Abstract
Post-ovulatory aging of oocytes results in the progressive loss of fertilization and developmental competence. This degradation of oocyte quality has been the object of numerous investigations, primarily focused on individual signaling pathways – which provide limited insight into the status of global signaling events. The purpose of the present investigation was to comprehensively assess broad patterns of signaling pathway activity during in vitro aging as an initial step in defining control points that can be targeted to prevent the reduction in oocyte quality during prolonged culture. An antibody microarray-based phospho-proteome analysis performed on oocytes before and after eight hours of culture revealed significant changes in the abundance or activation state of 43 proteins that function in a wide variety of protein kinase-mediated signaling pathways. Several of the most significantly affected kinases were studied by Western blot and confocal immunofluorescence to corroborate the array results. Prolonged culture resulted in global changes in the abundance and activity of protein kinases that regulate the response to calcium, stress, and cell-cycle control. Examination of intracellular structures revealed a previously unrecognized increase in the abundance of large autophogagic lysosomes, which correlates with changes in protein kinase pathways. These results provide insight into the stresses experienced by oocytes during culture and the diversity of responses that results from them. The observed increase in autophagy-related activity together with the disruptions in calcium signaling, cell-cycle, and stress-response pathways have the potential to negatively impact oocyte quality by interfering with the normal sequence of biochemical changes that constitute egg activation following fertilization.
Keywords: kinase, antibody microarray, PTK2B, autophagy, oocyte
Introduction
Mature mammalian oocytes maintain optimal developmental competence for a very limited time following ovulation. Mice treated with human chorionic gonadotropin (hCG) will ovulate about 10 hr following the administration. The optimal timing for fertilization ranges from 14-16 hr post-hCG (Marston and Chang 1964). Changes to oocyte viability occur gradually, such that 18-22 hr post-hCG, mouse oocytes exhibit abnormal calcium oscillations, reduce fertilization, and lower embryonic developmental potential – independent of whether the oocytes were aged in the oviduct (Igarashi et al. 1997; Takahashi et al. 2003) or cultured in vitro (Badenas et al. 1989; Gordo et al. 2002; Takahashi et al. 2009; Zhang et al. 2011). The window of optimal fertilization for primate and human oocytes, on the other hand, is not as well known, although there is clear evidence that they also undergo post-ovulatory aging. During clinical assistant reproduction, oocytes are usually collected from large antral follicles (27-36 hr post-hCG for humans and non-human primates) (Mansour et al. 1994; Stouffer and Zelinski-Wooten 2004; Wolf 2004) prior to ovulation, which usually occurs about 38 hr post-hCG (Andersen et al. 1995). The majority of these pre-ovulatory oocytes have matured to the metaphase-II (MII) stage, but still require 3-4 hr of in vitro culture to obtain optimal fertilization and developmental competence (Harrison et al. 1988; Khan et al. 1989; Trounson et al. 1982). Human oocytes collected from antral follicles retain developmental competence up to 16 hr in culture, but oocytes fertilized at 20-26 hr after collection resulted in zero pregnancies (Harrison et al. 1988).
Post-ovulatory aging of mammalian oocytes either in vivo or in vitro is associated with the failure to arrest at metaphase, abnormal-spindle development, displaced chromosomes, disruption of organelles, and other cytological defects resulting in the loss of developmental potential and induction of apoptotic cell death (Abbott et al. 1998; Ducibella et al. 1990; Fissore et al. 2002; Gordo et al. 2002; Huang et al. 2007; Miao et al. 2009; Steuerwald et al. 2005; Szollosi 1971; Takahashi et al. 2013; Tarin 1996; Xu et al. 1997). When in vitro-aged oocytes are fertilized, they frequently exhibit abnormal cell-cycle activation and fragmentation (Gordo et al. 2002; Takahashi et al. 2009). Normal, healthy oocytes respond to fertilization via sequential activation of multiple protein kinase signaling cascades triggered by fertilization-induced calcium oscillations (McGinnis et al. 2011a; Parrington et al. 2007; Runft et al. 2002; Swann and Lai 2013), whose timing and amplitude are critical for the initiation of development. Calcium signaling is disrupted in post-ovulatory in vitro-aged oocytes, however, and their altered pattern activate pathways of apoptosis, fragmentation, and cell death instead of normal development (Fissore et al. 2002; Gordo et al. 2002). Some of the early signs of cellular distress that lead to apoptosis and cell death include activation of lysosome biogenesis and autophagy (Moore et al. 2006b). But in general, our knowledge of how post-ovulatory aging influences global kinase signaling cascades or lysosomal-autophagic pathways remains rudimentary.
The goal of the present study was to define the signal transduction pathways in oocytes that are significantly up- or down-regulated in response to prolonged in vitro culture (“in vitro aging”). An unbiased antibody microarray method was used to detect potential changes in expression and activation state of protein kinases involved in a wide variety of signaling pathways. The results, some of which have been confirmed by Western blots, revealed that prolonged in vitro culture caused very significant changes in signal transduction pathways contributing to oocyte quality. These changes correlated with known and newly discovered changes in oocyte cytology, and provide insights into the global impact of post-ovulatory aging on oocyte developmental potential.
Results
In vitro aging induced changes in oocyte signal transduction proteins
To test the hypothesis that in vitro aging involves complex global changes in protein kinase signaling machinery, antibody microarray analyses of protein kinase-mediated signal transduction pathways in oocytes were performed before and after prolonged (8 hr) culture. The high-density design of the antibody microarray enabled the simultaneous analysis of multiple signaling pathways based on the abundance of protein kinases and their targets, as well as the phosphorylation state of key regulatory sites. This approach provided a valuable complement to RNA expression arrays because the abundance of signaling proteins can change independently of transcript abundance, and their catalytic activity (often indicated by their phosphorylation state) can change more rapidly than transcript abundance in response to changing conditions. The microarray data was, in some cases, confirmed by immunoblotting with antibodies to several pathways known to be important for egg activation and/or stress response in other cell types.
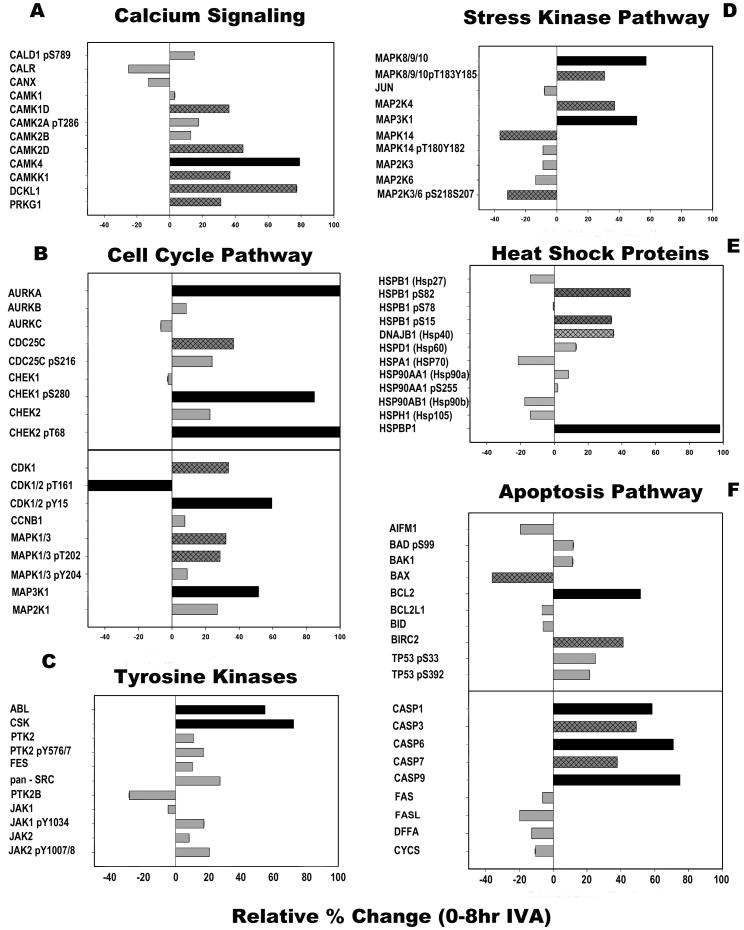
The antibody microarray analyses were performed on mature oocytes collected after 0 hr (14 hr post-hCG) and 8 hr of in vitro culture. Two samples, containing 2000 oocytes each, were analyzed by the Kinex™ KAM-850 Antibody Microarray, which makes use of over 850 commercial antibodies to detect changes in the expression or phosphorylation of protein kinases, phosphatases, their regulatory subunits and target proteins. The results demonstrated that oocytes collected after 8 hr of in vitro culture underwent extensive changes in signaling protein expression or phosphorylation (Table S1). Forty-three protein kinases exhibited changes in expression or phosphorylation state that were greater than or equal to 25% relative to control oocytes. A subset of these results, which reflect inappropriate activation of pathways involved in fertilization or culture-induced stress, are presented in Figure 1.
Fig. 1. Antibody microarray detected changes in protein kinase signaling pathways following 8 hr in vitro aging.
Oocytes collected at 14-hr post-human chorionic gonadotrophin and then aged in vitro for 8 hr were analyzed with the Kinex™ KAM-850 antibody microarray, and compared to control oocytes collected 14 hr post-hCG without in vitro aging (0 hr). Data were grouped according to signaling pathways known to function in the maintenance of meiotic arrest, fertilization, and/or embryonic development. These pathways include (A) calcium signaling, (B) cell cycle, (C) protein tyrosine kinases, (D) stress-activated protein kinases, (E) heat shock proteins, and (F) apoptosis. Bars with hatch marks indicate proteins that changed by 30-49%; solid black bars show proteins changed by 50% or more.
Numerous studies have detailed the effect of prolonged in vitro culture on oocyte calcium homeostasis and signaling (recently reviewed in Takahashi et al. 2013). Examination of the antibody-microarray data revealed that the calcium-calmodulin-dependent kinase family members CAMK1D, CAMK2D, CAMK4, and CAMKK1 were expressed at increased levels (>30% increase over controls) following 8 hr of in vitro aging (Fig. 1A). In contrast, the calcium binding proteins calreticulin (CALR) and calnexin (CANX) were expressed at a lower level following in vitro aging.
The cell-cycle regulatory kinase pathways are also activated in response to fertilization, and could potentially be activated inappropriately during prolonged in vitro culture. The cell-cycle-related kinase pathways (Fig. 1B) were significantly modified during prolonged in vitro culture. For example, CHEK2 levels increased over 20% during prolonged in vitro culture while the phosphorylation level of CHEK1 (at S280) and CHEK2 (at T68) increased over 80%. Increased phosphorylation of CHEK1 at S280 is associated with its inactivation, whereas phosphorylation of CHEK2 at T68 is consistent its activation. The CDC25C phosphatase (a substrate of CHEK-family kinases) increased in protein content and in phosphorylation at S216 (Fig. 1B), which is consistent with the above activation of CHEK2 and would be expected to impair resumption of the cell-cycle. Aurora kinase A (AURKA), which is implicated in the regulation of spindle assembly and stability, exhibited a large increase in protein expression while AURKB and AURKC, which function in chromosomal movement, showed little change (Fig. 1B). The culture-dependent rise in AURKA levels was confirmed by Western blot analysis of oocytes cultured in KSOMaa medium for different periods of time; indeed AURKA protein content increased progressively during culture, reaching a level over 50% higher than that of control oocytes (Fig. 2H). The increase in AURKA levels after 12 hr of culture was statistically significant (P<0.05) (Fig. 1A). The expression level of cyclin-dependent kinase CDK1 increased over 30% during prolonged culture, which would be predicted to promote cell-cycle resumption. Yet phosphorylation of CDK1 at Y15 increased over 60% and phosphorylation of T161 declined by over 50%, which together inactivate CDK1. The expressions of MAP2K1 and MAP3K1 (MEK1and MEKK1), which are serine/threonine kinases that act in a stimulatory way upstream of the MAPK pathway, increased 20% and 40%, respectively, during prolonged culture. The expression levels and phosphorylation status of MAPK1/3 (ERK2/ERK1), which are thought to be essential for spindle assembly and maintenance of metaphase arrest in oocytes, also increased during prolonged culture. Confirmation of this microarray result by Western blot demonstrated that MAPK1/3 protein content increased progressively during the first 12 hr in culture, then declined (Fig. 2B). Protein levels at 4 hr of culture were significantly higher than controls.
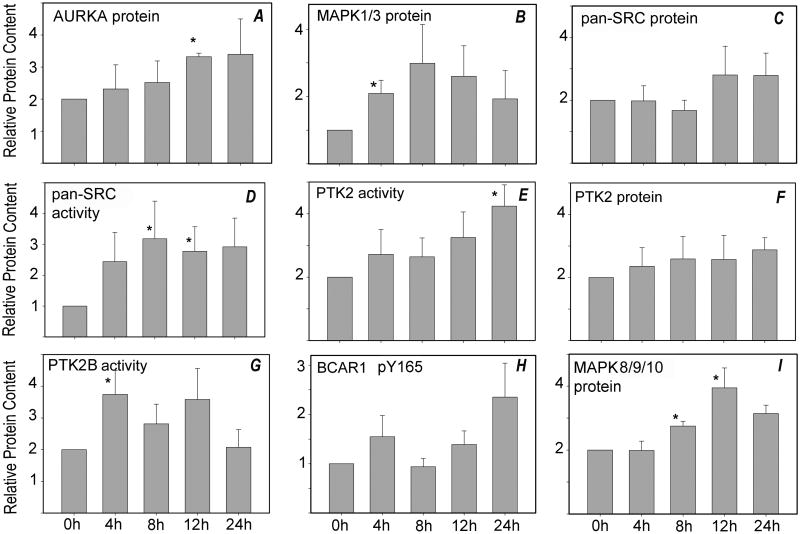
Fig. 2. Western blot analysis of oocytes aged in vitro.
Oocytes were cultured for periods of up to 24 hr (x-axis), then solubilized in SDS-containing sample buffer. Samples (each from a different pool of females) containing 3-5 oocytes were analyzed by SDS-PAGE, as described in ‘Materials and Methods’, and transferred to nylon membranes for Western blot analysis. Blots were probed with antibodies to (A) anti-AURKA (4 samples), (B) anti-MAPK1/3 (6 samples), (C) anti-pan-SRC (7 samples), (D) activated pan-SRC-family kinases (dephosphorylated-Y528; 6-9 samples), (E) anti-PTK2 phospho-Y576 (5 samples), (F) anti-PTK2 protein (4 samples), (G) anti-PTK2B phospho-Y579 (7 samples), (H) anti-BCAR1 phospho-Y165 (6 samples), or (I) anti-MAPK8/9/10 protein (6 samples). Each sample was also probed with anti-GAPDH as a loading control. The bound antibody was detected and quantified as described in ‘Materials and Methods’. Values represent the mean quantity of kinase protein corrected for GAPDH content, which are expressed relative to that present at 0 hr of culture (arbitrarily set as 1.0 on the y–axis). Error bars indicate standard error of the mean. Asterisks (*) indicates that the value was significantly different from 0 hr control (P<0.05).
The SRC and focal adhesion kinase (FAK) family of protein kinases are thought to be involved in maintaining oocyte cytoskeletal organization, cortical polarity, and spindle organization (Luo et al. 2009; Luo et al. 2010; Talmor-Cohen et al. 2004), and in somatic cells they are known to participate in stress responses (Cohen 2005). While the microarray data did not identify individual SRC family members, the array contained pan-specific antibodies for the entire SRC family (pan-SRC). The screen revealed an increase in SRC-family protein expression together with the closely related protein-tyrosine kinase ABL (Fig. 1C). Expression of the C-terminus SRC kinase (CSK), which down regulates SRC-family kinase activity, was also increased — and was likely counteracting SRC-family activation in the oocyte. Western blot analysis of SRC-family protein-tyrosine kinases (PTKs) confirmed that the expression of total SRC-family proteins tended to increase during prolonged culture (Fig. 2C), although scatter in the data rendered these changes less than significant. When the activation state of SRC-family kinases was tested with an antibody to the activated form of SRC-family kinases, a significant increase in immunoreactivity was observed after 8 and 12 hr of culture (Fig. 2D). The antibody microarray also detected changes in the protein level or activation state of FAK family members PTK2 (FAK) and PTK2B (PYK2). An increase in both PTK2 protein level and the phosphorylation state of its regulatory Y576 was evident in the microarray results (Fig. 1C) and Western blot (Fig. 2E and 2F). This activating phosphorylation state of PTK2B increased between 4 and 12 hr of in vitro culture (Fig. 2G), even though total PTK2B protein level appeared to decline by the microarray results (Fig. 1C). One indicator of PTK2B activity is the phosphorylation status of BCAR1 (p130CAS) at Y165, which was not included in the microarray. Monitoring by Western blot, however, showed that the amount of phosphorylated BCAR1 tended to increase during in vitro aging, although the changes varied between groups of oocytes and did not reach statistical significance (Fig 2H). Finally, prolonged culture resulted in increases in phosphorylation of the protein-tyrosine kinases JAK1 and JAK2 at regulatory (activating) phosphorylation sites, based on microarray analysis; these results have not yet been confirmed by Western blotting.
Pathways relating to stress-response mechanisms exhibited some of the largest disruptions after prolonged in vitro culture. MAPK8/9/10 (also known as JNK1/2/3) expression increased approximately 60%, and phosphorylation of the regulatory (activating) sites at T183 and Y185 was also increased (Fig. 1D; 2I). The microarray results demonstrated that MAP2K4 (MKK4) and MAP3K1 (MEKK1) were present at higher levels following prolonged in vitro culture, whereas MAPK14 (p38-alpha) was lower (Fig. 1D). The phosphorylation of MAPK14 at its regulatory (activating) phosphorylation sites T180 and Y182 was also reduced, along with the abundance of the MAP kinase kinases MAPK2K3 (MKK3) and MAP2K6 (MKK6), which both target these phosphorylation sites.
Heat-shock proteins are also commonly involved in the response to cellular stress. Our protein microarray detected significant changes in select members of this pathway (Fig. 1E). Phosphorylation of HSPB1 (HSP27) at S82 and S15 was significantly increased after in vitro aging, indicating regulation in the chaperone activity of this protein, and was accompanied by a drop in HSPB1 protein levels. The expression level of the HSPA1 (HSP70) protein also decreased more than 20% whereas the inhibitory protein HSPBP1 was increased by twofold, thus indicating a suppression of HSPA1 function (Fig. 1E).
Finally, the microarray results identified significant changes in apoptosis-related pathways (Fig. 1F). Following 8 hr of in vitro aging, the level of BAX protein declined over 30%, while BCL2 and BIRC2 protein abundance increased. In addition, phosphorylation of TP53 at the regulatory (activating) phosphorylation sites S33 and S392 increased slightly (22% and 25%, respectively) (Fig. 1F). Caspases 1, 3, 6, 7, and 9 all increased in abundance whereas FAS, FASL, and the caspase substrate DFFA all decreased in abundance (Fig. 1F).
In vitro aging induced changes in the meiotic spindle
Previous reports demonstrated that prolonged in vitro culture of mature oocytes leads to cytological changes in the position and organization of the meiotic spindle, cortical cytoskeleton, and calcium signaling machinery (Meyer and Longo 1979; Takahashi et al. 2013; Zhang et al. 2011). The antibody microarray results indicated changes in kinase signaling pathways (for example, MAPKs, CDKs, and AURKA) that impact spindle morphology and position. Therefore, morphological changes in the structure and localization of the MII spindle were examined and quantified by confocal microscopy at specific times during prolonged in vitro culture. MII oocytes cultured up to 24 hr were fixed at specific times of culture, processed for confocal immunofluorescence, and labeled with an antibody against acetylated tubulin (TUBA1A).
The pattern of acetylated tubulin provided a measure of metaphase spindle microtubule stability as well as spindle length, organization, and position (Fig. 3; asterisks identify the location of spindles with chromosomes). Spindle length increased significantly (P<0.05) after 24 hr of culture (Figs. 3A-C; quantified in Fig. 4A, where the mean spindle length increased from 21.1 μm in freshly isolated oocytes to 25.8 μm at 4 hr, 27.1 μm at 8 hr, and 31.3 μm at 24 hr of culture). Most spindles remained organized and near the oocyte cortex through the first 8 hr of in vitro culture (Fig. 3A), but fell away from the cortex after 24 hr (Figs. 3C, 3D, 3E, and 4B). By 24 hr of culture, 97% of spindles had detached one (Fig. 3C) or both (Fig. 3D) spindle poles from the cortex (Fig. 4B). All control oocytes contained chromosomes align along the metaphase plate in the center of the spindle (100%). Chromosome misalignment, however, was evident after only 4 hr of culture, and remained detectable at all later time points (4 hr =11%; 8 hr = 6%; 24 hr = 14%) (Fig. 3E and 4B).
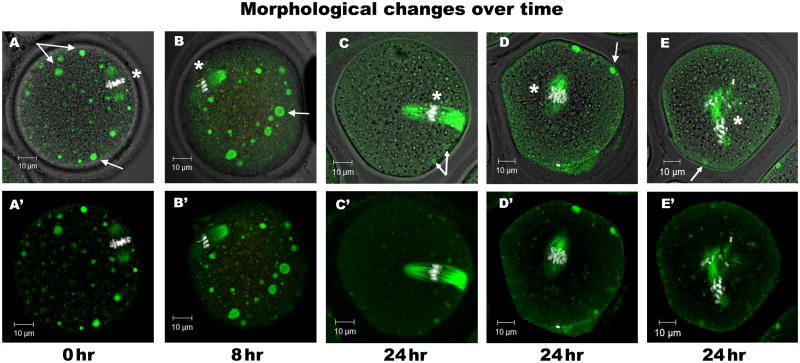
Fig. 3. Morphological changes in the metaphase spindle and lysosomes during in vitro aging.
LAMPA antibody was used to identify lysosomes and acetylated tubulin to label MII spindles in oocytes cultured in vitro for 0 hr (A, A′), 8 hr (B, B′), or 24 hr (C, C′, D, D′, E, E′). Confocal microscopy enabled the examination of intracellular structures throughout each oocyte. Freshly collected oocytes (0 hr) contained numerous large lysosomes (green spherical vesicles) dispersed throughout the cytoplasm and an intact MII spindle with both spindle poles located near the oocyte cortex. After 8 hr in culture, lysosomes appeared to increase in number and size. After 24 hr of culture, most spindles had released one (C, C′) or both poles (D, D′) from the cortex; some spindles had become disorganized and displace chromosomes (E, E′). Arrows indicate LAMPA (green) vesicles; asterisks indicate the metaphase spindle, with microtubules labeled for acetylated tubulin (green) and chromosomes labeled with Hoechst 33258 (white). The number of oocytes per group; 0 hr, 22; 4 hr, 28; 8 hr, 36; 24 hr, 33. Experiments were replicated a total of four times using 20 mice. Scale bars, 10 μm.
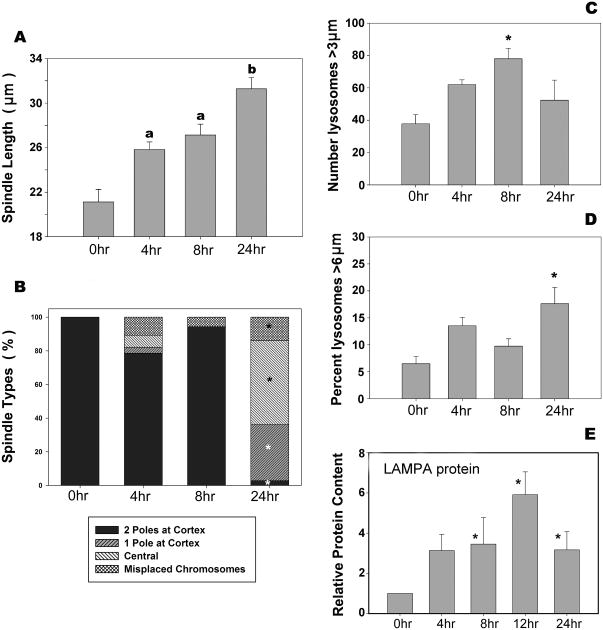
Fig. 4. Significant changes in spindle morphology and lysosome quantity and content.
Oocytes cultured in vitro for 0, 4, 8, or 24 hr were fixed, co-labeled for acetylated tubulin and LAMPA, and imaged by confocal microscopy (Fig. 3). (A) Average lengths of metaphase spindles at the time points shown. All aged oocytes (4, 8, and 24 hr) contained spindles that were significantly longer than 0 hr, and spindles in 24 hr in vitro-aged oocytes were significantly longer than at 0, 4, and 8 hr. ‘a’ and ‘b’ indicate significant differences among bars without letters (P<0.05). (B) Each oocyte was also graded according to spindle location –two spindle poles, one spindle pole or no spindle poles (central) visible near the oocyte cortex– and whether or not the spindle remained intact with chromosomes aligned on the metaphase plate or if the spindle was disorganized (misplaced chromosomes). After 24 hr of culture, there was a significant increase (*P>0.05) in all types of displaced spindles. (C-E) All large lysosomes (spherical green vesicles) within three confocal sections (top, bottom and central) of each oocyte were measured and classified by size (diameter). The total number of large lysosomes (>3 μm) in these three sections was calculated per oocyte. (C) The average number of large lysosomes (>3 μm) per oocyte after 8 hr of culture was significantly higher than at 0, 4, or 24 hr. (D) The percentage of large lysosomes that were (> 6 μm) was significantly higher at 24 hr than at earlier time points. (E) LAMPA is a protein commonly found in lysosomes. Western blot analysis was used to compare the levels of LAMPA protein in oocytes over time in vitro, and demonstrated a significant increase in LAMPA protein abundance after 8, 12, and 24 hr of culture. Error bars are standard error of the means. Number of oocytes per group: 0 hr, 22; 4 hr, 28; 8 hr, 36; 24 hr,33. Experiments were replicated a total of four times using 20 mice.
Effect of in vitro aging on lysosomal activity in oocytes
The number, size, and distribution of lysosomes were also assessed following different durations of in vitro aging by confocal immunofluorescence with an anti-LAMPA antibody (Fig. 3, arrows). The total number of large (>3 μm) lysosomes increased significantly (P<0.05) between 0 to 8 hr of culture, then declined after 24 hr (Fig. 4C). This decrease in the total number between 8 to 24 hr was due, at least in part, to the fusion of lysosomes to form larger lysosomes (>6 μm) (Fig. 4D). To determine if these morphological changes correlated with a change in total lysosomal mass, the lysosomal load of oocytes was estimated by Western blot analysis of LAMPA protein in groups of oocytes recovered after different periods of in vitro aging. The relative amount of LAMPA protein per oocyte detected by Western blots was found to increase progressively up to 12 hr, and then declined somewhat in total LAMPA content at 24 hr (Fig. 4E).
Comparison of in vitro and in vivo aging on the meiotic spindle and lysosome activity
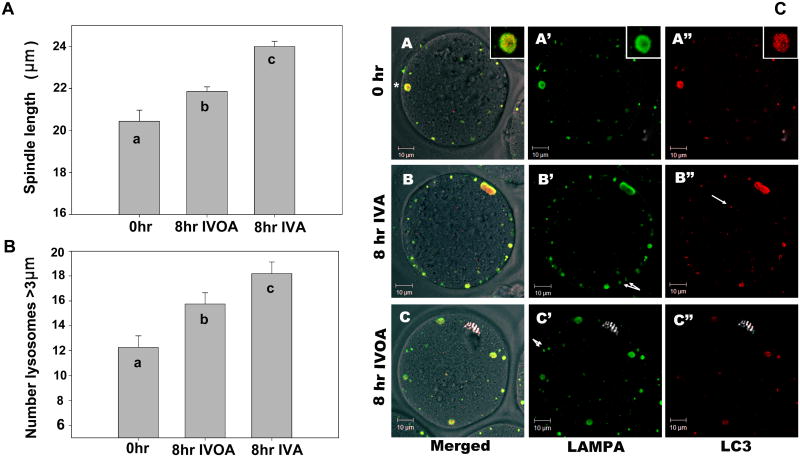
Previously published studies focused primarily on either in vivo or in vitro aging, without comparisons between the two. In order to establish if the same changes in spindle morphology and lysosomal content typical of oocytes subjected to in vitro aging occur during in vivo aging, we compared oocytes that had been subjected to 8 hr of either process. The aged oocytes were examined by confocal immunofluorescence microscopy to characterize spindle morphology, the relative number and size of lysosomes, as well as presence of the autophagy marker LC3. Spindle length during in vivo aging increased significantly compared to fresh controls (P<0.03) (Fig. 5A), but spindles in oocytes subjected to in vivo aging were significantly shorter than those present in oocytes following 8 hr of in vitro aging (P=0.0001). In addition, the majority of metaphase spindles maintained both poles at the oocyte cortex after 8 hr of in vivo aging (data not shown), similar to the phenotype following in vitro aging (Fig. 4B).
Fig. 5. Aging in vivo differs from in vitro, but all large lysosomes are autophagic.
Oocytes were recovered from the oviduct at 14 hr post-hCG administration, and either fixed immediately or cultured, similar to previous experiments. Additional oocytes were aged 8 hr in vivo, collected from oviducts at 22 hr post-hCG, and fixed immediately. Spindle length and lysosome biogenesis were compared between oocytes aged in vivo (IVOA) or in vitro (IVA). Oocytes at 0 hr, or after 8 hr in vivo or in vitro aging were co-labeled for acetylated tubulin and LAMPA (lysosomes), as described in Figures 1 and 2. Spindle length, organization, and lysosome size were measured as previously described. (A) Spindle length after 8 hr of in vivo aging was significantly longer than 0 hr controls, but shorter than those from 8 hr in vitro-aged populations. As seen in earlier studies, the number of large lysosomes was significantly increased after 8 hr of in vitro culture. (B) The number of large lysosomes in oocytes aged in vivo was also significantly increased above 0 hr, but this increase was less than occurred during in vitro aging (8hr IVA). ‘a’, ‘b’, ‘c’ indicate bars are significantly different (P<0.05). The number of oocytes used: 0 hr, 59; 8 hr in vivo-aged, 169; 8hr in vitro-aged, 130.)
To determine if the large lysosomes are indicative of autophagy, oocytes were co-labeled for LAMPA (lysosomes, green) and LC3 (autophagy, red) and imaged by confocal microscopy. All large (>3 μm) lysosomes in controls and 8 hr in vivo-aged and in vitro-aged oocytes were identified as being autophagic-lysosomes (co-labeled with LAMPA and LC3). (A*) is one such autophagic-lysosome (see A* enlarged inset). Some small vesicles were either lysosomes or autophagic, and labeled with only one marker. For example, the single arrow (B″) identifies a vesicle with LC3 but not LAMPA. Similarly, double arrows in B′ and C′ identify vesicles with LAMPA but no LC3. Scale bar, 10 μm.
Comparison of the number and size of lysosomes in oocytes aged in the oviduct revealed that 8 hr of in vivo aging resulted in a significant increase in the total number of large (>3 μm) lysosomes relative to freshly isolated oocytes (P>0.01). This increase during in vivo aging was lower than occurred in oocytes subjected to 8 hr of in vitro aging (P=0.06) (Fig. 5B). Similar results were obtained by Western blot analysis of LAMPA content (see below).
Large lysosomes in oocytes show markers of autophagy
The protein microarray results and the morphological analysis of aged oocytes indicated the activation of signaling pathways in response to cellular distress. Enlarged lysosomes in particular implicate possible autophagy, a mechanism of defense against oxidative and environmental stresses (Mehrpour et al. 2010; Moore et al. 2006a). To determine if the large lysosomes observed in aging oocytes were lysosomal autophagosomes, we co-labeled oocytes with antibodies against the lysosomal marker LAMPA (Fig. 5C-A′, B′, C′) and the autosomal marker LC3 (microtubule-associated protein 1A/1B-light chain 3) (Fig. 5C-A″, B″, C″, red). Regardless of the treatment, all large (>3 μm) lysosomes in oocytes (0 hr controls, or 8 hr of in vitro or in vivo aging) co-labeled with LAMPA and LC3 (Fig. 5C-A) – therefore all large lysosomes were autophagic. Vesicles smaller than 3 μm were a mixture of organelle types, with some co-labeling similarly to the large vesicles while others labeled with either LAMPA (Fig. 5C-B″, arrows) or LC3 (Fig. 5C-B″, arrow), indicating that these had not fused to form lysosomal autophagosomes.
Comparison of the effects of in vitro and in vivo oocyte aging on signaling components
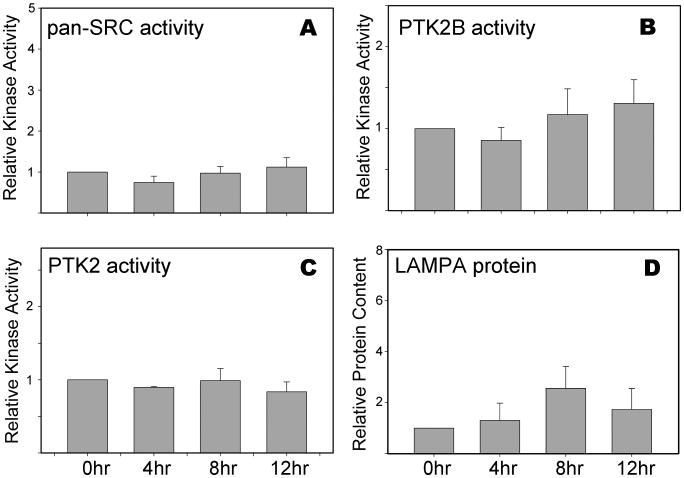
The significant changes in signaling pathway components and the morphological changes observed in response to in vitro aging were less dramatic during in vivo aging. This difference raised the question of whether or not changes in kinase signaling pathways also occur during prolonged residence in the oviduct. To test the effect of in vivo aging on oocyte signaling components, we performed Western blot analysis for several proteins found to respond significantly to in vitro aging. As seen in Figure 6, the activation state of SRC-family kinases, PTK2, and PTK2B did not change significantly during 12 hr of aging in the oviduct. The lysosome protein LAMPA, however, appeared to increase slightly in abundance after 8 hr in the oviduct, but the change was not significant (Fig. 6D). These results indicate that prolonged residence in the oviduct does not induce the same changes in oocyte signaling pathways that occur in response to in vitro culturing.
Fig 6. SRC, PTK2, and PTK2B signaling remains quiescent after 12 hr of in vivo aging.
Oocytes recovered from the oviduct at 14, 18, 22, and 26 hr post-hCG administration were analyzed as in Figure 5; these times corresponded to 0, 4, 8, and 12 hr of in vitro aging. Blots were probed with antibodies to activated (A) pan-SRC-family kinases (B) anti-PTK2B phospho-Y579, (C) anti-PTK2 phospho-Y576, or (D) anti-LAMPA proteins. Values represent the mean of kinase protein corrected for GAPDH content, and expressed relative to that present at 0 hr of culture (arbitrarily set as 1.0 on the y-axis). Three to four sets of oocytes were done for each bar. Error bars indicate standard error of the mean. While some minor changes in protein signal were observed at different times for the in vivo-aged oocytes, none were significantly different from the controls (P>0.05).
Discussion
Effect of in vitro aging on oocyte signaling pathways
Many cytological changes occur in oocytes during post-ovulatory aging, but little is known about the signaling pathways involved in this process. We proposed that changes in the expression or activation state of protein kinases key to known signaling pathways would report the extent to which specific pathways were activated or suppressed during the process of in vitro aging. The approach correlated cytological changes in the oocyte that were typical of in vitro aging under standard culture conditions with an unbiased antibody microarray-based quantitation of various signaling pathways that are regulated by protein kinases known to respond to stimuli. The microarray output reported changes in protein expression and post-translational modification (phosphorylation).
The antibody microarray is a powerful tool for interrogating cell-signaling mechanisms because of its broad coverage of proteins that can be screened for expression and phosphorylation, economy of scale, and high sensitivity. This methodology is limited, however, by the number of high-quality, specific antibodies as well as the requirement that the captured dye-labeled proteins from cell lysates are not denatured. Antibody-captured, native proteins can also reside in complexes, so changes in protein levels or phosphorylation status in different model systems may also reflect alterations in protein-protein interactions and cross-reactivity with non-target proteins. Consequently, key changes detected with antibody microarrays should be validated by alternative approaches, including immunoblotting performed under denaturing conditions. Given that antibody microarrays can be several orders of magnitude more sensitive than Western blots, the validation procedure may fail in some cases. Nevertheless, we were able to successfully confirm several of the leads provided by the antibody microarray analyses.
Our experimental design involved culturing ovulated oocytes from which cumulus cells were removed prior to culture. While cumulus contact with the oocyte during maturation is critical for oocyte viability, the presence of these somatic cells does not improve developmental potential during in vitro aging (Takahashi et al. 2009) and may, in fact, impair oocyte quality (Miao et al. 2009). During in vivo aging in the oviduct, however, cumulus cells are expected to lose direct contact with the oocyte, although they remain in close proximity to the oocytes along with other oviductal cells and content (follicular fluid, oviductal fluid, etc). Considering that the complex oviduct environment reduced many aspects of the oocyte stress response observed to occur after in vitro culture (see below), further study should focus on which oviductal factors are involved in this suppressed response.
In order to facilitate comparison of our results with those of other studies, we used the morphology and position of the meiotic spindle, which is consistently reported to undergo significant changes in morphology and position during in vitro aging (reviewed in Miao et al. 2009; Takahashi et al. 2013), to monitor and quantify the progress of in vitro aging under our culture conditions and to provide a baseline for comparison with potential changes in cell signaling. We also correlated change in meiotic spindle cytology to lysosome biogenesis, which change consistently during in vitro aging. The dynamics of lysosomal compartmentalization represent a novel aspect of oocyte homeostasis during in vitro aging, and thus may be as important an indicator as spindle structure for reporting the health of aged oocytes.
Effects of in vitro aging on spindle size and orientation
Metaphase spindle integrity and location are critical for the progression of development following fertilization. During MII arrest, the spindle aligns with both poles near the oocyte cortex. Following fertilization and egg activation, one pole of the spindle swings away from the oocyte cortex while the synchronous initiation of anaphase induces chromosome separation and polar body extrusion. Failure to maintain proper localization of the spindle at the cortex leads to abnormal cell division with large polar bodies and aneuploidy – phenotypes typically associated with post-ovulatory aging of oocytes (Mailhes et al. 1998; Santalo et al. 1987). Our results demonstrate that spindle elongation, which was previously reported in aged oocytes (Takahashi et al. 2013), begins early in culture (before 4 hr), and the spindle continues to lengthen with extended culture times (Fig. 4A). Oocyte spindles lengthened significantly even while both poles remained near to the oocyte cortex (Fig. 4B), indicating that the elongation process is not simply due to the loss of cortical anchorage. We chose to label spindles for acetylated alpha tubulin since the acetylation is thought to stabilize microtubules (de Pennart et al. 1988) whereas decreased acetylation is associated with the in vitro maturation of mouse oocytes (Sanfins et al. 2003). Meiotic spindles were acetylated in all oocytes, thus acetylation of alpha tubulin did not prevent spindle elongation. Studies in somatic cells have shown that CDK1 kinase activity is associated with microtubule shortening in the mitotic phase (Verde et al. 1992), therefore the changes in CDK1 activity detected by microarray (Fig. 1B) might be contributing to the continuous spindle lengthening observed in the in vitro-aged oocytes (see Supplemental Discussion). Similarly, deletion of MAPK14 or altered disruption of TPX2 (of the AURKA pathway) can lead to spindle elongation (Brunet et al. 2008; Ou et al. 2010).
It is interesting that the spindle maintains its overall integrity and location at the cortex for the first 8 hr of in vitro aging. This correlates with the increased activity of SRC-family kinases (Fig. 2D) as these kinases are involved in the maintenance of normal spindle organization while their suppression consistently results in meiotic spindle disorganization (Luo et al. 2009; McGinnis et al. 2007). Thus the high levels of SRC-family kinase activity that occur during the first 8 hr of in vitro aging may actually help maintain spindle integrity. Indeed, the levels of active SRC-family kinase were declining after 24 hr of culture, which corresponded to the age when the spindles fall away from the cortex and become less organized. We thus hypothesize that spindle changes in in vitro-aged oocytes are the result of shifts in intracellular signaling cascades such as SRC-family kinases, CDK1, MAPK14, and/or AURKA. By controlling these pathways during in vitro culture, it may be possible to prevent spindle elongation and, ultimately, loss of spindle integrity and chromosome displacement.
Selective changes in cell-cycle kinase pathways
Pathways that regulate spindle dynamics are closely tied to cell cycle control pathways. As in vitro aging resulted in imbalances in the protein kinase / phosphatase-mediated cell-cycle pathways, might these perturbations also affect the processes that maintain the oocyte in meiotic arrest? The antibody microarray suggest that multiple cell-cycle pathway components were modified during in vitro aging; in several cases, components that activate as well as repress cell-cycle progression were both stimulated. Thus, the activation of these opposing pathways during in vitro aging may reflect an effort by the oocyte to remain in meiotic arrest.
MAPK1/3
In freshly ovulated oocytes, fertilization and egg activation lead to a rapid decline in CDK1 kinase activity (90 min), but a much slower decline in MAPK1/3 kinase activity (8 hr) (Moos et al. 1995). The decline in CDK1 correlates with meiotic resumption while the later decline of MAPK1/3 is required for the formation of pronuclear envelopes (Moos et al. 1996) (CDK1; see Supplemental Discussion). Previous studies indicate that MAPK1/3 associates with the oocyte meiotic spindle and regulates microtubule characteristics (Verlhac et al. 1996). In the present study, our antibody microarray analysis detected an increase in MAPK1/3 protein levels after 8 hr of in vitro aging as well as increased phosphorylation at both regulatory (activating) phosphorylation sites (T202/Y204) (Fig. 1B), which is required for kinase activation (Ehses et al. 2002). This increased MAPK1/3 kinase activity may be responsible for the increased phosphorylation of CDC25C (Fig. 1B) (Wang et al. 2007). Thus, in vitro-aged oocytes appear to be following a similar pattern as fresh oocytes that are resuming meiosis, exhibiting declining CDK1 activity but increased MAPK1/3 activity.
AURKA
AURKA localizes to centrosomes and spindle poles, and is required for normal metaphase-spindle formation (Glover et al. 1995; Kimura et al. 1997; Sasai et al. 2008) and plays a critical role in the meiotic cell cycle (Crane et al. 2004; Liu and Ruderman 2006; Yao et al. 2004). In oocytes, AURKA phosphorylates and activates the translational regulatory protein CPEB, leading to the polyadenylation of stored mRNA and increased protein synthesis (Crane et al. 2004). Our antibody microarray data and follow-up immunoblots showed an increase in the level of AURKA protein (Fig. 1B; 2A), although the smaller difference in AURKA protein observed by Western blot possibly reflects the antibody's affinity for non-denatured (microarray) versus denatured (Westerm blot) proteins. This increased abundance of AURKA may support the increases in both CDK1 and MAPK1/3 proteins observed in the in vitro-aged oocyte, as it also normally regulates the synthesis of these two kinases in oocytes (Nishimura et al. 2009). Since AURKA helps maintain meiotic arrest, in-depth studies are needed to further explore how AURKA maintains high levels of cell cycle factors and spindle integrity during extended oocyte culture.
SRC and PTK2B kinase pathways
Protein tyrosine kinases of the SRC and FAK families can significantly impact oocyte quality. Oocytes express the SRC-family kinase FYN at higher levels than other SRC-family members, and require FYN during oocyte maturation as well as pronuclear congression (Levi et al. 2010; Luo et al. 2010; McGinnis et al. 2007; McGinnis et al. 2009; Mehlmann and Jaffe 2005; Meng et al. 2006). FYN is also important for the maintenance of cortical cytoskeletal organization and spindle positioning (Luo et al. 2009). The results of the antibody microarray and Western blot analyses demonstrated an increase in abundance and activity of SRC-family kinases during in vitro aging (Figs. 1C; 2). Interestingly, CSK, the negative regulator of SRC-family kinase activity, increased over 80% as measured by microarray, which could represent an effort by the oocyte to respond to elevated SRC-family kinase activity by deactivating it. The closely related ABL kinase also increased in abundance during in vitro aging (Fig. 1C). While SRC-family kinase activity normally increases in a highly localized manner following fertilization (McGinnis et al. 2007), fertilization-induced changes in ABL kinase are associated primarily with the actin cytoskeleton (Walker et al. 1996), and have only been reported in sea urchin oocytes (Moore and Kinsey 1994). In mammalian oocytes, active ABL kinase has been most closely tied to DNA repair following genotoxic stress (Kim et al. 2013; Maiani et al. 2011).
Both SRC-family and PTK2B kinases figure prominently in the cellular response to different forms of stress. For example, both SRC-family and PTK2B kinases are activated in response to hyper- and hypo-osmotic stress (Cohen 2005; Reinehr et al. 2005; Tai et al. 2002) and participate in the regulation of voltage and volume-dependent ion channels (Nilius and Droogmans 2001; Samak et al. 2011). SRC-family kinases may also act downstream of the TRPV4 channel following hypo-osmotic challenge (Shahidullah et al. 2012a; Shahidullah et al. 2012b). SRC-family and PTK2B kinases are also critical in cellular responses to oxidative stress (Martel-Gallegos et al. 2013). The increased activity of SRC-family kinases and PTK2B during in vitro aging thus indicates that oocytes experience significant stress resulting from osmotic and/or redox-related insults. The elevated SRC-family kinases and PTK2B activity could, in turn, impact ion channel permeability (Byron and Lucchesi 2002; Heidkamp et al. 2005) and thereby contribute to the known detrimental effects of in vitro aging on calcium homeostasis and signaling capability (Takahashi et al. 2009; Zhang et al. 2011).
In addition to the well-established role in stress response, PTK2B functions in cell survival. PTK2B-mediated inhibition of TP53 is an intrinsic mechanism promoting cell survival in many cell types (Lim et al. 2010; Strappazzon et al. 2007). If a similar situation exists in oocytes, the PTK2B protein may assist in protecting against TP53-stimulated cell death during in vitro aging.
Selective activation of cell-death pathways
In vitro aging of oocytes causes an increase in the production of reactive oxygen species during extended in vitro culture (Takahashi et al. 2009). Oxidative-damage-induced protein unfolding leads to the upregulation of molecular chaperones, such as heat-shock proteins that activate many kinase signaling pathways including those involved in autophagy and cell death (Agarwal et al. 2005; de Lamirande and O'Flaherty 2008; Hoffman and Brookes 2009; Moore et al. 2006a; Storz 2006). Abnormal calcium signaling following the fertilization of aged oocytes is also known to induce apoptosis (Gordo et al. 2002). Therefore, we examined our antibody microarray results for pathways associated with stress and cell death including MAPK14, MAPK8/9/10, heat shock proteins, apoptosis cascades, and DNA damage repair.
p38 MAPK and JNK pathways
Stress-activated MAP kinases, including JNK isoforms (MAPK8/9/10) and p38 MAPK (MAPK14), play critical roles in maintaining cell survival. Our antibody microarray data indicated a selective increase or decrease of specific stress-activated MAPKs. MAPK8/9/10 expression increased 60%, with a simultaneous increase in activating phosphorylation at T183 and Y185 after 8 hr of in vitro culture (Fig. 1D; 2I), whereas many of the factors involved in MAPK14 signaling were significantly decreased (Fig. 1D). Thus, it appears that in vitro-aged oocytes have selectively deactivated the MAPK14 stress pathway while increasing the MAPK8/9/10 pathway. Intriguingly, a similar phenomenon is seen in some proliferative human cancers down-regulating MAPK14 and up-regulating MAPK8/9/10 (Matesic et al. 2012). It is clear that the JNK pathway could play several different roles in response to in vitro aging, the details of which have yet to be worked out.
Lysosomes and autophagy
Autophagy is a critical pathway by which cells remove damaged organelles and proteins. The mammalian oocyte contains the machinery necessary for autophagy: This process contributes to the early loss of oocytes following birth in mice and rats (Escobar et al. 2008; Escobar et al. 2010; Rodrigues et al. 2009) and plays an essential role in oocyte maturation and preimplantation development (Song et al. 2012). Aging of oocytes in vitro is also detrimental to organelles such as mitochondria and endoplasmic reticulum (Gordo et al. 2002; Takahashi et al. 2013), so an increase in autophagy in the aged oocyte may help maintain oocyte intracellular homeostasis. In the present study, LAMPA was used to identify lysosomes, an indicator of both apoptosis and autophagy, and a marker of environmental stress (Moore et al. 2006b). During in vitro aging, the number of large (>3 μm) lysosomes increased significantly during the first 8 hr of culture (Figs. 3 and 4). Many of the lysosomes fused to form extremely large lysosomes (>6 μm). Between 8 and 24 hr of in vitro aging, the overall number of lysosomes decreased, although the percentage of large one (>6 μm) increased significantly (Fig. 4C). In the later stages of autophagy, autophagocytic vesicles fuse with lysosomes to form very large vesicles that contained both lysosomal protein LAMPA and LC3 protein, a marker of autophagy. To determine if the extremely large lysosomes present in aging oocytes are signs of increased autophagy, we co-labeled oocytes after 8 hr of in vitro or in vivo aging for LC3 and LAMPA. Regardless of treatment (0 hr control or 8 hr in vivo or in vitro aging), all lysosomes >3 μm in diameter contained both LAMPA and LC3 proteins, indicating they are lysosomal-autophagosomes (Fig 5C). Therefore, the overall increase in large lysosomes during extended oocyte culture represented an increase in autophagy. Further studies are needed to better understand the roles of autophagy in aging oocyte.
Comparison of in vitro and in vivo effects on signaling pathways
The conditions typical of in vitro oocyte culture are quite different from those in the oviduct. The oviductal milieu includes enriched oviductal fluids that cannot be reproduced in current culture systems. The primary goal of this study was to define the impact of extended periods of in vitro culture on oocyte biochemistry. The above phospho-proteomic results for in vitro oocyte aging demonstrated significant changes in 43 of the proteins tested, with 9 of these validated by Western blot. Many of the observed changes appear to correlate with known deleterious effects of in vitro aging on oocyte quality.
In vivo aging of oocytes has also been shown to cause changes to specific pathways including calcium homeostasis, cell cycle, and apoptosis (Takahashi et al. 2013). Our analysis of the impact of intra-oviduct aging on the SRC and FAK family kinases failed to detect significant changes in these specific enzymes after 8 hr of aging (Fig. 6). Interestingly, the morphological analysis of lysosomes and spindle determined that in vivo-aged oocytes undergo similar but less dramatic changes than were detected in the in vitro-aged oocytes. There was a significant increase in spindle length and lysosome number compared to control (0 hr) oocytes, but the in vivo-aged spindles were still significantly shorter than their in vitro-aged counterparts (Fig. 5A-B). There was a slight, but insignificant, increase in LAMPA after 8 hr of in vitro aging (Fig. 6D) that correlated with an increase in the number of large lysosomes formed during in vivo aging (Fig. 5B). Thus, changes in kinase activity and pathways during in vivo aging are more subtle and difficult to detect by Western blot. To our knowledge, few studies (Abbott et al. 1998) have directly compared the effects of post-ovulatory aging in vivo versus in vitro; more of these types of studies need to be done to determine the biological significance of the aging process. A more global analysis of signaling changes during in vivo aging might also reveal other defects not addressed within the limits of our study.
Conclusions
The mature mammalian oocyte awaits fertilization in a quiescent state of meiotic arrest that is characterized by suppressed gene expression and protein synthesis. Using an antibody microarray to detect simultaneous changes in multiple pathways, the present study demonstrated that mouse oocytes respond to prolonged in vitro culture by initiating diverse changes in the activity of the protein kinases that control critical cellular processes, including calcium homeostasis, cell-cycle control, responses to osmotic and/or oxidative stress, and lysosome-mediated autophagy. While more detailed analyses of the time course of these changes need to be done, these results are consistent with the following sequence of events: Exposure of mature oocytes to the culture environment triggers the rapid activation of SRC-family and PTK2-family kinases within 4 hr, possibly reflecting osmotic or oxidative stress. These activated kinases could modify the activity of cation channels in the plasma membrane and endoplasmic reticulum, causing an imbalance in calcium homeostasis and possibly impairing the ability of oocytes to respond to fertilization. Changes in cytoplasmic calcium, together with the capacity of FYN and PTK2B to modify the cortical actin cytoskeleton, could trigger inappropriate cortical vesicle exocytosis, leading to zona pellucida ‘hardening’ and reduced fertilizing capacity – all known symptoms of post-ovulatory aging.
The fact that MAPK8/9/10 activities increased, together with phosphorylation of HSPB1, indicates that a pro-apoptotic response to stress was underway by 8 to 12 hr. This pathway was countered in 8-hr in vitro-aged oocytes by elevated levels of BLC2 and decreased BAX. Our findings also provide the first demonstration that in vitro-aged oocytes exhibit a progressive increase in the number of large lysosomes and abundance of LAMPA protein. Large lysosomes were enriched in LC3 protein, indicating that they were lysosomal autophagosomes and part of an autophagic process that would likely reduce oocyte quality significantly. Together these results demonstrate that in vitro culture for as little as 8 hr caused significant changes in protein kinase-mediated pathways and can trigger an environmental stress response involving escalating conflict between pro- and anti-survival forces within the oocytes. The inappropriate activation of kinases normally involved in fertilization, together with the progressive increase in lysosomal autophagy, would significantly reduce fertilization and developmental potential of in vitro-aged oocytes.
Materials and Methods
Reagents
All reagents were purchased from Sigma Chemical Corp., St Louis, MO, USA, unless otherwise stated.
Oocyte Collection
Cumulus-enclosed oocytes were collected from CF1 female mice aged 6-7 weeks (Harlan Sprague-Dawley, Indianapolis, IN). Mice were housed in a temperature- and light-controlled room on a 14 hr light:10 hr dark cycle, and experiments were conducted in accordance with National Institutes of Health guidelines for the Care and Use of Laboratory Animals (National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. et al. 2011). All experimental procedures were approved by the University of Kansas Medical Center Internal Animal Care and Use Committee. Mice were euthanized by isoflurane inhalation anesthesia, followed by cervical dislocation. Females were stimulated with 5 IU of equine chorionic gonadotropin (PG600) (Intervet Inc, Millsboro, DE, USA) followed 48 hr later with 5 IU of hCG) (Chorulon) (Intervet Inc, Millsboro, DE, USA). Oviducts were collected at 13-14 hr post-hCG. Cumulus-oocyte complexes were released into HEPES-buffered KSOM medium (FHM) (Chemicon-Millipore, Billerica, MA) supplemented with 4 mg/ml fatty acid-free bovine serum albumin (BSA) (Sigma catalog #A7030). Cumulus-oocyte complexes were washed briefly in low dose (0.3 mg/ml) hyaluronidase in FHM to remove cumulus cells.
Oocyte Culture
Cumulus cell were removed from oocytes prior to culture, a necessary step in assisted reproductive technology protocols, for polar body biopsy and in scientific studies of oocyte biology or any procedures that require visualization of the oocyte prior to culture. While the presence of cumulus cells provides critical nourishment to the oocyte during in vitro maturation, the loose, expanded cumulus matrix does not improve oocyte developmental potential during in in vitro culture (Takahashi et al. 2009); in fact, the presence of cumulus cells accelerates and increases the effects of in vitro aging (Miao et al. 2009). Denuded oocytes were washed once in KSOMaa (Evolve; Zenith Biotech, Guilford, CT, USA) with 4 mg/ml BSA, and then transferred to 500 μl KSOMaa in 4-well dishes (NUNC from Thermo Scientific, Rochester, NY USA). Oocytes were cultured at 37.2°C in a humidified incubator with 6% CO2 in air for the appropriate times determined by the assay – in vitro-aged oocytes were collected at 13-14h post-hCG, then cultured for 0-24 hr; control oocytes were freshly collected (0 hr) and processed at 14 hr post-hCG. Culture periods for this study were chosen based on previously published reports that demonstrated clear post-ovulatory aging (loss of developmental competence) by 20-22 hr post-hCG. Therefore, oocytes collected at 14 hr post-hCG and aged 8 hr (22 hr post-hCG) were used for the protein phospho-array. The other times (4 or 12 hr) were designed to fit around the 8-hr time point used for the phospho-array study. A 24-hr aging period is commonly used as an extreme of in vitro aging (Fissore et al. 2002).
Western Blots
Oocytes processed for Western blots were transferred to FHM with 4 mg/ml polyvinyl alcohol and no BSA (Biggers et al. 1997). Sets of 20 oocytes were transferred to 0.6-ml tubes (Avant #AVSS65, Midsci Corp, St Louis, MO USA), and then centrifuged to pool the oocytes. Media were removed, Dulbecco's phosphate buffered saline (DPBS) was added, and the samples were then centrifuged again for 2 minutes; this washing was repeated three times. Following the last wash, all DPBS was removed and 2 μl of 2× SDS-PAGE buffer was added to each tube. Samples were heated at 90°C for 5 minutes, and then frozen at -20°C. SDS-PAGE buffer was supplemented with phosphatase inhibitors (40 μM phenylarsine oxide, 100 μM sodium orthovanadate, and 1 μM Calyculin-A) to prevent the loss of protein phosphorylation.
Aliquots (3-5 oocytes per lane) of each sample set were loaded in adjacent lanes of a microgel (lane width 1 mm × 0.75 mm) and, after electrophoresis, were transferred to Immobilon-P membranes (Millipore, Billerica, MA). Primary antibodies used for Western blots are listed in Table 1. Blots where the target protein kinase was larger than 57-kDa were probed with an antibody to the desired protein kinase, combined with anti-GAPDH as an internal control. Blots where the target protein was less than 55-kDa were prepared in duplicate, and probed separately with anti-GAPDH. The resulting X-ray films were scanned, and band intensities were quantified by measurement of integrated pixel intensity (band intensity less background) using Metamorph 7.1 software (Molecular Devices, Sunnydale, CA). The band intensity of each sample was divided by the intensity of GAPDH in that sample to correct for oocyte collection and loading errors. The values at different times of culture were expressed relative to the value of the 0 hr control, which was arbitrarily set at 1.0. The values obtained from 4-8 sample sets were analyzed by the Students' t-test or Mann-Whitney Rank Sum test to determine if they were significantly different from the 0 hr value (P<0.05; SigmaPlot, Systat Software, Inc, Chicago IL USA)
Table 1. Antibodies used for Western blots and immunofluorescence studies.
| Primary Antibody List | Catalog # | Supplier |
|---|---|---|
| AURKA | 4718S | Cell Signaling Technology, Danvers MA USA |
| LAMPA (LAMP1) | 1D4B | Developmental Studies Hybridoma Bank, Iowa City IA USA |
| LC3 | 2775 | Cell Signaling Technology, Danvers MA USA |
| MAPK3 (MAP Kinase 1/3) (Erk1/2) | 06-182 | Upstate Biotechnology Inc (now Millipore), Billerica MA USA |
| MAPK8/9/10 (JNK) | sc-571 | Santa Cruz Biotechnology Inc, Dallas TX USA |
| P130CAS (BCAR1) phospho-Y165 | 4015 | Cell Signaling Technology, Danvers MA USA |
| PTK2 (FAK) | C20 | Santa Cruz Biotechnology Inc, Dallas TX USA |
| PTK2 pY576 | 700013 | Invitrogen Corp., Camarillo CA USA |
| PTK2B phospho-Y579 (PYK2) | 44632G | Invitrogen Corp., Camarillo CA USA |
| SRC dephos-Y530 (clone 28) | AHO0051 | Invitrogen Corp., Camarillo CA USA |
| SRC (pan-SRC) | SC-18 | Santa Cruz Biotechnology Inc, Dallas TX USA |
| TUBA1A - acetylated (alpha-tubulin) | T7451 | Sigma Chemical Corp, St Louis, MO USA |
Fixation and immunofluorescence labeling
Methods for fixation and immunohistochemistry of whole oocytes were similar to those previously reported (McGinnis et al. 2011b; McGinnis et al. 2013). Briefly, oocytes were fixed in 2% paraformaldehyde (PFA) and permeabilized for 15 min in wash solution (DPBS + 4 mg/ml BSA + 0.5% Triton-X100). All fixatives and wash solutions were supplemented with 40 μM phenylarsine oxide, 100 μM sodium orthovanadate, and 1 μM Calyculin-A to inhibit phosphatase activity. Primary antibodies used are listed in Table 1. DNA and F-actin were labeled with Hoechst 33258 and Alexa 586-conjugated phalloidin, respectively (Molecular Probes, Eugene OR USA). Secondary antibodies for immunofluorescence were Alexa Fluor 488 or Alexa Fluor 568; goat anti-mouse or goat anti-rabbit, depending on the source of the primary antibody (Molecular Probes-Invitrogen, Eugene OR USA). Oocytes were imaged either at 63× on a Nikon T2000 inverted confocal or at 40× on a Zeiss LSM500 Pascal inverted confocal microscope.
Analysis of lysosome and spindle structure during in vitro and in vivo aging
To determine if in vitro aging affects overall size and distribution of lysosomes, oocytes were labeled for LAMPA and examined by immunofluorescence confocal microscopy by methods described above. Oocytes cultured for 0, 4, 8, or 24 hr in vitro were fixed in 2% paraformaldehyde. Oocytes from each culture period were mounted on glass slides in groups of 5-9. Each group was imaged in three planar sections: (1) equator, (2) top, and (3) bottom. Spindle length and large-lysosome diameters were measured using the analyses tools within the Zeiss LSM Image Browser (Carl Zeiss MicroImaging GmbH, Oberkochen, Germany). All lysosomes >3 μm detected within the above three images were counted for each oocyte. The total number of large lysosomes in these three sections was calculated as representative of the total number of large lysosomes per oocyte. In addition, each oocyte was imaged at the plane central to each spindle to enable analysis of spindle integrity and location within the oocyte. The values obtained from 5-18 sample sets were analyzed by Students' t-test or Mann-Whitney Rank Sum test to determine if they were significantly different from the 0 hr value (P<0.05; SigmaPlot, Systat Software, Inc, Chicago IL USA)
KAM-850 antibody microarray
Oocytes were processed similar to those for Western blots (see above). After the last wash, all DPBS was removed and tubes frozen at -80°C. Both control and 8-hr in vitro-aged oocytes were collected and processed on the same days. Collections were repeated daily until 2,000 oocytes per treatment (0 hr controls or 8 hr in vitro-aged) had been collected and frozen. The frozen samples were then shipped on dry ice to Kinexus Bioinformatics Corp. (Vancouver BC, Canada) for processing. The Kinex™ Antibody Microarray KAM-850 analyses were performed with detergent-solubilized protein lysates as described previously (Pelech et al. 2008). Briefly, protein lysates from control and treated oocytes were labeled with a fluorescent dye, and unincorporated dye molecules were removed by ultrafiltration. Purified, labeled proteins from the control and its corresponding treated sample were incubated simultaneously on opposite ends of a Kinex™ KAM-850 Antibody Microarray. Each Kinex™ antibody microarray analysis was based on duplicate measurements with 2 identical fields of 16 grids containing antibodies for 854 signaling proteins (517 pan-specific for expression; 337 phosphosite-specific). After probing, arrays were scanned using a ScanArray scanner (Perkin Elmer, Wellesley, USA) with a resolution of 10 μm. The resulting images were quantified using ImaGene (BioDiscovery, El Segundo, CA). To represent the data, we used the reported percentage change from control and the percentage error in the duplicate measures values from the final report generated by Kinexus Bioinformatics Corp.
Supplementary Material
Supplemental Fig. S1. Images of representative Western blots.
Oocytes cultured for 0, 4, 8, 12, or 24 hr of culture were analyzed by SDS-PAGE, as described in ‘Materials and Methods’. Blots were probed with antibodies to (A) activated pan-SRC Y529, (B) activated kinase anti-PTK2 phospho-Y576, (C) activated kinase anti-PTK2B phospho-Y579, (D) anti-pan-SRC protein, (E) anti-PTK2 protein, (F) anti-AURKA, (G) anti-MAPK 1/3 protein, (H) anti-MAPK8/9/10 protein, (I) anti-LAMPA, (J) anti-BCAR1 phospho-Y165, (K) anti-GAPDH. The bound antibody was detected and quantified as described in ‘Materials and Methods’.
Supplemental Table S1. KINEX™ KAM-850 antibody microarray report for analyses of in vitro culture mouse oocytes.
Data were analyzed by Kinexus Bioinformatics Corp. and selected columns from the original report are presented here in table format. “Antibody Code” represents the Kinexus proprietary antibody database record; “Globally Normalized” indicates fluorescence signal at the indicated time point, normalized to the global fluorescence signal for proteins in the sample; “%CFC” is the percentage change from 0 hr control; “Z scores” ((ratio 8 hr signal – 0 hr signal)/ sample standard deviation) reflects how large the changes are and how close the duplicate measurements are for each set of samples being compared for the same protein. Samples highlighted in pink represent those exhibiting signal increases judged to be significant based on the %CFC and Z scores, while blue represents samples exhibiting significant decreases in signal.
Acknowledgments
The LAMPA monoclonal antibody, developed by J. Thomas August, was purchased from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Iowa City IA USA. The authors thank Drs. Jane Shi and Hong Zhang for completing the antibody microarray, and Carol Carton for assistance with animals and Western blots. Thank you also to Drs. Margaret Petroff and Lane Christenson for the JNK and LC3 antibodies, respectively. This work was supported by NICHD grant HD062860 to WH Kinsey.
Funding: NICHD: HD062860 to WHK
Abbreviations
- FAK
focal adhesion kinase
- hCG
human chorionic gonadotropin
- MII
metaphase II
Footnotes
The authors have no conflict of interest with this research.
References
- Abbott AL, Xu Z, Kopf GS, Ducibella T, Schultz RM. In vitro culture retards spontaneous activation of cell cycle progression and cortical granule exocytosis that normally occur in in vivo unfertilized mouse eggs. Biol Reprod. 1998;59(6):1515–1521. doi: 10.1095/biolreprod59.6.1515. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen AG, Als-Nielsen B, Hornnes PJ, Franch Andersen L. Time interval from human chorionic gonadotrophin (HCG) injection to follicular rupture. Hum Reprod. 1995;10(12):3202–3205. doi: 10.1093/oxfordjournals.humrep.a135888. [DOI] [PubMed] [Google Scholar]
- Badenas J, Santalo J, Calafell JM, Estop AM, Egozcue J. Effect of the degree of maturation of mouse oocytes at fertilization: a source of chromosome imbalance. Gamete Res. 1989;24(2):205–218. doi: 10.1002/mrd.1120240208. [DOI] [PubMed] [Google Scholar]
- Biggers JD, Summers MC, McGinnis LK. Polyvinyl alcohol and amino acids as substitutes for bovine serum albumin in culture media for mouse preimplantation embryos. Hum Reprod Update. 1997;3(2):125–135. doi: 10.1093/humupd/3.2.125. [DOI] [PubMed] [Google Scholar]
- Brunet S, Dumont J, Lee KW, Kinoshita K, Hikal P, Gruss OJ, Maro B, Verlhac MH. Meiotic regulation of TPX2 protein levels governs cell cycle progression in mouse oocytes. PLoS ONE. 2008;3(10):e3338. doi: 10.1371/journal.pone.0003338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byron KL, Lucchesi PA. Signal transduction of physiological concentrations of vasopressin in A7r5 vascular smooth muscle cells. A role for PYK2 and tyrosine phosphorylation of K+ channels in the stimulation of Ca2+ spiking. J Biol Chem. 2002;277(9):7298–7307. doi: 10.1074/jbc.M104726200. [DOI] [PubMed] [Google Scholar]
- Cohen DM. SRC family kinases in cell volume regulation. American journal of physiology Cell physiology. 2005;288(3):C483–493. doi: 10.1152/ajpcell.00452.2004. [DOI] [PubMed] [Google Scholar]
- Crane R, Gadea B, Littlepage L, Wu H, Ruderman JV. Aurora A, Meiosis and Mitosis. Biology of the Cell. 2004;96(3):215. doi: 10.1016/j.biolcel.2003.09.008. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, O'Flaherty C. Sperm activation: Role of reactive oxygen species and kinases. Biochimica et Biophysica Acta (BBA) - Proteins & Proteomics. 2008;1784(1):106. doi: 10.1016/j.bbapap.2007.08.024. [DOI] [PubMed] [Google Scholar]
- de Pennart H, Houliston E, Maro B. Post-translational modifications of tubulin and the dynamics of microtubules in mouse oocytes and zygotes. Biol Cell. 1988;64(3):375–378. doi: 10.1016/0248-4900(88)90012-3. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Duffy P, Reindollar R, Su B. Changes in the distribution of mouse oocyte cortical granules and ability to undergo the cortical reaction during gonadotropin-stimulated meiotic maturation and aging in vivo. Biol Reprod. 1990;43(5):870–876. doi: 10.1095/biolreprod43.5.870. [DOI] [PubMed] [Google Scholar]
- Ehses JA, Pelech SL, Pederson RA, McIntosh CH. Glucose-dependent insulinotropic polypeptide activates the Raf-Mek1/2-ERK1/2 module via a cyclic AMP/cAMP-dependent protein kinase/Rap1-mediated pathway. J Biol Chem. 2002;277(40):37088–37097. doi: 10.1074/jbc.M205055200. [DOI] [PubMed] [Google Scholar]
- Escobar ML, Echeverria OM, Ortiz R, Vazquez-Nin GH. Combined apoptosis and autophagy, the process that eliminates the oocytes of atretic follicles in immature rats. Apoptosis : an international journal on programmed cell death. 2008;13(10):1253–1266. doi: 10.1007/s10495-008-0248-z. [DOI] [PubMed] [Google Scholar]
- Escobar ML, Echeverria OM, Sanchez-Sanchez L, Mendez C, Pedernera E, Vazquez-Nin GH. Analysis of different cell death processes of prepubertal rat oocytes in vitro. Apoptosis : an international journal on programmed cell death. 2010;15(4):511–526. doi: 10.1007/s10495-009-0448-1. [DOI] [PubMed] [Google Scholar]
- Fissore RA, Kurokawa M, Knott JG, Zhang M, Smyth J. Mechanisms underlying oocyte activation and postovulatory ageing. Reproduction. 2002;124(6):745–754. doi: 10.1530/rep.0.1240745. [DOI] [PubMed] [Google Scholar]
- Glover DM, Leibowitz MH, McLean DA, Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81(1):95. doi: 10.1016/0092-8674(95)90374-7. [DOI] [PubMed] [Google Scholar]
- Gordo AC, Rodrigues P, Kurokawa M, Jellerette T, Exley GE, Warner C, Fissore R. Intracellular calcium oscillations signal apoptosis rather than activation in in vitro aged mouse eggs. Biol Reprod. 2002;66(6):1828–1837. doi: 10.1095/biolreprod66.6.1828. [DOI] [PubMed] [Google Scholar]
- Harrison KL, Wilson LM, Breen TM, Pope AK, Cummins JM, Hennessey JF. Fertilization of human oocytes in relation to varying delay before insemination. Fertil Steril. 1988;50(2):294–297. doi: 10.1016/s0015-0282(16)60076-6. [DOI] [PubMed] [Google Scholar]
- Heidkamp MC, Scully BT, Vijayan K, Engman SJ, Szotek EL, Samarel AM. PYK2 regulates SERCA2 gene expression in neonatal rat ventricular myocytes. American journal of physiology Cell physiology. 2005;289(2):C471–482. doi: 10.1152/ajpcell.00130.2005. [DOI] [PubMed] [Google Scholar]
- Hoffman DL, Brookes PS. Oxygen sensitivity of mitochondrial reactive oxygen species generation depends on metabolic conditions. J Biol Chem. 2009;284(24):16236–16245. doi: 10.1074/jbc.M809512200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JC, Yan LY, Lei ZL, Miao YL, Shi LH, Yang JW, Wang Q, Ouyang YC, Sun QY, Chen DY. Changes in histone acetylation during postovulatory aging of mouse oocyte. Biol Reprod. 2007;77(4):666–670. doi: 10.1095/biolreprod.107.062703. [DOI] [PubMed] [Google Scholar]
- Igarashi H, Takahashi E, Hiroi M, Doi K. Aging-related changes in calcium oscillations in fertilized mouse oocytes. Mol Reprod Dev. 1997;48(3):383–390. doi: 10.1002/(SICI)1098-2795(199711)48:3<383::AID-MRD12>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Khan I, Staessen C, Van den Abbeel E, Camus M, Wisanto A, Smitz J, Devroey P, Van Steirteghem AC. Time of insemination and its effect on in-vitro fertilization, cleavage and pregnancy rates in GnRH agonist/HMG-stimulated cycles. Hum Reprod. 1989;4(8):921–926. doi: 10.1093/oxfordjournals.humrep.a137013. [DOI] [PubMed] [Google Scholar]
- Kim SY, Cordeiro MH, Serna VA, Ebbert K, Butler LM, Sinha S, Mills AA, Woodruff TK, Kurita T. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ. 2013;20(8):987–997. doi: 10.1038/cdd.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Kotani S, Hattori T, Sumi N, Yoshioka T, Todokoro K, Okano Y. Cell cycle-dependent expression and spindle pole localization of a novel human protein kinase, Aik, related to Aurora of Drosophila and yeast Ipl1. J Biol Chem. 1997;272(21):13766–13771. doi: 10.1074/jbc.272.21.13766. [DOI] [PubMed] [Google Scholar]
- Levi M, Maro B, Shalgi R. The involvement of Fyn kinase in resumption of the first meiotic division in mouse oocytes. Cell Cycle. 2010;9(8) doi: 10.4161/cc.9.8.11299. [DOI] [PubMed] [Google Scholar]
- Lim ST, Miller NL, Nam JO, Chen XL, Lim Y, Schlaepfer DD. Pyk2 inhibition of p53 as an adaptive and intrinsic mechanism facilitating cell proliferation and survival. J Biol Chem. 2010;285(3):1743–1753. doi: 10.1074/jbc.M109.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Ruderman JV. Aurora A, mitotic entry, and spindle bipolarity. PNAS. 2006;103(15):5811–5816. doi: 10.1073/pnas.0601425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, McGinnis LK, Kinsey WH. Fyn kinase activity is required for normal organization and functional polarity of the mouse oocyte cortex. Mol Reprod Dev. 2009;76(9):819–831. doi: 10.1002/mrd.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, McGinnis LK, Kinsey WH. Role of Fyn kinase in oocyte developmental potential. Reprod Fertil Dev. 2010;22(6):966–976. doi: 10.1071/RD09311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiani E, Diederich M, Gonfloni S. DNA damage response: the emerging role of c-Abl as a regulatory switch? Biochemical pharmacology. 2011;82(10):1269–1276. doi: 10.1016/j.bcp.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Mailhes JB, Young D, London SN. Postovulatory ageing of mouse oocytes in vivo and premature centromere separation and aneuploidy Biol Reprod. 1998;58(5):1206–1210. doi: 10.1095/biolreprod58.5.1206. [DOI] [PubMed] [Google Scholar]
- Mansour RT, Aboulghar MA, Serour GI. Study of the optimum time for human chorionic gonadotropin-ovum pickup interval in in vitro fertilization. J Assist Reprod Genet. 1994;11(9):478–481. doi: 10.1007/BF02215712. [DOI] [PubMed] [Google Scholar]
- Marston JH, Chang MC. The fertilizable life of ova and their morphology following delayed insemination in mature and immature mice. J Exp Zool. 1964;155:237–251. doi: 10.1002/jez.1401550211. [DOI] [PubMed] [Google Scholar]
- Martel-Gallegos G, Casas-Pruneda G, Ortega-Ortega F, Sanchez-Armass S, Olivares-Reyes JA, Diebold B, Perez-Cornejo P, Arreola J. Oxidative stress induced by P2X7 receptor stimulation in murine macrophages is mediated by c-Src/Pyk2 and ERK1/2. Biochim Biophys Acta. 2013;1830(10):4650–4659. doi: 10.1016/j.bbagen.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Matesic DF, Sidorova TS, Burns TJ, Bell AM, Tran PL, Ruch RJ, May SW. p38 MAPK activation, JNK inhibition, neoplastic growth inhibition, and increased gap junction communication in human lung carcinoma and Ras-transformed cells by 4-phenyl-3-butenoic acid. J Cell Biochem. 2012;113(1):269–281. doi: 10.1002/jcb.23353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis LK, Albertini DF, Kinsey WH. Localized activation of Src-family protein kinases in the mouse egg. Dev Biol. 2007;306(1):241–254. doi: 10.1016/j.ydbio.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis LK, Carroll DJ, Kinsey WH. Protein tyrosine kinase signaling during oocyte maturation and fertilization. Mol Reprod Dev. 2011a;78(10-11):831–845. doi: 10.1002/mrd.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis LK, Hong X, Christenson LK, Kinsey WH. Fer tyrosine kinase is required for germinal vesicle breakdown and meiosis-I in mouse oocytes. Mol Reprod Dev. 2011b;78(1):33–47. doi: 10.1002/mrd.21264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis LK, Kinsey WH, Albertini DF. Functions of Fyn kinase in the completion of meiosis in mouse oocytes. Dev Biol. 2009;327(2):280–287. doi: 10.1016/j.ydbio.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis LK, Luo J, Kinsey WH. Protein tyrosine kinase signaling in the mouse oocyte cortex during sperm-egg interactions and anaphase resumption. Mol Reprod Dev. 2013;80(4):260–272. doi: 10.1002/mrd.22160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, Jaffe LA. SH2 domain-mediated activation of an SRC family kinase is not required to initiate Ca2+ release at fertilization in mouse eggs. Reproduction. 2005;129(5):557–564. doi: 10.1530/rep.1.00638. [DOI] [PubMed] [Google Scholar]
- Mehrpour M, Esclatine A, Beau I, Codogno P. Autophagy in health and disease. 1. Regulation and significance of autophagy: an overview. American journal of physiology Cell physiology. 2010;298(4):C776–785. doi: 10.1152/ajpcell.00507.2009. [DOI] [PubMed] [Google Scholar]
- Meng L, Luo J, Li C, Kinsey WH. Role of Src homology 2 domain-mediated PTK signaling in mouse zygotic development. Reproduction. 2006;132(3):413–421. doi: 10.1530/rep.1.01151. [DOI] [PubMed] [Google Scholar]
- Meyer NL, Longo FJ. Cytological events associated with in vitro aged and fertilized rabbit eggs. Anat Rec. 1979;195(2):357–374. doi: 10.1002/ar.1091950209. [DOI] [PubMed] [Google Scholar]
- Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 2009;15(5):573–585. doi: 10.1093/humupd/dmp014. [DOI] [PubMed] [Google Scholar]
- Moore KL, Kinsey WH. Identification of an abl-related protein tyrosine kinase in the cortex of the sea urchin egg: possible role at fertilization. Dev Biol. 1994;164(2):444–455. doi: 10.1006/dbio.1994.1214. [DOI] [PubMed] [Google Scholar]
- Moore MN, Allen JI, Somerfield PJ. Autophagy: role in surviving environmental stress. Marine environmental research. 2006a;62(Suppl):S420–425. doi: 10.1016/j.marenvres.2006.04.055. [DOI] [PubMed] [Google Scholar]
- Moore MN, Icarus Allen J, McVeigh A. Environmental prognostics: an integrated model supporting lysosomal stress responses as predictive biomarkers of animal health status. Marine environmental research. 2006b;61(3):278–304. doi: 10.1016/j.marenvres.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Moos J, Visconti PE, Moore GD, Schultz RM, Kopf GS. Potential role of mitogen-activated protein kinase in pronuclear envelope assembly and disassembly following fertilization of mouse eggs. Biol Reprod. 1995;53(3):692–699. doi: 10.1095/biolreprod53.3.692. [DOI] [PubMed] [Google Scholar]
- Moos J, Xu Z, Schultz RM, Kopf GS. Regulation of Nuclear Envelope Assembly/Disassembly by MAP Kinase. Dev Biol. 1996;175(2):358. doi: 10.1006/dbio.1996.0121. [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) Guide for the care and use of laboratory animals. Washington, D.C.: National Academies Press; 2011. p. xxv.p. 220. [Google Scholar]
- Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81(4):1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Endo T, Kano K, Naito K. Porcine Aurora A accelerates Cyclin B and Mos synthesis and promotes meiotic resumption of porcine oocytes. Anim Reprod Sci. 2009;113(1-4):114–124. doi: 10.1016/j.anireprosci.2008.05.074. [DOI] [PubMed] [Google Scholar]
- Ou XH, Li S, Xu BZ, Wang ZB, Quan S, Li M, Zhang QH, Ouyang YC, Schatten H, Xing FQ, Sun QY. p38alpha MAPK is a MTOC-associated protein regulating spindle assembly, spindle length and accurate chromosome segregation during mouse oocyte meiotic maturation. Cell Cycle. 2010;9(20):4130–4143. doi: 10.4161/cc.9.20.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrington J, Davis LC, Galione A, Wessel G. Flipping the switch: how a sperm activates the egg at fertilization. Dev Dyn. 2007;236(8):2027–2038. doi: 10.1002/dvdy.21255. [DOI] [PubMed] [Google Scholar]
- Pelech S, Jelinkova L, Susor A, Zhang H, Shi X, Pavlok A, Kubelka M, Kovarova H. Antibody microarray analyses of signal transduction protein expression and phosphorylation during porcine oocyte maturation. J Proteome Res. 2008;7(7):2860–2871. doi: 10.1021/pr800082a. [DOI] [PubMed] [Google Scholar]
- Reinehr R, Becker S, Eberle A, Grether-Beck S, Haussinger D. Involvement of NADPH oxidase isoforms and Src family kinases in CD95-dependent hepatocyte apoptosis. J Biol Chem. 2005;280(29):27179–27194. doi: 10.1074/jbc.M414361200. [DOI] [PubMed] [Google Scholar]
- Rodrigues P, Limback D, McGinnis LK, Plancha CE, Albertini DF. Multiple mechanisms of germ cell loss in the perinatal mouse ovary. Reproduction. 2009;137(4):709–720. doi: 10.1530/REP-08-0203. [DOI] [PubMed] [Google Scholar]
- Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertilization: where it all begins. Dev Biol. 2002;245(2):237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- Samak G, Narayanan D, Jaggar JH, Rao R. CaV1.3 channels and intracellular calcium mediate osmotic stress-induced N-terminal c-Jun kinase activation and disruption of tight junctions in Caco-2 CELL MONOLAYERS. J Biol Chem. 2011;286(34):30232–30243. doi: 10.1074/jbc.M111.240358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfins A, Lee GY, Plancha CE, Overstrom EW, Albertini DF. Distinctions in Meiotic Spindle Structure and Assembly During In Vitro and In Vivo Maturation of Mouse Oocytes. Biol Reprod. 2003;69(6):2059–2067. doi: 10.1095/biolreprod.103.020537. [DOI] [PubMed] [Google Scholar]
- Santalo J, Badenas J, Catala V, Egozcue J. Chromosomes of mouse embryos in vivo and in vitro: effect of manipulation, maternal age and gamete ageing. Hum Reprod. 1987;2(8):717–719. doi: 10.1093/oxfordjournals.humrep.a136620. [DOI] [PubMed] [Google Scholar]
- Sasai K, Parant JM, Brandt ME, Carter J, Adams HP, Stass SA, Killary AM, Katayama H, Sen S. Targeted disruption of Aurora A causes abnormal mitotic spindle assembly, chromosome misalignment and embryonic lethality. Oncogene. 2008;27(29):4122–4127. doi: 10.1038/onc.2008.47. [DOI] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Beimgraben C, Delamere NA. Hyposmotic stress causes ATP release and stimulates Na,K-ATPase activity in porcine lens. J Cell Physiol. 2012a;227(4):1428–1437. doi: 10.1002/jcp.22858. [DOI] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Delamere NA. TRPV4 in porcine lens epithelium regulates hemichannel-mediated ATP release and Na-K-ATPase activity. American journal of physiology Cell physiology. 2012b;302(12):C1751–1761. doi: 10.1152/ajpcell.00010.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BS, Yoon SB, Kim JS, Sim BW, Kim YH, Cha JJ, Choi SA, Min HK, Lee Y, Huh JW, Lee SR, Kim SH, Koo DB, Choo YK, Kim HM, Kim SU, Chang KT. Induction of autophagy promotes preattachment development of bovine embryos by reducing endoplasmic reticulum stress. Biol Reprod. 2012;87(1):8, 1–11. doi: 10.1095/biolreprod.111.097949. [DOI] [PubMed] [Google Scholar]
- Steuerwald NM, Steuerwald MD, Mailhes JB. Post-ovulatory aging of mouse oocytes leads to decreased MAD2 transcripts and increased frequencies of premature centromere separation and anaphase. Mol Hum Reprod. 2005;11(9):623–630. doi: 10.1093/molehr/gah231. [DOI] [PubMed] [Google Scholar]
- Storz P. Reactive oxygen species-mediated mitochondria-to-nucleus signaling: A key to aging and radical-caused diseases. Sci STKE 2006. 2006;2006(332):re3. doi: 10.1126/stke.3322006re3. [DOI] [PubMed] [Google Scholar]
- Stouffer RL, Zelinski-Wooten MB. Overriding follicle selection in controlled ovarian stimulation protocols: quality vs quantity. Reprod Biol Endocrinol. 2004;2:32. doi: 10.1186/1477-7827-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strappazzon F, Torch S, Trioulier Y, Blot B, Sadoul R, Verna JM. Survival response-linked Pyk2 activation during potassium depletion-induced apoptosis of cerebellar granule neurons. Molecular and cellular neurosciences. 2007;34(3):355–365. doi: 10.1016/j.mcn.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Swann K, Lai FA. PLCzeta and the initiation of Ca(2+) oscillations in fertilizing mammalian eggs. Cell calcium. 2013;53(1):55–62. doi: 10.1016/j.ceca.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Szollosi D. Morphological changes in mouse eggs due to aging in the fallopian tube. Am J Anat. 1971;130(2):209–225. doi: 10.1002/aja.1001300207. [DOI] [PubMed] [Google Scholar]
- Tai LK, Okuda M, Abe J, Yan C, Berk BC. Fluid shear stress activates proline-rich tyrosine kinase via reactive oxygen species-dependent pathway. Arteriosclerosis, thrombosis, and vascular biology. 2002;22(11):1790–1796. doi: 10.1161/01.atv.0000034475.40227.40. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Igarashi H, Amita M, Hara S, Matsuo K, Kurachi H. Molecular mechanism of poor embryo development in postovulatory aged oocytes: Mini review. The journal of obstetrics and gynaecology research. 2013;39(10):1431–1439. doi: 10.1111/jog.12111. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Igarashi H, Kawagoe J, Amita M, Hara S, Kurachi H. Poor embryo development in mouse oocytes aged in vitro is associated with impaired calcium homeostasis. Biol Reprod. 2009;80(3):493–502. doi: 10.1095/biolreprod.108.072017. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Takahashi E, Igarashi H, Tezuka N, Kurachi H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol Reprod Dev. 2003;66(2):143–152. doi: 10.1002/mrd.10341. [DOI] [PubMed] [Google Scholar]
- Talmor-Cohen A, Tomashov-Matar R, Tsai WB, Kinsey WH, Shalgi R. Fyn kinase-tubulin interaction during meiosis of rat eggs. Reproduction. 2004;128(4):387–393. doi: 10.1530/rep.1.00266. [DOI] [PubMed] [Google Scholar]
- Tarin JJ. Potential effects of age-associated oxidative stress on mammalian oocytes/embryos. Mol Hum Reprod. 1996;2(10):717–724. doi: 10.1093/molehr/2.10.717. [DOI] [PubMed] [Google Scholar]
- Trounson AO, Mohr LR, Wood C, Leeton JF. Effect of delayed insemination on in-vitro fertilization, culture and transfer of human embryos. J Reprod Fertil. 1982;64(2):285–294. doi: 10.1530/jrf.0.0640285. [DOI] [PubMed] [Google Scholar]
- Verde F, Dogterom M, Stelzer E, Karsenti E, Leibler S. Control of microtubule dynamics and length by cyclin A- and cyclin B-dependent kinases in Xenopus egg extracts. J Cell Biol. 1992;118(5):1097–1108. doi: 10.1083/jcb.118.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlhac MH, Kubiak JZ, Weber M, Geraud G, Colledge WH, Evans MJ, Maro B. Mos is required for MAP kinase activation and is involved in microtubule organization during meiotic maturation in the mouse. Development. 1996;122(3):815–822. doi: 10.1242/dev.122.3.815. [DOI] [PubMed] [Google Scholar]
- Walker G, Burgess D, Kinsey WH. Fertilization promotes selective association of the Abl [correction of AbI] kinase with the egg cytoskeleton. Eur J Cell Biol. 1996;70(2):165–171. [PubMed] [Google Scholar]
- Wang R, He G, Nelman-Gonzalez M, Ashorn CL, Gallick GE, Stukenberg PT, Kirschner MW, Kuang J. Regulation of Cdc25C by ERK-MAP Kinases during the G2/M Transition. Cell. 2007;128(6):1119. doi: 10.1016/j.cell.2006.11.053. [DOI] [PubMed] [Google Scholar]
- Wolf DP. Assisted reproductive technologies in rhesus macaques. Reprod Biol Endocrinol. 2004;2:37. doi: 10.1186/1477-7827-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Abbott A, Kopf GS, Schultz RM, Ducibella T. Spontaneous activation of ovulated mouse eggs: time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and inositol 1,4,5-trisphosphate sensitivity. Biol Reprod. 1997;57(4):743–750. doi: 10.1095/biolreprod57.4.743. [DOI] [PubMed] [Google Scholar]
- Yao LJ, Zhong ZS, Zhang LS, Chen DY, Schatten H, Sun QY. Aurora-A Is a Critical Regulator of Microtubule Assembly and Nuclear Activity in Mouse Oocytes, Fertilized Eggs, and Early Embryos. Biol Reprod. 2004;70(5):1392–1399. doi: 10.1095/biolreprod.103.025155. [DOI] [PubMed] [Google Scholar]
- Zhang N, Wakai T, Fissore RA. Caffeine alleviates the deterioration of Ca(2+) release mechanisms and fragmentation of in vitro-aged mouse eggs. Mol Reprod Dev. 2011;78(9):684–701. doi: 10.1002/mrd.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1. Images of representative Western blots.
Oocytes cultured for 0, 4, 8, 12, or 24 hr of culture were analyzed by SDS-PAGE, as described in ‘Materials and Methods’. Blots were probed with antibodies to (A) activated pan-SRC Y529, (B) activated kinase anti-PTK2 phospho-Y576, (C) activated kinase anti-PTK2B phospho-Y579, (D) anti-pan-SRC protein, (E) anti-PTK2 protein, (F) anti-AURKA, (G) anti-MAPK 1/3 protein, (H) anti-MAPK8/9/10 protein, (I) anti-LAMPA, (J) anti-BCAR1 phospho-Y165, (K) anti-GAPDH. The bound antibody was detected and quantified as described in ‘Materials and Methods’.
Supplemental Table S1. KINEX™ KAM-850 antibody microarray report for analyses of in vitro culture mouse oocytes.
Data were analyzed by Kinexus Bioinformatics Corp. and selected columns from the original report are presented here in table format. “Antibody Code” represents the Kinexus proprietary antibody database record; “Globally Normalized” indicates fluorescence signal at the indicated time point, normalized to the global fluorescence signal for proteins in the sample; “%CFC” is the percentage change from 0 hr control; “Z scores” ((ratio 8 hr signal – 0 hr signal)/ sample standard deviation) reflects how large the changes are and how close the duplicate measurements are for each set of samples being compared for the same protein. Samples highlighted in pink represent those exhibiting signal increases judged to be significant based on the %CFC and Z scores, while blue represents samples exhibiting significant decreases in signal.