Abstract
Standardization of sample collection, shipping, and storage has been a major focus of biorepositories servicing large, multi-institute studies. The standardization of total protein concentration measurements may also provide an important metric for characterizing biospecimens. The measurement of total protein concentration in urine is challenging because of widely variable sample dilutions obtained in the clinic and the lack of a reference matrix for use with a standard curve and blank subtraction. Urinary proteins are therefore typically precipitated and reconstituted in a reference solution before quantitation. We have tested three different methods for protein precipitation and evaluated them using variability in total protein concentration measurement as a metric. The methods were tested on four urine samples ranging from very concentrated to very dilute. A method using a commercially available kit provided the most reproducible results, with average coefficients of variation <10%. Addition of a freeze/thaw did not lead to significant protein loss or additional variability. Samples were titrated and the measurements obtained appeared to be linearly correlated with sample starting volume. This method was applied to analysis of 77 urine biorepository samples and provided reproducible results when the same sample was assayed on different microwell plates.
Keywords: chemical precipitation, proteomics, quantitation, urine
INTRODUCTION
One of the biggest challenges in conducting translational research has been the lack of sufficient quantities of suitable biopsy tissue or uniformly handled serum and urine specimens to allow objective monitoring of histologic, serologic, and/or molecular changes associated with disease progression or therapeutic intervention. Large-scale, multi-institute studies and associated biorepositories have emerged to address this need. By standardizing sample collection, shipping, and storage, biorepositories provide researchers opportunities to use well-characterized samples from large cohorts to discover and characterize unique phenotypic and molecular biomarkers for disease progression that may provide targets for clinical intervention.1–5
Urine is an attractive biospecimen for biomarker discovery. Comprised primarily of shed cells, debris, and secreted components from the urinary tract, as well as blood components that have passed through glomerular filtration and renal tubule reabsorption, urinary components may reflect local renal or urogenital disease, as well as more systemic alterations in distant organs. Urinary biospecimens are typically plentiful, can be obtained noninvasively and repeatedly sampled, and have less complexity than blood-derived biospecimens.6 With the advent of modern, highly sensitive mass spectrometers, proteomics approaches have been used extensively for biomarker discovery in urine. Initial methods optimization efforts centered on one- or two-dimensional (1D or 2D, respectively) gel electrophoresis approaches,7–12 where techniques for protein concentration and removal of salts and other contaminants from urine were evaluated using spot resolution and number of proteins identified as metrics. More recently, highly accurate mass spectrometers and liquid chromatography (LC)-based approaches have been used. Here, the metrics have typically been proteome coverage6,7,13–16 or quantitation of individual proteins using label or label-free methods.17–21
Notwithstanding urine's advantages, the composition and the concentration of components within urine are influenced by a number of factors, such as hydration and elimination frequency, which are not easily controllable within the clinical setting.22 Therefore, it is extremely important to normalize the samples in order to make meaningful comparisons among subjects. Creatinine concentration has long been used for normalization of analyte concentrations in urine, particularly for toxicology analysis. However, creatinine levels can be influenced by age, gender, ethnicity, diet, exercise, body mass index, muscle mass, medications, tubular secretions, and glomerular filtration rate.17,23 For proteomics applications, total protein concentration of the samples, obtained using Bradford or bicinchoninic acid (BCA) assays, is more typically used to equalize sample “loading” conditions before sample processing or mass spectrometric analysis. As urine samples do not have a reference matrix, samples are generally precipitated6,7,11,13,15,21 or buffer exchanged17 to obtain an accurate measure of total protein concentration. In proteomics studies, most optimization efforts evaluated the utility of different approaches using an endpoint such as the number of proteins that could be identified.6–16 However, to the best of our knowledge, these studies did not investigate total protein concentration as a possible metric. Variability in total protein concentration, as a result of sample preparation, could lead to erroneous conclusions, particularly when pooled samples or small sample sets are analyzed.
The goal of this study was to evaluate urinary protein precipitation methods to use for preparing samples for obtaining total protein concentration measurements. The selected method will be used to characterize biorepository samples in a uniform manner; additionally, protein concentration values will be archived and available for researchers who wish to use samples collected by the Multi-Disciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network for biomarker discovery.
MATERIALS AND METHODS
Reagents
Eighty percent TCA was obtained from High Valley Chemical (Centerville, UT, USA). Acetone was purchased from Sigma-Aldrich (St. Louis, MO, USA). 2D Clean-up Kits were acquired from Thermo Scientific (Rockford, IL, USA). Axygen Maximum Recovery tubes (VWR, Radnor, PA, USA) and GeneMate ultimate low-retention pipette tips (ISC BioExpress, Kaysville, UT, USA) were used to reduce variability resulting from protein binding to the plastic. A BCA assay kit was obtained from Thermo Scientific and a DetectX Creatinine Urinary Kit was purchased from Arbor Assays (Ann Arbor, MI, USA).
Samples
Clean-catch urine samples for methods development were obtained from male and female adult volunteers using an Institutional Review Board-approved protocol at the University of Colorado Anschutz Medical Center. This project has been approved by the Colorado Multiple Institutional Review Board, and all human subjects participating in this study provided informed consent. Samples were frozen at −80°C, thawed for 3 h at room temperature, and then centrifuged at 3000 g for 10 min at 4°C to remove debris. Supernatants were divided into 1–3 mL aliquots and stored at −80°C for one time use. Initial development was performed on four samples, which based on color, appeared to range from very dilute to very concentrated; we subsequently used fairly dilute samples from two male and one female donor for scaling studies. The optimized method was tested on 77 samples stored in the Tissue Analysis and Technology Core (TATC) biorepository that were collected by MAPP Network Discovery Sites. Three of those samples were analyzed twice on different plates to assess reproducibility.
Protein Precipitation
Protein precipitation is generally carried out using salts, organics, or pH change. TCA and acetone are often used for protein precipitation in urine and were used by MAPP Network Discovery Site researchers performing proteomics experiments. Therefore, we compared methods provided to us by two of the network laboratories with the use of a commercial kit for protein precipitation which had worked effectively for other applications in our core facility. Urine (1–3 mL aliquot) was thawed at room temperature and vortexed until no precipitate was present.
Method 1
Eighty percent TCA (1:10 v/v) was added to thawed urine and samples were incubated on ice for 1 h. Samples were then centrifuged at 10,000 g for 15 min at 4°C. The supernatant was discarded and the pellet resuspended in 1 mL ice-cold acetone. Samples were incubated at −20°C for 30 min. Following centrifugation at 14,000 g for 15 min at 4°C, the supernatant was removed and pellets washed twice with 1 mL ice-cold acetone, centrifuging each time at 14,000 g for 15 min at 4°C. Washed pellets were resuspended in ice-cold acetone and incubated at −20°C overnight. In the morning, samples were centrifuged at 14,000 g for 15 min at 4°C, supernatant was removed, and pellets were allowed to air dry before reconstitution in 100 mM ammonium bicarbonate.
Method 2
Thawed urine samples were chilled on ice for 15 min. Ice-cold, 80% TCA (1:10 v/v) was added to thawed urine and samples were incubated on ice for 1 h or up to 3 days at −20°C. Samples were then centrifuged at 14,000 g for 15 min at 4°C. Supernatants were removed and 100 uL ice-cold acetone was used to resuspend pellets. Following a 5 min incubation on ice, samples were centrifuged at 14,000 g for 15 min at 4°C. Supernatants were removed and pellets allowed to air dry before resuspension in 100 mM ammonium bicarbonate.
Method 3
Urine was thawed, and 1 mL aliquots were vacuum centrifuged to dryness. Samples were resuspended in 100 ul water by repeated pipetting and placed on ice. A 2D Clean-up Kit was used for precipitation following the manufacturer's directions, with the exception of incubating in wash buffer overnight at −20°C. In the morning, samples were vortexed for 30 s and centrifuged at 12,000 g for 5 min at 4°C, supernatant was removed, and pellets were air dried. Samples were reconstituted in 100 mM ammonium bicarbonate by sonicating for 5 min at room temperature.
Protein Quantitation
Following precipitation and reconstitution, total protein concentration was assessed using the BCA assay, following the manufacturer's instructions. Colorimetric changes were measured using a wavelength of 562 nm with blank subtraction with a Synergy H1 microplate reader. Urine samples were reconstituted initially in a minimal volume (30 uL). Samples were tested on Parafilm by using 1 ul of sample mixed with 40 ul of BCA working reagent. If a purple color was evident immediately, the urine was diluted and retested until only a slight purple color appeared (Fig. 1) Following appropriate dilution, 5 ul of sample were used for quantitation in each well. A multichannel pipettor was used to reduce loading variability, and samples were measured in triplicate.
Figure 1.
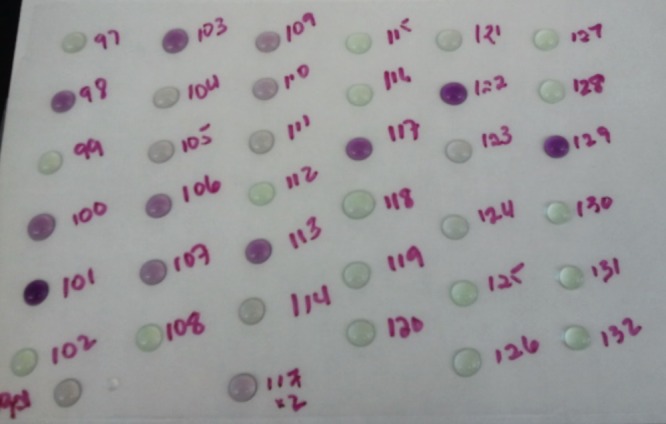
The rapid appearance of a color change for urine samples in BCA working solution facilitated determination of suitable sample dilution volumes. This allowed for detection of a majority of samples within the limits of the assay and reduced the need for repeated analysis of out-of-range samples. The immediate color change appears to be specific to urine samples and was not observed for plasma samples processed in an identical manner.
Creatinine Measurements
A 10-uL aliquot of thawed urine was removed and used immediately or stored at −20°C until used. Samples were diluted and incubated following the manufacturer's instructions and a colorimetric change measured at 490 nm.
Data Analysis
Data were analyzed with GraphPad Prism V5. A Mann-Whitney two-tailed t-test or a one-way ANOVA (Kruskal-Wallis test) was used to compare among conditions. Significance resulted for P < 0.05.
RESULTS
Generation of Pellets
Four trials were performed using each method, and generation of a precipitated pellet was visually assessed for each sample. Visible pellets likely consisted of salts, as well as precipitated protein, and pellets obtained using Method 3 may have included detergent as well. Three replicates were typically used for each sample; therefore, 12 assays were performed/trial, and 48 assays were attempted/method. Method 1 did not generate any visible pellets; Method 2 generated pellets that were visible in 31 of the 48 assays, generally for only the two most concentrated samples; and Method 3 generated visible pellets in all 48 assays. Relative pellet size, determined by inspection, appeared to be similar among multiple preparations only for Method 3. With the use of Methods 1 and 2, attempts to incubate samples overnight in TCA led to sample discoloration (a brownish tinge was observed) and interference with the BCA assay. Method 3 implemented following the manufacturer's instructions yielded pellets for all samples; however, the approximate pellet size, determined by inspection, appeared to be more reproducible when samples were allowed to incubate overnight.
Measurement Variability
Samples from four different donors were assayed using each of the methods. At least three technical replicates were performed/method, and all measurements were made in triplicate. Figure 2 plots the average total protein concentration obtained for each of the donors using each of the methods. Visual inspection by color suggested that urine from Donors 1 and 4 was highly concentrated (deep yellow color), urine from Donor 3 was much more dilute (pale yellow color), and urine from Donor 2 was extremely dilute (clear). Protein concentrations obtained using Method 3 support the visual evaluation. Average concentrations obtained using Methods 1 and 2 were extremely variable compared with those obtained using Method 3. The normalization with creatinine yielded similar results (Supplemental Fig. 1). Although Method 2 appeared to precipitate more protein than Method 3 for some donors, the wide variability in concentration measurements/donor, plotted in Fig. 2, suggests that Method 3 may reflect the actual protein concentration more accurately than Method 2.
Figure 2.
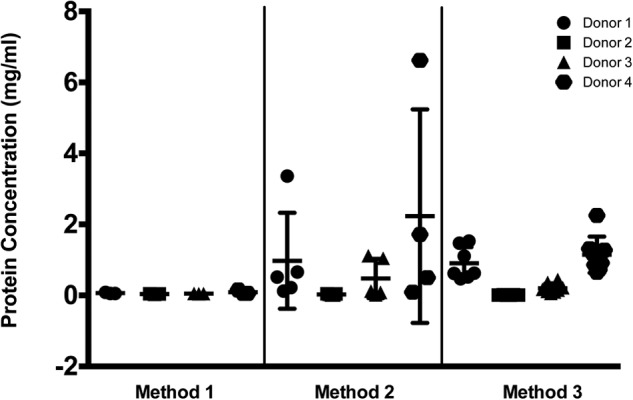
Protein concentration measurements obtained following three different methods of protein precipitation were compared using sample from four different donors. Method 1 did not produce visible pellets, and the resultant protein concentrations measured were below the standard curve and calculated by extrapolation. Replicate measurements were obtained on different days for each of the methods. Method 1, n = 3; Method 2, n = 4; Method 3, n = 9.
As Donor 2 urine was the most dilute, it was reconstituted in 30 uL ammonium bicarbonate and was not diluted further; therefore, repeated measures of this sample on different days should show the least amount of variability. Plotted in Figure 3A is a comparison of the average coefficients of variation (CVs) for measurements of Donor 2 protein concentration using the three different methods. These CVs were obtained from three technical replicates/data point. Additionally, protein concentrations and CVs obtained over a 7-month period from several different aliquots of Donor 2 urine (Figs. 3B and C) using Method 3 suggest that this method provided the least amount of variability and the most measurement consistency.
Figure 3.

(A) Average CV of protein concentration of Donor 2 urine from three technical replicates/data point. Comparison of measurement variability among the methods revealed that Method 3 provided the most consistent results. Method 1, n = 5; Method 2, n = 4; Method 3, n = 8. With the use of Method 3, different aliquots of Donor 2 urine were measured over several months using different kits. (B) Protein concentration measurements and (C) variability were plotted.
Effect of Freeze/Thaw
With the use of Method 3, analysis of samples from Donors 1, 3, and 4 undergoing one or two freeze-thaws showed only slight but significant differences in protein concentration (Fig. 4A) and no significant difference in measurement variability (Fig. 4B) for doubly thawed samples compared with singly thawed samples.
Figure 4.

Comparison of samples that were thawed and processed immediately (“Fresh”) with those that were thawed, refrozen during processing, then rethawed, and measured (“Thawed”). (A) Protein concentration measurements from three different donors, measured on the same day, show a slight but significant decrease in protein concentration of doubly thawed samples compared with singly thawed samples. (B) Average CV of protein concentration measurements from three different donors, measured on the same day, reveals no significant increase in variability when samples undergo an additional freeze-thaw. A Mann-Whitney two-tailed t-test did not yield a significant P value for individual donor comparisons or the grouped comparison plotted here.
Scalability of the Method
Using Method 3, sample from one donor was separated into 1000, 500, and 250 ul aliquots and total protein concentration measured. Scaled average concentrations from triplicate measurements are summarized in Table 1. The measured concentrations were multiplied by the relative dilution factor (one, two, or four, respectively) to obtain the calculated concentrations tabulated, normalized to a 1-mL sample. The average CV reflects the combined variation of nine separate measurements for each volume of sample. As might be expected, the samples with the smallest starting volume (i.e., the most dilute) exhibited the greatest variability. Differences from the expected ratio ranged ±14%, on average, suggesting the method is scalable. MicroBCA was used for this quantitation, which provided slightly more reproducible results than the BCA method; however, differences were not significant (Supplemental Fig. 2).
TABLE 1.
Calculated Protein Concentration (mg/mL) for Three Replicate Experiments Scaled to a 1-mL Starting Volume
| 1000 ul | 500 ul | 250 ul | |
|---|---|---|---|
| Experiment 1 | 0.0233 | 0.0255 | 0.0207 |
| Experiment 2 | 0.0212 | 0.0219 | 0.0270 |
| Experiment 3 | 0.0218 | 0.0247 | 0.0225 |
| Average | 0.0221 | 0.0240 | 0.0234 |
| sd | 0.0011 | 0.0019 | 0.0032 |
| CV | 5% | 8% | 14% |
Plate-to-Plate Reproducibility
Method 3 was used to obtain protein and creatinine concentrations for 77 samples collected by the MAPP Network Discovery Sites and stored in the TATC biorepository. Three different samples were chosen randomly for repeat analysis on two different microwell plates. Results from two of the samples are summarized in Table 2. The measured protein concentrations were found to vary by 5–7% between plates (note that the first 3 measurements reported for each sample were from plate 1 and the last three were from plate 2). Variance in measurement within the plate ranged from 2% to 6% for these samples. Plotted in Fig. 5 are the measured creatinine and protein concentrations for the biorepository samples. Average concentrations were not significantly different between male and female subjects for either measure, although subsequent stratification revealed that creatinine levels were decreased significantly in men with high pelvic pain scores (Supplemental Fig. 3).
TABLE 2.
Plate-to-Plate Reproducibility for Biorepository Samples
| Sample | Concentration (mg/ml) | sd | CV (%) |
|---|---|---|---|
| URI0000374 | |||
| 0.0510 | |||
| 0.0528 | |||
| 0.0519 | |||
| 0.0463 | |||
| 0.0440 | |||
| 0.0479 | |||
| Average | 0.0490 | 0.0035 | 7% |
| URI0002140 | |||
| 0.0764 | |||
| 0.0857 | |||
| 0.0846 | |||
| 0.0790 | |||
| 0.0787 | |||
| 0.0838 | |||
| Average | 0.0814 | 0.0038 | 5% |
Figure 5.
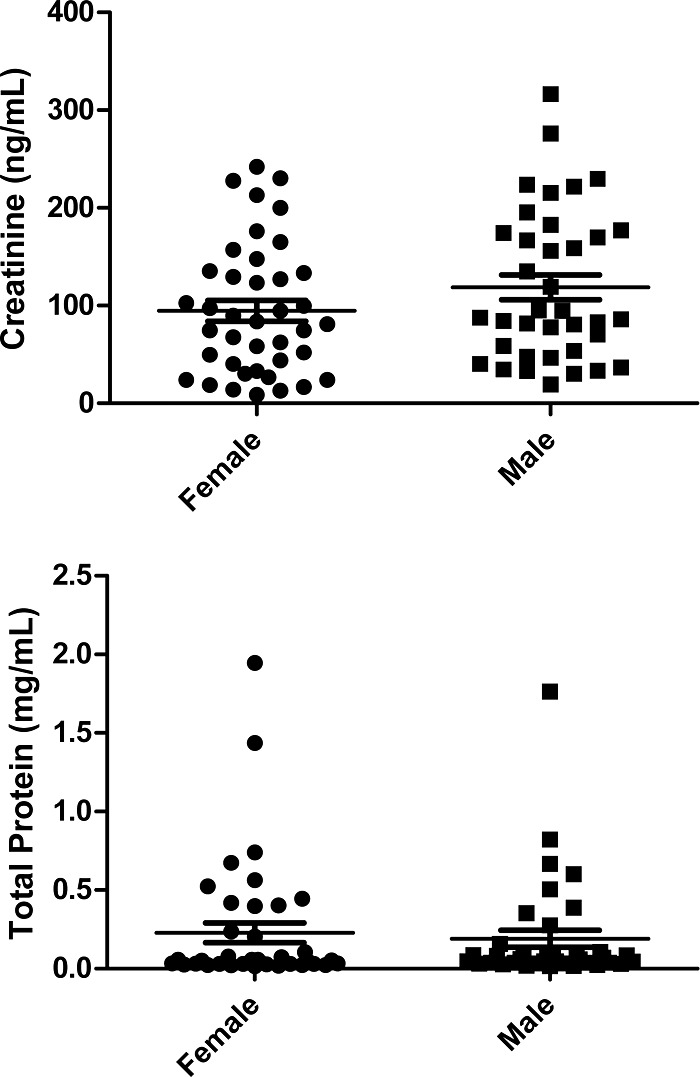
Method 3 was used to measure protein concentrations from 77 biorepository samples. Neither creatinine (upper) or protein (lower) concentrations were significantly different for men (n=37) versus women (n=40) participating in the study.
DISCUSSION
The measurement of total protein concentration in urine can be challenging as a result of the variability in dilution and the lack of a reference matrix. Precipitating proteins and resolubilizing them in a common, well-defined matrix that is also used for a calibration curve may potentially allow for more accurate measures of total protein concentration, improving the reliability of downstream tests. However, precipitation is not always straightforward, and protein loss may result from incomplete precipitation and difficulty with resolubilization. In this work, we compared variability in measurement of total protein concentration in urine obtained following protein precipitation using TCA/acetone or the 2D Clean-up Kit, modified by adding an overnight incubation in organic wash buffer. Samples from four donors, ranging from very concentrated to very dilute, were used to establish conditions that would be appropriate for a wide range of urine biospecimens. Although many different methods for precipitating proteins have been compared, as described in more detail below, we wished to use approaches that were being used by the MAPP network laboratories for urinary proteomics sample preparation so that the samples would be analyzed in a similar fashion throughout the network.
In our hands, TCA/acetone precipitation, a very common method reported in the literature24 and the basis for the protocols provided to us, did not reliably generate reproducible pellets that could be resolubilized to obtain total protein concentrations with low variability. Method 1 used a short incubation in 80% TCA at room temperature, as well as an overnight incubation with ice-cold acetone. We found this method to be the least effective in precipitating protein. Method 2 used a long incubation in ice-cold 80% TCA with a short incubation in ice-cold acetone. We investigated incubating overnight, over the weekend, and incubation for 6 days in TCA. Pellets were observed for all but the most dilute sample (Donor 2), and the lowest CVs were obtained when incubating over the weekend. However, samples took on a brown color following overnight incubation in TCA; this color deepened the longer the samples were incubated, suggesting the potential for the generation of artifacts or degradation products that might interfere with downstream proteomics approaches. Although this method yielded the highest protein concentrations in some cases, the worrisome color change and the large measurement variation discouraged its use. TCA is used routinely for protein precipitation; however, it has been shown to be less effective in precipitating unfolded states of proteins.24 This may be a particular problem when dealing with urinary proteins, where urea present in the matrix may have a denaturing effect.
In the present study, the most reproducible measurements were obtained using the 2D Clean-up Kit following the manufacturer's protocol, but with an overnight incubation in wash buffer added. Pellets were observed for all samples and the CV values were very consistent, typically averaging <10% from run to run. CVs were well below 5% for sample from Donor 2, which did not require further dilution. We also did not see significant loss when samples were subjected to an additional freeze-thaw (recapitulating results reviewed by Thongboonkerd).25 Although mean protein concentration values were lower for thawed samples, the difference was not significant and no additional variance was observed (Fig. 4). It should be noted that the range of measured concentrations was more broad for samples subjected to an additional freeze/thaw and was also increased for less concentrated samples, such as that from Donor 3 (Supplemental Fig. 4), although not significantly. Measurement of raw urine samples provided extremely variable results—CV of 34% for Donor 3 urine and 170% for Donor 4 urine—suggesting that some type of processing may be necessary to obtain reliable protein concentration measurements in urine when BCA is used. It should be noted that processing is not used within a clinical setting to assess proteinuria, where turbimetry and dye binding have been reported to yield very precise results (<3% CV).26 However, proteomics analyses require sample cleanup; therefore, such approaches were not used in our study.
Although some components of the 2D Clean-up Kit are proprietary, they consist of a precipitant with ∼10% TCA in a salt buffer and an acetone wash buffer. The addition of a coprecipitant, which includes sodium carbonate and sodium deoxycholate monohydrate, appears to enhance the protein precipitation process; the proprietary wash additive aids in resolubilization. We found that the initial pellets formed after addition of the precipitant and the coprecipitant were fairly stable in urine, and the supernatant could be easily removed without losing parts of the pellet. In contrast, pellets from plasma samples (5 uL), prepared in an identical manner, tended to loosen quickly. Great care had to be taken so as not to lose sample when removing supernatants when processing the plasma samples. In our hands, pellets from very concentrated samples were more difficult to resolubilize in 100 mM ammonium bicarbonate after drying. We resorted to placing those samples in an ultrasonicator for 5–10 min and followed up by repeated pipetting to dissolve the pellets completely. Those from dilute samples were very easy to resolubilize with only a few steps of repeated pipetting. Plasma samples, with far more protein content than is present in urine samples, were much more difficult to resolubilize and often required several steps of sonication and repeated pipetting. In our experience, pellets were easy to resolubilize in a buffer for 1D or 2D gel electrophoresis; however, we chose ammonium bicarbonate so the method would be immediately compatible with downstream LC-tandem mass spectrometry (MS/MS) analyses. It is possible that another buffer choice, such as Tris, might yield different results.
In this study, we focused on developing a method for reproducibly obtaining protein concentration measurements for urine samples. However, we did not follow up this work with subsequent experiments to evaluate proteome coverage or quantitation, as other studies have done. This was because the use of precipitation approaches reported in the literature appears to vary depending on the downstream analytical method and any subsequent sample fractionation. Most generally, sample processing approaches used in prior optimization studies used ultrafiltration, with or without depletion of albumin, or protein precipitation. For example, Lee et al.15 found no significant difference between ultrafiltration and precipitation with 90% ethanol when limited proteome coverage was assessed using GeLC/MS/MS, whereas Afkarian et al.6 observed that ultrafiltration resulted in a loss of 70–75% of the sample and increased variability compared with precipitation. Bakun and coworkers18 used a 10-kDa MW cutoff filter and observed poor correlation of iTRAQ and selected reaction monitoring ratios for a limited set of proteins, likely as a result of variability introduced by the additional steps of sample handling for the iTRAQ experiments. Some groups reported improvements gained by immunodepleting albumin,16,20 whereas others reported no significant difference.6 Precipitation approaches were also quite varied, with TCA,7,14,21 acetone,20,22 methanol,6 or ethanol6,13 used as precipitating agents; only one study performed a comparison, which was between methanol and ethanol.6 Virtually all of the studies cited above used metrics, such as number of spots on a 2D gel or number of proteins identified by LC-MS/MS, to assess the efficacy of method optimization; total protein concentration values were typically not reported. Therefore, a comparison between our observation and other reported studies is not completely straightforward. It is possible that proteome coverage analysis would reveal deficiencies in the use of our current method based on the 2D Clean-up Kit for urine, and it would be informative to determine whether the kit yields biased proteome coverage results for 2D gel or LC-based analyses. However, that is beyond the scope of our current study. Notwithstanding, it is important to note that we have used this kit for preparation of other biofluids, such as cervico-vaginal fluid for downstream LC-MS-based proteomics analyses,27 and detected many of the same proteins reported in other studies.
An important characteristic of a biorepository sample is its total protein concentration, which when measured uniformly for all samples, can be used by individual researchers for normalization of results and for multi-institute studies, enabling data comparison across a research network. We have developed a highly reproducible, scalable and sensitive method for precipitating urinary proteins in biorepository samples. A surprising aspect of our method development was the immediate color change that we observed when adding BCA working solution to our reconstituted urine samples. We have not observed a similar response from plasma, lavage fluid, cervico-vaginal fluid, or tissue samples precipitated with the same kit and reconstituted in the same buffer. However, the color change was very useful for enabling us to dilute samples easily and ensure that they fell within the linear range of the BCA assay, reducing the number of reruns required. Another surprise was the differences that we observed between urine and plasma samples; pellets from urine appeared to be much more stable and easier to resolubilize than those from plasma. The optimized method was applied to obtain total protein concentrations from 77 biorepository samples. Although creatinine is often used to normalize urine samples and account for dilution, our results show that much more consistent values were obtained for protein concentration compared with creatinine, suggesting that protein might be better than creatinine for normalizing urine samples. Further refinements of the method may improve the ease of use of this method with plasma samples. The cost of using the 2D Clean-up Kit may deter some researchers from its use; this must be weighed against the potential for improved total protein concentration measurement and resultant ability to compare among disparate samples.
ACKNOWLEDGMENTS
Funding for the MAPP Research Network was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), U.S. National Institutes of Health (NIH; DK82370, DK82342, DK82315, DK82344, DK82325, DK82345, DK82333, and DK82316.). In addition, this work was supported, in part, by the NIH/National Center for Advancing Translational Sciences (NCATS) Colorado County Technical Services, Inc. (CTSI; Grant UL1 TR001082). The authors appreciate the contribution to this research made by staff members of the University of Colorado Anschutz Medical Campus Biorepository Core Facility. There are no conflicts of interest to disclose.
REFERENCES
- 1. Christensen H, Nielsen JS, Sorensen KM, Melbye M, Brandslund I. New national Biobank of The Danish Center for Strategic Research on Type 2 Diabetes (DD2). Clin Epidemiol 2012;4:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Palmirotta R, Barbanti P, Ludovici G, et al. Establishment of a biorepository for migraine research: the experience of Interinstitutional Multidisciplinary BioBank (BioBIM). Neurol Sci 2013;34:1659–1663. [DOI] [PubMed] [Google Scholar]
- 3. Turner CF, Pan H, Silk GW, et al. The NIDDK Central Repository at 8 years—ambition, revision, use and impact. Database (Oxford) 2011;2011:bar043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vehik K, Fiske SW, Logan CA, et al. Methods, quality control and specimen management in an international multi-center investigation of type 1 diabetes: TEDDY. Diabetes Metab Res Rev 2013;29:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feng Z, Kagan J, Pepe M, et al. The Early Detection Research Network's Specimen reference sets: paving the way for rapid evaluation of potential biomarkers. Clin Chem 2013;59:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Afkarian M, Bhasin M, Dillon ST, et al. Optimizing a proteomics platform for urine biomarker discovery. Mol Cell Proteomics 2010;9:2195–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kentsis A, Monigatti F, Dorff K, Campagne F, Bachur R, Steen H. Urine proteomics for profiling of human disease using high accuracy mass spectrometry. Proteomics Clin Appl 2009;3:1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oh J, Pyo JH, Jo EH, et al. Establishment of a near-standard two-dimensional human urine proteomic map. Proteomics 2004;4:3485–3497. [DOI] [PubMed] [Google Scholar]
- 9. Pieper R, Gatlin CL, McGrath AM, et al. Characterization of the human urinary proteome: a method for high-resolution display of urinary proteins on two-dimensional electrophoresis gels with a yield of nearly 1400 distinct protein spots. Proteomics 2004;4:1159–1174. [DOI] [PubMed] [Google Scholar]
- 10. Zerefos P, Prados J, Kossida S, Kalousis A, Vlahou A. Sample preparation and bioinformatics in MALDI profiling of urinary proteins. J Chromatogr B 2007;853:20–30. [DOI] [PubMed] [Google Scholar]
- 11. Thongboonkerd V, Chutipongtanate S, Kanlaya R. Systematic evaluation of sample preparation methods for gel-based human urinary proteomics: quantity, quality, and variability. J Proteome Res 2006;5:183–191. [DOI] [PubMed] [Google Scholar]
- 12. Magistroni R, Ligabue G, Lupo V, et al. Proteomic analysis of urine from proteinuric patients shows a proteolitic activity directed against albumin. Nephrol Dialysis Transplant 2009;24:1672–1681. [DOI] [PubMed] [Google Scholar]
- 13. Froehlich JW, Vaezzadeh AR, Kirchner M, et al. An in-depth comparison of the male pediatric and adult urinary proteomes. Biochim Biophys Acta 2014;1844:1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kentsis A, Lin YY, Kurek K, et al. Discovery and validation of urine markers of acute pediatric appendicitis using high-accuracy mass spectrometry. Ann Emerg Med 2010;55:62–70.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee RS, Monigatti F, Briscoe AC, Waldon Z, Freeman MR, Steen H. Optimizing sample handling for urinary proteomics. J Proteome Res 2008;7:4022–4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaezzadeh AR, Briscoe AC, Steen H, Lee RS. One-step sample concentration, purification, and albumin depletion method for urinary proteomics. J Proteome Res 2010;9:6082–6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jantos-Siwy J, Schiffer E, Brand K, et al. Quantitative urinary proteome analysis for biomarker evaluation in chronic kidney disease. J Proteome Res 2009;8:268–281. [DOI] [PubMed] [Google Scholar]
- 18. Bakun M, Niemczyk M, Domanski D, et al. Urine proteome of autosomal dominant polycystic kidney disease patients. Clin Proteomics 2012;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sigdel TK, Lau K, Schilling J, Sarwal M. Optimizing protein recovery for urinary proteomics, a tool to monitor renal transplantation. Clin Transplant 2008;22:617–623. [DOI] [PubMed] [Google Scholar]
- 20. Chen CL, Lin TS, Tsai CH, et al. Identification of potential bladder cancer markers in urine by abundant-protein depletion coupled with quantitative proteomics. J Proteomics 2013;85:28–43. [DOI] [PubMed] [Google Scholar]
- 21. Court M, Selevsek N, Matondo M, et al. Toward a standardized urine proteome analysis methodology. Proteomics 2011;11:1160–1171. [DOI] [PubMed] [Google Scholar]
- 22. Li M, Xu Y, Xu M, et al. Association between nonalcoholic fatty liver disease (NAFLD) and osteoporotic fracture in middle-aged and elderly chinese. J Clin Endocrinol Metab 2012;97:2033–2038. [DOI] [PubMed] [Google Scholar]
- 23. Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 2005;113:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rajalingam D, Loftis C, Xu JJ, Kumar TK. Trichloroacetic acid-induced protein precipitation involves the reversible association of a stable partially structured intermediate. Protein Sci 2009;18:980–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thongboonkerd V. Practical points in urinary proteomics. J Proteome Res 2007;6:3881–3890. [DOI] [PubMed] [Google Scholar]
- 26. Martin H. Laboratory measurement of urine albumin and urine total protein in screening for proteinuria in chronic kidney disease. Clin Biochem Rev 2011;32:97–102. [PMC free article] [PubMed] [Google Scholar]
- 27. Klein LL, Jonscher KR, Heerwagen MJ, Gibbs RS, McManaman JL. Shotgun proteomic analysis of vaginal fluid from women in late pregnancy. Reprod Sci 2008;15:263–273. [DOI] [PubMed] [Google Scholar]


