Fig. 3A), there was a significant ‘session’ x ‘dose’ interaction (F10,26 = 4.0; p = 0.002). Individual ANOVAs indicated that there was a significant ‘dose’ effect during the 1st relapse session (F2, 16 = 13.0; p < 0.001). Post-hoc comparisons indicated that all groups were different from each other. Comparing baseline EtOH responding to the responding observed during the 1st relapse session for the groups injected immediately prior to the session (top panel, Fig. 3A) indicated: a) saline treated rats increased responding; b) 1 mg/kg cocaine injected rats responded similar to baseline levels; and c) rats administered 10 mg/kg cocaine reduced EtOH self-administration. In rats injected 30 min prior to EtOH relapse (middle panel, Fig. 3A), there was a significant ‘session’ by ‘dose’ interaction (F10, 22 = 3.6; p = 0.037). An ANOVA performed on the 1st relapse session (p = 0.005) indicated that the 10 mg/kg cocaine group responded significantly more than the saline group, but not more than the 1 mg/kg cocaine group. All groups responded more during the 1st relapse session compared to baseline values (p values < 0.008). For rats injected 4 hr prior to EtOH relapse testing (bottom panel, Fig. 3A), there was a significant effect of ‘dose’ during the 1st relapse session (p = 0.006) with post-hoc comparisons indicating that all groups were different from each other and were higher than baseline.
Fig. 3.
(A) Mean (±S.E.M.) responses per session on the EtOH lever by P rats given i.p. saline (n = 4/time point) or 1 or 10 mg/kg cocaine (n = 7–8/dose/time point) immediately, 30 minute or 4 hour prior to only the 1st EtOH relapse session. Upper Panel: (*) indicates that rats administered saline responded significantly (p < 0.05) more on the EtOH lever during the 1st relapse session compared to baseline levels. (#) indicates that 10 mg/kg dose cocaine decreased EtOH lever responding during the 1st relapse session compared to the saline group or baseline values. Middle Panel: (*) indicates that rats administered saline, 1 mg/kg and 10 mg/kg cocaine responded significantly (p < 0.05) more on the EtOH lever during the 1st relapse session compared to baseline levels. (#) indicates that responses by the 10 mg/kg cocaine group during the 1st relapse session were higher than the saline group (p<0.05). Lower Panel: (*) indicates that rats administered saline, 1.0 and 10 mg/kg of cocaine responded significantly (p < 0.05) more on the EtOH lever during the 1st EtOH relapse session compared to baseline levels. (#) indicates that responding by the 10 mg/kg cocaine group was significantly more during the 1st relapse session compared to the saline and 1 mg/kg groups (p<0.05), and responding by the 1 mg/kg cocaine group was higher than the saline group. (B) Mean (±S.E.M.) responses per session on the water lever by P rats given i.p. saline (n = 4/time point) or 1 or 10 mg/kg cocaine (n = 7–8/dose/time point) immediately, 30 minute or 4 hour prior to only the 1st water relapse session. Lower Panel: (*) indicates that rats administered 1 or 10 mg/kg cocaine 4 hours prior to the session responded significantly (p < 0.05) less on the EtOH lever compared to saline.
An additional way to decompose the significant 3-way interaction term would be to hold ‘dose’ constant. In rats injected with cocaine, there was a significant ‘session’ by ‘time of injection’ interaction (F10, 32 = 2.3; p = 0.037); responding in rats injected with cocaine was different between ‘time of injection’ cohorts (significant differences; 4 hr > 30 min > immediate).
The average baseline for EtOH intake for the last 7 days of maintenance prior to the 1st relapse session for the immediate, 30-min and 4-hr groups was estimated to be approximately 1.3 g/kg in the 60-min session. These intakes are similar to those previously reported and should produce blood EtOH levels greater than 80 mg% (Bell et al., 2014). The saline groups’ average EtOH intakes for all time points increased to approximately 1.8 g/kg during the 1st relapse session. The immediate effects of 1 mg/kg of cocaine on EtOH intake remained the same compared to baseline, whereas 10 mg/kg cocaine reduced EtOH intake to 0.4 g/kg during the 1st relapse session. The 30-min and 4-hr delayed effects of cocaine increased the estimated EtOH intakes as high as 2.5 and 3.5 g/kg/session, respectively.
For responses on the water lever during relapse, there was a significant effect of ‘session’ (F7,31 = 7.16; p < 0.05), ‘dose’ (F 2,32 = 4.5; p < 0.05), and ‘session time’ x ‘dose’ interaction (F 14,64 = 2.44; p < 0.05). The ANOVA revealed that water lever responses during 1st relapse session for the 4 hr cocaine group were significantly lower than the saline group on (p < 0.05, Fig. 3B). However, there were no significant differences between groups for the other time points (p > 0.05).
Time Course of Effects of Cocaine on EtOH Relapse Drinking
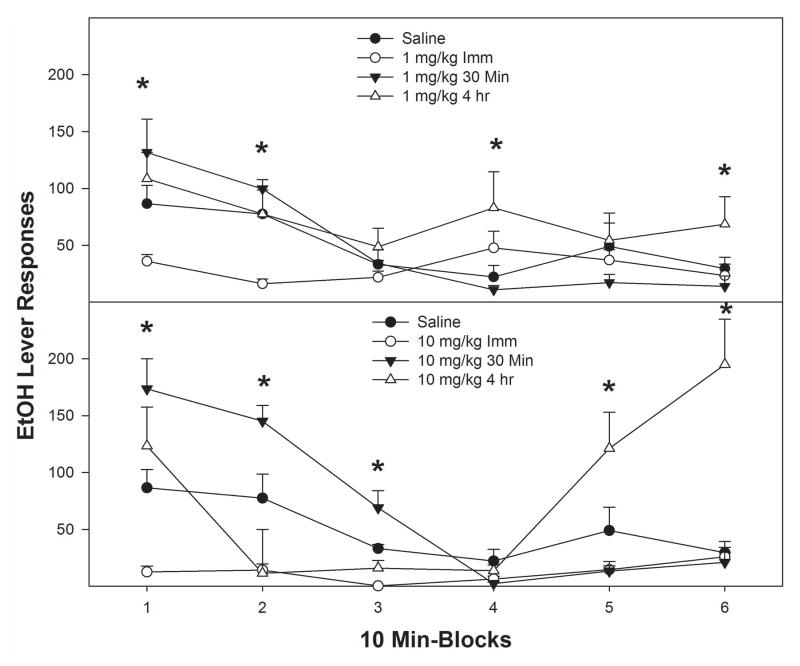
Examining the time course effects of cocaine on EtOH relapse during the 1st relapse session (Fig. 4) indicated that the temporal profile of responding during EtOH relapse was influenced by the dose of cocaine and ‘time of injection’. An analysis of the EtOH relapse responding indicated by holding ‘dose’ constant, there was significant ‘time of injection’ x ‘session time’ interaction (F10,32 > 2.2; p < 0.043) in rats administered either 1 or 10 mg/kg cocaine (saline group was not different, p = 0.68). Cocaine administered immediately prior to EtOH relapse reduced responding throughout the 60 min session (top and bottom panel, Fig. 4). Administering 1 mg/kg cocaine 30 min before the session, increased responding compared to saline during the first 10 min (top panel, Fig. 4). P rats administered 1 mg/kg cocaine 4 hr prior to EtOH relapse increased responding compared to saline during the 4th and 6th 10-min blocks. Rats administered 10 mg/kg cocaine 30 min prior to testing (bottom panel, Fig. 4), responded more than saline controls during the 1st, 2nd, and 3rd 10-min blocks. Administration of 10 mg/kg cocaine 4 hr prior to EtOH relapse increased responding during the 5th and 6th 10-min periods compared to saline values.
Fig. 4.
Mean (±S.E.M.) responses per 10-minute block during the 1st relapse session on the EtOH lever by P rats given i.p. saline (n = 4/time point) or 1 or 10 mg/kg of cocaine (n = 7– 8/dose/time point) immediately, 30 minute or 4 hour prior to only the session. Upper Panel: (*) indicates that rats administered 1 mg/kg cocaine immediately prior to the relapse test responded significantly (p < 0.05) less on the EtOH lever during 1st and 2nd 10-minute block compared to all other groups, and the group given cocaine 4 hour prior to the session increased EtOH responding during 4th and 6th 10-minute block compared to saline values. Lower Panel: (*) indicates that rats (a) administered 10 mg/kg cocaine immediately prior to the session responded significantly (p < 0.05) less on the EtOH lever during the 1st 10-minute block compared to all the other groups; (b) administered 10 mg/kg cocaine immediately or 4 hour prior to the session had lower responding compared to other 2 groups during the 2nd 10-minute block; (c) 10 mg/kg cocaine given 30-minute prior to relapse testing increased EtOH responding significantly more compared to saline during the 1st, 2nd, and 3rd 10-minute block ; and (d) 10 mg/kg cocaine given 4 hour prior to session increased EtOH responding during the 5th and 6th 10-minute block compared to saline.
DISCUSSION
The results of the current studies indicate that exposure to cocaine in a P rat can enhance the expression of EtOH-seeking and relapse. The cross-reactivity between cocaine and EtOH-seeking supports the assertion of common pathways for drug-seeking. The data also indicate that a single exposure to cocaine can have a persisting effect on EtOH-seeking since cocaine enhanced EtOH-seeking in P rats in a dose-dependent manner if administered immediately, 30 min, or 4 hr prior to the 1st PSR session (Fig.1A). The transference from drug-seeking to drug-intake is complex between EtOH and cocaine, indicated by the current results that cocaine enhanced EtOH relapse only if administered 30 min or 4 hr prior to the 1st EtOH relapse session (Fig. 3A).
The cross-reactivity of cocaine to stimulate seeking behaviors for other drugs of abuse (in cocaine naïve animals) has been observed previously. Priming injections of cocaine can enhance cocaine and heroin drug-seeking (De Vries et al., 1998). The present results demonstrate that priming doses of cocaine can increase EtOH-seeking and relapse drinking. These findings parallel human studies that indicate that cocaine can enhance alcohol relapse behaviors (Fox et al., 2005).
Interestingly, priming injections of cocaine can reinstate natural rewards such as food-seeking (c.f. Nair et al., 2009). However cocaine does not reliably induce food-seeking behavior (c.f. Nair et al., 2009), suggesting that cocaine’s effect may be more specific to drugs of abuse than natural awards.
Previous studies suggest that cocaine’s effect on EtOH intake (McMillan and Snodgrass, 1991; Hudzik et al., 1993; Stromberg et al., 2002) may be dependent upon the time interval between cocaine administration and testing. Immediate administration of cocaine prior to the 1st EtOH relapse session prevented enhanced responding, which resulted in no effect or a reduction of relapse self-administration (Fig. 3A). It was only as the time interval increased between the administration of cocaine and EtOH access that there was an enhancement in EtOH-relapse responding, with the 4-hr interval showing the most robust responding (Fig. 3A). In addition, the results revealed that for the 4-hr interval group, EtOH responding was highest during the 1st 10 min and the last 40–60 min of the 1st relapse session (Fig. 4), suggesting that the effects of cocaine on the reinforcing actions of EtOH drinking persist for at least 5 hr. Overall, the present findings are in line with previous studies that demonstrated cocaine may initially reduce EtOH intake (McMillan and Snodgrass, 1991; Hudzik et al., 1993) and that time of exposure may play a factor in shifting preference from cocaine during the 1st hour to EtOH during 2nd-6th hour (Stromberg et al., 2002).
Administration of cocaine at all 3 time points prior to the 1st PSR session enhanced EtOH-seeking (Fig. 1A). The difference observed between immediate cocaine prior to relapse and EtOH–seeking is likely due to the availability of EtOH during relapse. Although there did not appear to be a time-dependent factor on PSR responding (Fig.1A), examination of the 10-min blocks (Fig. 2) indicated that the effects of the immediate injections of cocaine to enhance EtOH-seeking were more pronounced after 40 min, a similar effect was not observed with relapse drinking (Fig. 4) In addition, it appears that the activation of EtOH-seeking behavior by cocaine can persist for at least 4 hr after initial exposure to the lower dose and up to 24 hr with the higher dose (Fig. 1A), effects which were not observed with relapse drinking (Fig. 3A). Previous studies demonstrated that a single administration of cocaine can induce long-lasting effects on glutamatergic synaptic long-term potentiation on DA neurons in the VTA (Ungless et al., 2001) and increase extracellular glutamate 24 hr after administration (McKee and Meshul, 2005). Therefore, it is possible that prolonged heighten activation of certain neuronal pathways by cocaine is the basis of the persistent effect on EtOH-seeking behavior.
Cocaine and EtOH reinforcing effects are mediated primarily via the mesolimbic DA system and they can act on similar neuronal mechanisms and neural substrates that may contribute to co-abuse of both drugs. Intracranial self-administration studies have shown that DA and/or 5-hydroxytryptamine-3 (5-HT3) receptor mediated neuronal activity within the posterior ventral tegmental area (pVTA) and/or nucleus accumbens shell are involved in mediating the reinforcing actions of EtOH (Rodd-Henricks et al., 2000; Rodd-Henricks et al., 2003; Rodd et al., 2004; Engleman et al., 2009) and cocaine (Rodd et al., 2002c; Rodd et al., 2005c). In addition, other studies indicated the involvement of 5-HT3 receptors in the actions of cocaine (Briscione et al., 2013; Kankaanpaa et al., 2002; King et al., 2002). The activation of the mesolimbic DA system is also thought to be involved in mediating compulsive drug seeking and relapse behaviors (Robinson and Berridge, 1993). Evidence indicates that the activation DA (Hauser et al., 2011) and 5-HT3 (Hauser et al., 2014) receptors within the mesolimbic DA system are involved in mediating EtOH-seeking behavior. Furthermore, it is thought that drugs of abuse can prime responding by activating the mesolimbic DA system, which can become sensitized upon repeated drug use in a long lasting manner (Robinson and Berridge, 1993).
Neuroadaptations of the mesolimbic DA system can occur after chronic drinking. Prolonged or repeated EtOH exposure in P rats can lead to neuroadaptive changes in basal DA (Thielen et al., 2004; Smith and Weiss, 1999) and 5-HT neurotransmission (Thielen et al., 2004), as well as alterations in D2 autoreceptors (Thielen et al., 2004; Engleman et al., 2003) and 5-HT3 receptors (Thielen et al., 2004) and increased sensitivity of the mesolimbic DA system to the reinforcing effects of EtOH (Rodd et al., 2005a, b). Collectively, the reinforcing effects of cocaine priming doses and the increased sensitivity of the mesolimbic DA system induced by a prior history of EtOH may be factors contributing to the immediate and delayed effects of cocaine on EtOH-seeking and relapse. However, it is likely that other pathways may be involved that differentially mediate EtOH-seeking and EtOH-relapse drinking behaviors.
The effects of immediate administration of cocaine on relapse behavior may be due to several factors. Pharmacokinetics studies have shown that EtOH can elevate concentrations of cocaine (Perez-Reyes and Jeffcoat 1992; Dean et al., 1992; Farre et al., 1993; Pan and Hedaya, 1999), suggesting that EtOH may increase bioavailability of cocaine when both drugs are administered together. An increase bioavailability of cocaine may result in the animals needing less EtOH. However, others have not found any pharmacokinetics changes of cocaine by EtOH (Fowler et al., 1992). The co-administration of low doses of cocaine with EtOH can potentiate EtOH induced excitation of DA VTA neurons (Bunney et al., 2000), leading to higher DA neurotransmission levels compared to cocaine or EtOH alone (Lindholm et al., 2001). However, higher cocaine concentrations can inhibit EtOH induced excitation of DA VTA neurons (Bunney et al., 2000). Therefore, it is possible that the combined effects of low-dose cocaine with EtOH may prevent enhanced responding for EtOH during relapse because cocaine may be substituting for EtOH. The reduced responding for EtOH during relapse with the higher dose of cocaine may be a result of cocaine preventing the effects of EtOH on VTA DA neurons. The finding that the immediate injection of the high dose of cocaine enhanced responding in the PSR test but inhibited responding under EtOH relapse conditions suggests that different mechanisms may be mediating EtOH -seeking and –relapse drinking behaviors.
A report by Ding et al., (2012) indicated interactions between EtOH and cocaine within the brain reward system. These findings indicated that Wistar rats will self-administer sub-threshold concentrations of EtOH and cocaine directly into the pVTA when given together, suggesting that EtOH and cocaine can act synergistically to promote reinforcing effects. These results support an interpretation that an interaction between the two drugs may be altering EtOH relapse behaviors (i.e, low concentrations of cocaine enhance the rewarding properties of EtOH).
The half-life of i.p. administration of cocaine in rats is approximately 30 to 50 min (Lau et al., 1991; Pan and Hedaya, 1999). One major metabolite of cocaine is norcocaine, which increases gradually following administration of cocaine, although it has a half-life of only 30–50 min (Pan and Hedaya, 1999). Norcocaine is thought to be more potent than cocaine because it can induce higher DA levels at much lower dose than cocaine (Pan and Hedaya, 1999) and this may contribute to the delayed response following the immediate injection, as well to early responses following the 30-min and 4-hr injection intervals in the PSR test (Fig. 2).
Cocaine’s robust enhancing effects were specific to responses on the EtOH lever compared to responses on the water lever. In the 1st PSR test session, there were no significant effects of cocaine compared to saline controls on responses on the water lever at either dose or at any of the time intervals (Fig. 1B). In the 1st relapse session, responses on the water lever were not significantly elevated above saline values at either dose or at any of the time intervals (Fig. 4). Taken together, the lack of effect of cocaine on responding on the water lever during EtOH-seeking (Fig. 1B) or relapse (Fig. 3B) supports the idea that cocaine is stimulating EtOH goal-directed behavior and is not merely stimulating general motor activity.
In conclusion, the present findings suggest that a single dose of cocaine can have persisting effects on neuronal circuits regulating EtOH-seeking and EtOH relapse. These persisting effects could contribute to the co-use and co-abuse of alcohol and cocaine. The data also supports the hypothesis that exposure to a different agent that activates the drug reward pathway can elicit/enhance seeking behaviors for a previously obtainable reinforcer. This evidence of cross-reactivity between drugs of abuse on seeking behaviors supports the theory of a common biological system regulating drug-seeking.
Acknowledgments
The skillful technical assistance of Tylene Pommer and Victoria McQueen is gratefully acknowledged. This research was supported in part by NIAAA grants AA07611, AA022287, AA07462, and AA020908.
References
- Bell RL, Rodd ZA, Engleman EA, Toalston JE, McBride WJ. Scheduled access alcohol drinking by alcohol-preferring (P) and high-alcohol-drinking (HAD) rats: modeling adolescent and adult binge-like drinking. Alcohol. 2014;48:225–34. doi: 10.1016/j.alcohol.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscione MA, Serafine KM, Merluzzi AP, Rice KC, Riley AL. The effects of the 5-HT3 receptor antagonist tropisetron on cocaine-induced conditioned taste aversion. Pharmacol Biochem Behav. 2013;105:112–7. doi: 10.1016/j.pbb.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookoff D, Rotondo MF, Shaw LM, Campbell EA, Fields L. Coacaethylene levels in patients who test positive for cocaine. Ann Emerg Med. 1996;27:316–20. doi: 10.1016/s0196-0644(96)70266-4. [DOI] [PubMed] [Google Scholar]
- Bunney EB, Appel SB, Brodie MS. Cocaine potentiates ethanol-induced excitation of dopaminergic reward neurons in the ventral tegmental area. J Pharmacol Exp Ther. 2000;293:383–9. [PubMed] [Google Scholar]
- Cailhol S, Mormede P. Effects of cocaine-induced sensitization on ethanol drinking: sex and strain differences. Behav Pharmacol. 2000;11:387–394. doi: 10.1097/00008877-200008000-00004. [DOI] [PubMed] [Google Scholar]
- Dean RA, Harper ET, Dumaual N, Stoeckel DA, Bosron WF. Effects of ethanol on cocaine metabolism: formation of cocaethylene and norcocaethylene. Toxicol Appl Pharmacol. 1992;117:1–8. doi: 10.1016/0041-008x(92)90210-j. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. Drug induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci. 1998;10:3565–71. doi: 10.1046/j.1460-9568.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- Dhaher R, Hauser SR, Getachew B, Bell RL, McBride WJ, McKinzie DL, Rodd ZA. The Orexin-1 Receptor Antagonist SB-334867 Reduces Alcohol Relapse Drinking, but not Alcohol Seeking, in Alcohol-Preferring (P) Rats. J Addict Med. 2010;4:153–9. doi: 10.1097/ADM.0b013e3181bd893f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Oster SM, Hauser SR, Toalston JE, Bell RL, McBride WJ, Rodd ZA. Synergistic self-administration of ethanol and cocaine directly into the posterior ventral tegmental area: involvement of serotonin-3 receptors. J Pharmacol Exp Ther. 2012;340:202–9. doi: 10.1124/jpet.111.187245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, Ding ZM, Oster SM, Toalston JE, Bell RL, Murphy JM, McBride WJ, Rodd ZA. Ethanol is self-administered into the nucleus accumbens shell, but not the core: evidence of genetic sensitivity. Alcohol Clin Exp Res. 2009;33:2162–71. doi: 10.1111/j.1530-0277.2009.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, McBride WJ, Li TK, Lumeng L, Murphy JM. Ethanol drinking experience attenuates (−)sulpiride-induced increases in extracellular dopamine levels in the nucleus accumbens of alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2003;27:424–31. doi: 10.1097/01.ALC.0000056618.57931.A5. [DOI] [PubMed] [Google Scholar]
- Farre M, de la Torre R, Llorente M, Lamas X, Ugena B, Segura J, Camí J. Alcohol and cocaine interactions in humans. J Pharmacol Exp Ther. 1993;266:1364–73. [PubMed] [Google Scholar]
- Filbery FM, DeWitt SJ. Cannabis cue-elivited craving and the reward neurocircuitry. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:30–35. doi: 10.1016/j.pnpbp.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology. 2005;30:880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Fox HC, Tuit KL, Sinha R. Stress system changes associated with marijuana dependence may increase craving for alcohol and cocaine. Hum Psychopharmacol Clin Exp. 2013;28:40–53. doi: 10.1002/hup.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, MacGregor RR, Wang GJ, Wolf AP. Alcohol intoxication does not change [11C]cocaine pharmacokinetics in human brain and heart. Synapse. 1992;12:228–35. doi: 10.1002/syn.890120308. [DOI] [PubMed] [Google Scholar]
- Hauser SR, Deehan GA, Jr, Toalston JE, Bell RL, McBride WJ, Rodd ZA. Enhanced Alcohol-Seeking Behavior by Nicotine in the Posterior Ventral Tegmental Area of Female Alcohol-Preferring (P) rats: Modulation by Serotonin-3 and Nicotinic Cholinergic Receptors. Psychopharmacology. 2014 doi: 10.1007/s00213-014-3508-3. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Getachew B, Oster SM, Dhaher R, Ding ZM, Bell RL, McBride WJ, Rodd ZA. Nicotine Modulates Alcohol-Seeking and Relapse by Alcohol-Preferring (P) Rats in a Time-Dependent Manner. Alcohol Clin Exp Res. 2012;36:43–54. doi: 10.1111/j.1530-0277.2011.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Ding ZM, Getachew B, Toalston JE, Oster SM, McBride WJ, Rodd ZA. The Posterior Ventral Tegmental Area Mediates Alcohol-Seeking Behavior in the Alcohol Preferring P Rats. Journal of Pharmacology and Experimental Therapeutics. 2011;336:857–65. doi: 10.1124/jpet.110.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudzik TJ, Wessinger WD, McMillan DE. Effects of cocaine self-administration on ethanol, food and water intake in the rat. Drug Alcohol Depend. 1993;33:225–233. doi: 10.1016/0376-8716(93)90109-4. [DOI] [PubMed] [Google Scholar]
- Ikegami A, Olsen CM, Fleming SM, Guerra EE, Bittner MA, Wagner J, Duvauchelle CL. Intravenous ethanol/cocaine self-administration initiates high intake of intravenous ethanol alone. Pharmacol Biochem Behav. 2002;72:787–794. doi: 10.1016/s0091-3057(02)00738-4. [DOI] [PubMed] [Google Scholar]
- Kankaanpaa A, Meririnne E, Seppala T. 5-HT3 receptor antagonist MDL 72222 attenuates cocaine- and mazindol- but not methylphenidate-induced neurochemical and behavioral effects in the rat. Psychopharmacology. 2002;159:341–50. doi: 10.1007/s00213-001-0939-4. [DOI] [PubMed] [Google Scholar]
- Katner SN, Oster SM, Ding ZM, Deehan GA, Jr, Toalston JE, Hauser SR, McBride WJ, Rodd ZA. Alcohol-preferring (P) rats are more sensitive than Wistar rats to the reinforcing effects of cocaine self-administered directly into the nucleus accumbens shell. Pharmacol Biochem Behav. 2011;99:688–95. doi: 10.1016/j.pbb.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GR, Pinto G, Konen J, Castro G, Tran S, Hilburn C. The effects of continuous 5-HT3 receptor antagonist administration on the subsequent behavioral response to cocaine. Eur J Pharmacol. 2002;449:253–9. doi: 10.1016/s0014-2999(02)02036-8. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Ben-Shahar O, Ettenberg A. Alcohol consumption is preferred to water in rats pretreated with intravenous cocaine. Pharmacol Biochem Behav. 2006;85:281–286. doi: 10.1016/j.pbb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs- a quantitative meta-analysis of cue-reactivity brain response. E J Neurosci. 2011;33:1318–26. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Lau CE, Imam A, Ma F, Falk JL. Acute effects of cocaine on spontaneous and discriminative motor functions: relation to route of administration and pharmacokinetics. J Pharmacol Exp Ther. 1991;257:444–56. [PubMed] [Google Scholar]
- Lindholm S, Rosin A, Dahlin I, Georgieva J, Franck J. Ethanol administration potentiates cocaine-induced dopamine levels in the rat nucleus accumbens. Brain Res. 2001;915:176–84. doi: 10.1016/s0006-8993(01)02847-5. [DOI] [PubMed] [Google Scholar]
- Magura S, Rosenblum A. Modulating effect of alcohol use on cocaine use. Addict Behav. 2000;25:117–22. doi: 10.1016/s0306-4603(98)00128-2. [DOI] [PubMed] [Google Scholar]
- McKee BL, Meshul CK. Time-dependent changes in extracellular glutamate in the rat dorsolateral striatum following a single cocaine injection. Neuroscience. 2005;133:605–613. doi: 10.1016/j.neuroscience.2005.02.020. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Yorger L, McBride WJ, Murphy JM, Lumeng L, Li TK. The alcohol deprivation effect in the alcohol-preferring P rat under free drinking and operant access conditions. Alcohol Clin Exp Res. 1998;22:1170–1176. [PubMed] [Google Scholar]
- McMillan DE, Snodgrass SH. Effects of acute and chronic administration of delta 9- tetrahy-drocannabinol or cocaine on ethanol intake in a rat model. Drug Alcohol Depend. 1991;27:263–74. doi: 10.1016/0376-8716(91)90009-n. [DOI] [PubMed] [Google Scholar]
- Mikkola JA, Honkanen A, Piepponen TP, Kiianmaa K, Ahtee L. Effects of repeated cocaine treatment on striatal dopamine release in alcohol-preferring AA and alcohol-avoiding ANA rats. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:209–14. doi: 10.1007/s002100000367. [DOI] [PubMed] [Google Scholar]
- Miller NS, Millman RB, Keskinen S. The diagnosis of alcohol, cocaine, and other drug dependence in an inpatient treatment population. J Subst Abuse Treat. 1989;6:37–40. doi: 10.1016/0740-5472(89)90018-4. [DOI] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y. The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Prog Neurobiol. 2009;89:18–45. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WJ, Hedaya MA. Cocaine and alcohol interactions in the rat: contribution of cocaine metabolites to the pharmacological effects. J Pharm Sci. 1999;88:468–476. doi: 10.1021/js980283h. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Jeffcoat AR. Ethanol/cocaine interaction: cocaine and cocaethylene plasma concentrations and their relationship to subjective and cardiovascular effects. Life Sci. 1992;51:553–63. doi: 10.1016/0024-3205(92)90224-d. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD, McBride WJ. The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res. 2006;171:207–215. doi: 10.1016/j.bbr.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li TK, Lumeng L, McBride WJ. Prolonged increase in the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol following repeated exposure to cycles of ethanol access and deprivation. J Pharmacol Exp Ther. 2005a;315:648–57. doi: 10.1124/jpet.105.084350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li TK, Lumeng L, McBride WJ. Chronic ethanol drinking by alcohol-preferring rats increases the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol. Alcohol Clin Exp Res. 2005b;29:358–66. doi: 10.1097/01.alc.0000156127.30983.9d. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self- administration of cocaine within the posterior ventral tegmental area of Wistar rats: evidence for involvement of serotonin-3 receptors and dopamine neurons. J Pharmacol Exp Ther. 2005c;313:134–45. doi: 10.1124/jpet.104.075952. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Li TK, McBride WJ. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology. 2003;28:1614–1621. doi: 10.1038/sj.npp.1300214. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self- administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol Clin Exp Res. 2002a;26:1632–1641. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self- administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: II. Adult exposure. Alcohol Clin Exp Res. 2002b;26:1642–1652. doi: 10.1097/01.ALC.0000036302.73712.9D. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Li TK, Murphy JM, McBride WJ. Cocaine is self- administered into the shell but not the core of the nucleus accumbens of Wistar rats. J Pharmacol Exp Ther. 2002c;303:1216–26. doi: 10.1124/jpet.102.038950. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology (Berl) 2000;149:217–24. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Anton SF, Carroll K, Budde D, Prusoff BA, Gawin F. Psychiatric diagnoses of treatment-seeking cocaine abusers. Arch Gen Psychiatry. 1991;48:43–51. doi: 10.1001/archpsyc.1991.01810250045005. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Perrott MA, Peters AR. The effects of alcohol on cigarette craving in heavy smokers and tobacco chippers. Psychol Addict Behav. 2005;19:263–70. doi: 10.1037/0893-164X.19.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, Weiss F. Ethanol exposure differentially alters central monoamine neurotransmission in alcohol-preferring versus -nonpreferring rats. J Pharmacol Exp Ther. 1999;288:1223–8. [PubMed] [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Sengpiel T, Mackler SA, Volpicelli JR, O'Brien CP, Vogel WH. Effect of naltrexone on oral consumption of concurrently available ethanol and cocaine in the rat. Alcohol. 2002;3:169–79. doi: 10.1016/s0741-8329(02)00280-x. [DOI] [PubMed] [Google Scholar]
- Thielen RJ, Engleman EA, Rodd ZA, Murphy JM, Lumeng L, Li TK, McBride WJ. Ethanol drinking and deprivation alter dopaminergic and serotonergic function in the nucleus accumbens of alcohol-preferring rats. J Pharmacol Exp Ther. 2004;309:216–25. doi: 10.1124/jpet.103.059790. [DOI] [PubMed] [Google Scholar]
- Uemura K, Li YJ, Ohbora Y, Fujimiya T, Komura S. Effects of repeated cocaine administration on alcohol consumption. J Stud Alcohol. 1998;59:115–118. doi: 10.15288/jsa.1998.59.115. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Williamson S, Gossop M, Powis B, Griffiths P, Fountain J, Strang J. Adverse effects of stimulant drugs in a community sample of drug users. Drug Alcohol Depend. 1997;44:87–94. doi: 10.1016/s0376-8716(96)01324-5. [DOI] [PubMed] [Google Scholar]