Abstract
Background
We sought to compare liver transplant waiting list access by demographics and geography relative to the pool of potential liver transplant candidates across the United States using a novel metric of access to care, termed a liver wait-listing ratio (LWR).
Methods
We calculated LWRs from national liver transplant registration data and liver mortality data from the Scientific Registry of Transplant Recipients and the National Center for Healthcare Statistics from 1999 to 2006 to identify variation by diagnosis, demographics, geography, and era.
Results
Among patients with ALF and CLF, African Americans had significantly lower access to the waiting list compared with whites (acute: 0.201 versus 0.280; pre-MELD 0.201 versus 0.290; MELD era: 0.201 versus 0.274; all, P<0.0001) (chronic: 0.084 versus 0.163; pre-MELD 0.085 versus 0.179; MELD 0.084 versus 0.154; all, P<0.0001). Hispanics and whites had similar LWR in both eras (both P >0.05). In the MELD era, female subjects had greater access to the waiting list compared with male subjects (acute: 0.428 versus 0.154; chronic: 0.158 versus 0.140; all, P<0.0001). LWRs varied by three-fold by state (pre-MELD acute: 0.122–0.418, chronic: 0.092–0.247; MELD acute: 0.121–0.428, chronic: 0.092–0.243).
Conclusions
The marked inequity in early access to liver transplantation underscores the need for local and national policy initiatives to affect this disparity.
Keywords: Access to transplantation, Health services, Organ allocation
Receipt of a liver transplant requires referral to a transplant center, evaluation of suitability for transplantation, registration on the liver transplant waiting list, and survival until allocated a suitable donor liver. For several years, the U.S. liver transplant waiting list has remained stable. Approximately 13,000 to 15,000 candidates are wait-listed at any given time, and approximately 6,000 patients receive a liver transplant and 2,000 patients die waiting (1). However, this view of transplant access is limited because many patients are never wait-listed.
An important goal in achieving equity in the care of patients with liver disease is assuring fair access to the liver transplant waiting list. Becoming a wait-listed liver transplant candidate is a critical step in the transplant process but is not well studied (2–4), primarily related to a lack of comprehensive national data on patients with CLF in the United States. (5, 6). African Americans were significantly less likely to be referred for liver transplantation, despite having similar disease burden as their white counterparts in the U.S. Veterans Administration medical system (7). State-based data demonstrate racial and sex-based inequities in transplant evaluation and wait-listing rates (8). The lack of consistent referral, evaluation, and listing practices undermines the ethical obligations of the transplant community and may withhold care from those who could derive benefit from liver transplantation.
We aimed to determine the extent of demographic and geographic variation in access to the liver transplant waiting list using national data. Comprehensive data are not available on all transplant-eligible patients to calculate absolute waiting list registration rates. We created an empirical measure of relative waiting list access termed the liver wait-listing ratio (LWR). This metric captures the rate of wait-listing for a given group relative to those potentially eligible for transplant, namely, those who died from liver disease and those who were listed, less those already transplanted. Intuitively, transplant providers understand that not all patients who die from liver disease are transplant-eligible. We set the “transplant-eligible” denominator on liver disease mortality because U.S. decedent data are comprehensive, available, epidemiologically explicable, and is applied in a variety of public health and policy contexts. The actual ratios are not as pertinent as their relative differences for comparison purposes, and we present this analysis to better understand disparities in the liver transplant process.
RESULTS
Liver waiting list activity and liver disease mortality are displayed in Table 1. Across the entire cohort, LWR were 1.7-fold higher for patients with ALF than for those with CLF (0.261 versus 0.152, respectively). For ALF, waiting list registrations declined by 19.8% from 1999 to 2006, although there was a 10% decrease in ALF deaths. Deaths after waiting list registration also remained relatively stable. The LWR for ALF remained relatively constant, ranging from 0.247 to 0.265, with overlap of confidence intervals across years and eras (pre-MELD 0.262 versus MELD 0.260, P=0.74). For CLF, waiting list registrations increased by 6.5% from 1999 to 2006, whereas the number of deaths attributed to CLF increased by 24.2%. The number of waiting list and posttransplant deaths also grew by 43.6%. The LWR for CLF remained relatively stable from 1999 to 2001 but was much lower beginning in 2002 and in subsequent years. The LWR was 12% higher in the pre-MELD era versus the MELD era among CLF patients (0.165 versus 0.146, P<0.0001).
TABLE 1.
Waiting list registrations, deaths from liver disease, and liver wait-listing ratios for acute liver failure and chronic liver disease by year, 1999–2006
| Year | Waiting list | NCHS deaths | Waiting list + posttransplant deaths | LWR (95% CI) |
|---|---|---|---|---|
| All acute | 3263 | 10,602 | 1358 | 0.261 (0.253–0.269) |
| 1999 | 450 | 1406 | 160 | 0.265 (0.244–0.286) |
| 2000 | 406 | 1374 | 167 | 0.252 (0.231,0.273) |
| 2001 | 443 | 1357 | 160 | 0.270 (0.249–0.292) |
| 2002 | 392 | 1368 | 179 | 0.248 (0.227–0.269) |
| 2003 | 376 | 1275 | 168 | 0.254 (0.231–0.276) |
| 2004 | 392 | 1226 | 177 | 0.272 (0.249–0.295) |
| 2005 | 443 | 1331 | 185 | 0.279 (0.257–0.301) |
| 2006 | 361 | 1265 | 162 | 0.247 (0.225–0.269) |
| All chronic | 68,982 | 412,932 | 29,351 | 0.152 (0.151–0.153) |
| 1999 | 8462 | 45,662 | 2920 | 0.165 (0.162–0.168) |
| 2000 | 8770 | 47,133 | 3135 | 0.166 (0.163–0.169) |
| 2001 | 8885 | 48,772 | 3550 | 0.164 (0.161–0.167) |
| 2002 | 7764 | 51,613 | 3711 | 0.139 (0.137–0.142) |
| 2003 | 8355 | 53,489 | 3774 | 0.144 (0.141–0.147) |
| 2004 | 8885 | 53,741 | 3981 | 0.152 (0.149–0.154) |
| 2005 | 8852 | 55,797 | 4087 | 0.146 (0.143–0.149) |
| 2006 | 9009 | 56,725 | 4193 | 0.146 (0.144–0.149) |
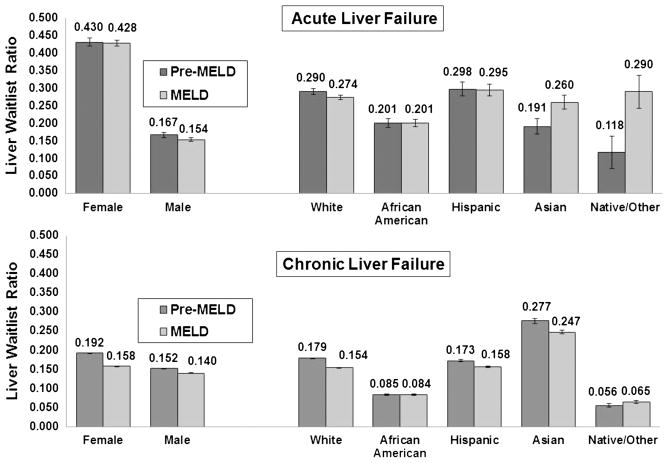
Liver wait-listing ratio for ALF varied by sex and by race/ethnicity (Fig. 1). Female subjects had a nearly threefold higher LWR compared with male subjects, and the magnitude of this difference remained relatively constant by era (both eras, P<0.0001). We observed a 25% to 30% lower LWR for African Americans with ALF compared with whites, and this difference did not change with time (both eras, P<0.0001). Asians had 36% higher LWR in the MELD era versus the pre-MELD era (0.260 versus 0.191, P=0.02). This trend was also noted among Native American/Other patients (0.118 versus 0.290, P=0.02).
FIGURE 1.
Racial/ethnic and sex-based variation in liver wait-listing ratios for acute and chronic liver failure, 1999–2006. For acute liver failure, there were vast differences between female and male subjects in LWRs but not much change by era. Female subjects had nearly three-fold higher LWRs compared with male subjects. By race/ethnicity, we observed a significant disparity for African Americans that did not improve by era and was 25% to 30% lower than for whites. Hispanics had similar LWRs to whites in both eras. Asians had significantly higher wait-listing rates in the latter part of the cohort, as did Native American/Other race patients. For chronic liver failure, a sex-based disparity was noted but with much smaller magnitude than observed with acute liver failure. The LWRs for both sexes declined significantly in the MELD era. With regards to race/ethnicity, African Americans had two-fold lower LWRs compared with whites, which did not improve in the latter part of the cohort. Asians had significantly higher LWRs for chronic liver disease than whites in both eras, and the LWRs for Native/Others were the lowest of the cohort. Hispanic patients retained similar LWRs as whites in both eras, but both observed some decline as time went on.
Figure 1 also demonstrates demographic differences in LWR for patients with CLF. Compared with male subjects, female subjects had a 20% higher LWR in the pre-MELD era (P<0.0001). This difference declined to 11% in the MELD era but remained significant (P<0.0001). African Americans were wait-listed half as often as whites across eras (both, P<0.0001). Hispanics and whites had similar LWRs, but Asians had a 50% higher LWR in both eras (both eras, P<0.0001) compared with whites. Native American/Others had the lowest LWR among those with CLF (pre-MELD era: 0.056; MELD era: 0.065).
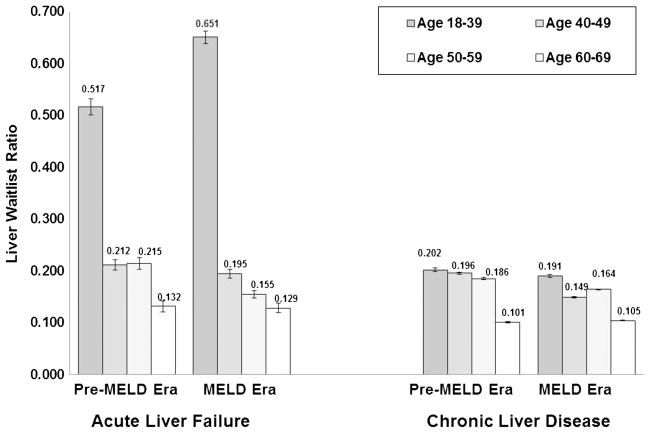
Age-based variation in liver wait-listing rates in both the pre-MELD and MELD eras was extensive (Fig. 2). For ALF, younger patients (18–39 years) had 2.4- to 3.3- fold higher LWR when compared with those aged 40 to 49 years and 50 to 59 years in the pre-MELD era (both, P<0.0001). For CLF, the degree of age-based variation was much less pronounced but still significant in the pre-MELD era. In the MELD era, younger adults had significantly higher LWR than all other age groups (0.191). Notably, patients aged 60 to 69 years had the lowest LWR (pre-MELD era: 0.101; MELD era: 0.105), regardless of era and category of liver disease.
FIGURE 2.
Age-based variation in liver wait-listing ratios by era, 1999–2006. Age-based variation in wait-listing rates in the pre-MELD and MELD eras was evident. For acute liver failure, young patients had two- to three-fold higher wait-listing rates than middle-aged adults, and patients older than 60 had the lowest LWRs, independent of diagnosis. For chronic liver disease, the degree of age-based variation was more compressed, but younger adults again had the highest wait-listing rates.
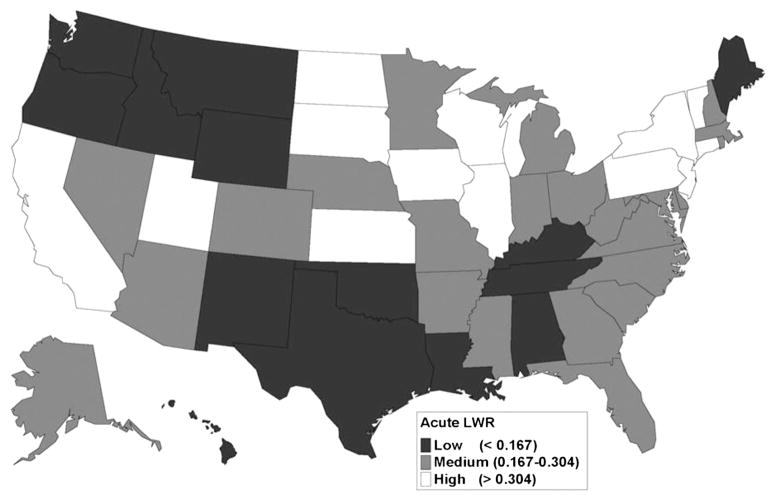
Geographic differences in LWR by state were notable for individuals with ALF and CLF (Figs. 3 and 4). The distribution of LWR by state varied widely. For ALF (Fig. 3), states with the lowest LWR quartile were mostly in the Pacific Northwest and the southern United States. High-access states included California, portions of the Midwestern United States, and parts of the mid-Atlantic area and New England. For CLF (Fig. 4), there was more geographic dispersion of the states comprising the lowest LWR quartile. Utah, Iowa, Wisconsin, Illinois, Pennsylvania, New York, and New Jersey were in the highest LWR quartiles for both acute and chronic liver disease.
FIGURE 3.
Geographic variation in liver wait-listing ratios for acute liver failure, 1999–2006. For patients with acute liver failure, access to the liver transplant waiting list was geographically variable across the United States. Low access states were concentrated in the Pacific Northwest, and in the South. High access states included California, several areas of the Midwest, and the middle Atlantic states.
FIGURE 4.
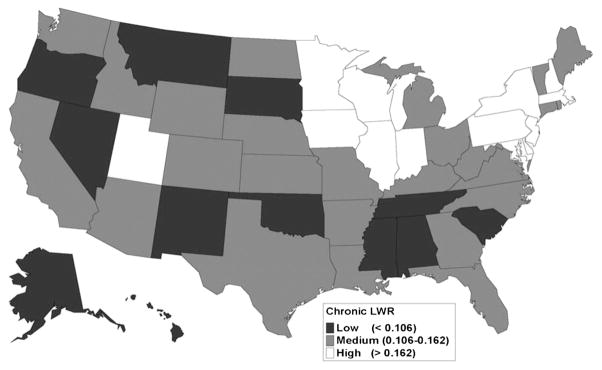
Geographic variation in wait-listing ratios for chronic liver disease, 1999–2006. For chronic liver disease patients, the middle two LWR quartiles (medium) were widely dispersed. High access areas were in the Midwest and the middle Atlantic. Low access states were also fairly dispersed geographically.
DISCUSSION
It is difficult to evaluate and study access to the liver transplant waiting list. Without a more sophisticated data source that holds longitudinal records on patients with cirrhosis and maintains linkages to national transplant data, the transplant community must rely on proxy measures to estimate liver wait-listing rates. We created an empirically derived measure of access to care; the ratio of waiting list registrations to those who are potentially transplant eligible, that is, those who died from liver diseases in a given period or who were put on the waiting list. This metric is understandable and generalizable and readily allows epidemiological analysis. Although there are inherent limitations to our method, we have demonstrated variation in access to the liver waiting list by age, sex, race/ethnicity, acuity of liver failure, and era. These differences may be related to a variety of factors but underscore an important issue in liver transplantation: access to the liver transplant waiting list is wrought with demographic and geographic inequity, and this has not appreciably changed with the adoption of MELD-based organ allocation policies.
We observed a significant degree of variation in wait-listing rates based on demographic characteristics. Younger patients had significantly higher LWRs compared with middle-aged and older adults for ALF, but this difference was much smaller in CLF. This observation is likely related to how providers make decisions regarding transplant candidacy. Our findings likely reflect selective referral of younger patients for transplant (9, 10). Poorer outcomes are described in patients older than 40 in fulminant hepatic failure (11), and data from the U.S. ALF Study Group demonstrate lower transplant rates among the elderly for non–acetaminophen-related failure (10). In CLF in the MELD era, wait-listing rates were significantly higher for younger patients compared with the pre-MELD era. The reasons for this are unclear, but it may reflect a change in medical decision making as a result of the introduction of MELD-based allocation rules. With regard to sex-based differences, a nearly three-fold higher female LWR (versus males) in both eras was observed for ALF and may be related to greater female predisposition to developing ALF when exposed to a noxious stimulus or sex differences in the phenotype of ALF (9, 10).
We also observed sex-based differences in wait-listing rates in the CLF population, with some attenuation in this difference from the pre-MELD to the MELD era, which could be linked to the utilization of MELD in U.S. liver allocation. For a given patient with CLF, the MELD score provides clinicians with a reasonable estimate of expected mortality over the next 3 months. This knowledge likely affects clinician behavior regarding waiting list registration, based on the clinical context and organ availability. The differences observed in our study may be related to differences in the type of liver disease and disease progression between sexes. These findings could also be related to differential access to health insurance. Adult female subjects make up more than 60% of Medicaid enrollees (12), which likely facilitates access to specialty liver disease care. Delays in referring male subjects to transplant centers or termination of the evaluation process at the transplant center for medical, surgical, or psychosocial reasons could account for a proportion of the differences observed. These findings are interestingly contrary to the sex-based differences present at the next step in the transplant process—receipt of a liver transplant from the waiting list. We have previously shown that wait-listed female subjects have lower transplant rates compared with male subjects (13, 14), even after accounting for differences in MELD scores and place of residence (15).
The persistent disparities from the pre-MELD to the MELD era underscore several points about access to the liver waiting list. Organ allocation system is based on MELD points and may affect a center’s behavior in listing someone based on their local environment. From the patient perspective, access to the waiting list likely has more to do with the potential personal resources, access to timely high-quality care, and ability to navigate through the liver transplant process. Centers decide to list a patient for transplant based on a nuanced medical, surgical, and psychosocial evaluation and not on MELD points alone. The allocation system plays a greater role in what happens to liver transplant candidates who are already on the list, and disparities exist in that phase of the process (16).
However, access to the waiting list is clearly related to where patients get their transplant care and may be at the root of the geographic patterns observed in this study. Transplant center wait-listing behavior is subject to multiple factors, including demand for transplant in the geographic area, competition with other local/regional centers, regulatory pressures on outcomes, practice patterns, and local organ availability (14, 17, 18). Liver disease mortality varies substantially across the United States as well (19), and there is some suggestion that transplant services and providers are poorly distributed relative to locations of need (1, 20). Furthermore, Bryce and colleagues have identified variation in access to transplant services within a fixed geographic area (8); given epidemiological and demographic differences in the U.S. population, differences in access between geographic areas are expected but are subject to many factors (21). Geographic variation in the accessibility of liver transplant care should be a clear policy priority and merits larger study on its own.
The racial disparity in LWR between African Americans and whites is consistent with broader patterns in liver disease. Several studies have shown that African Americans are disadvantaged compared with whites in earlier steps in the transplant process, including a worse response to hepatitis C therapy, inequitable treatment of cirrhosis complications, and lower rates of referral to transplant programs (2–4, 7, 22). In our study, for both acute and chronic liver disease, African Americans had significantly lower LWRs compared with whites in each era, by at least 25%. This may be related to several factors, including differential rates of referral to liver transplant programs, racial/ethnic differences in disease progression, delays in diagnosis, differences in the quality of hospitals and outpatient care offered to African American patients, patient preferences, mistrust of transplant providers, socioeconomic differences, or even provider bias (23–27). However, once African American patients are listed, recent data suggest that they have similar liver transplant rates as white candidates (16). This suggests that the critical step in alleviating racial/ethnic disparities in liver disease is to identify strategies that improve access to transplant centers and expedite waiting list registration for appropriate patients.
There are several limitations to our analysis. The inherent challenge in this analysis is estimating the true prevalent burden of patients living with liver disease. Our relative wait-listing ratio relies on a denominator derived from liver disease mortality records. Errors in the recording of causes of death have been reported, which may affect our results, particularly if error rates vary by any of the characteristics of interest (28–30). Specifically, ambiguity in administrative coding of ICD-9 and ICD-10 diagnoses for causes of death make it difficult in many cases to distinguish between deaths from chronic liver disease, acute liver failure, or the commonly used phrase of “acute-on-chronic” liver failure. This could skew results, but assumptions regarding the chronicity of liver failure are a necessary evil in this observational approach. Furthermore, the LWR we have provided is based on raw data, and we could not identify those who were evaluated by a transplant center and deemed ineligible for listing. It is unlikely that all of the never-wait-listed decedents in the denominator were ever truly “transplant eligible.” It is also possible that the number who would never have been eligible varies by patient demographics or geography. Such variation could bias the comparisons being made to some extent, but there is no better data available in the United States currently to answer this question.
Our study is novel in that it provides the transplant community and policy makers with a more comprehensive understanding of variation in access to an early step in the liver transplant process. Better data are needed. Considerable resources have been devoted to ensuring equitable access to liver transplantation among wait-listed candidates in the development of U.S. deceased donor organ allocation policies. A clinical registry or broad-based sampling study of patients with chronic liver disease would help to inform clinical decision making at one step earlier in the process and create a foundation for observational studies. Furthermore, data linkage with the SRTR would help create targeted policy interventions to improve access to liver transplantation. In large measure, this would parallel currently available census-level end-stage renal disease data from the Centers for Medicare and Medicaid Services, which can be linked to kidney transplant waiting list and transplant data. An additional area of future study that would contribute immensely to this area would be to determine what health-care structural factors affect rates of transplant center referral, including characteristics of referring providers and transplant centers, as well as the local insurance environment. Further efforts should also focus on interventions designed to reduce modifiable demographic and geographic variations in access to the liver transplant waiting list, as well as the creation of better sources of data to study this key step in the liver transplant process.
MATERIALS AND METHODS
The study database was created using data from the Scientific Registry of Transplant Recipients (SRTR) and the National Center for Healthcare Statistics (NCHS). All patient-level transplant candidate and recipient data are submitted by transplant centers via the Organ Procurement and Transplantation Network (OPTN) to the SRTR. The SRTR data are supplemented by additional data sources, including the Social Security Death Master File for candidate and recipient mortality (31, 32). Adult waiting list and post-transplant data from 1999 to 2006 were obtained from the SRTR, including waiting list registration, waiting list mortality, and posttransplant mortality, for patients with acute and chronic liver failure. Patients younger than 18 years and older than 69 years were excluded. Acute liver failure was defined as wait-listing as Status 1 at registration. These data were categorized by age, sex, race/ethnicity, and state of candidate residence.
National data on adult deaths attributable to liver disease were obtained from the NCHS using ICD-9 and ICD-10 codes. Deaths occurring at ages younger than 18 and older than 69 were excluded. Deaths identified in the NCHS data were classified as either from acute or chronic liver disease based on review of liver-related ICD-10 diagnoses recorded as primary or secondary causes of death (Table 1). Categorization as ALF or CLF was based on the primary or the first secondary liver code cause of death in NCHS. For ALF, we excluded those deaths due to alcoholism (about 800 deaths per year) or cancer. For CLF, we excluded nonliver cancer deaths. From 1999 to 2006, 423,534 patients in the United States died from liver disease, and the classification of diagnosis codes for ALF and CLF (Table S1, SDC, http://links.lww.com/TP/A930).
Our analytic approach was based on the assumption that the liver disease incidence, wait-listing, transplantation, and liver-related deaths were approximately in a steady state in the study. Since 1999, the annual number of new registrants, transplants, and waiting list deaths has been fairly consistent (1). For a given year, a liver wait-listing ratio (LWR) was calculated using the formula LWR=(SRTR waiting list registrations)/[(SRTR waiting list registrations +NCHS liver disease deaths)–(SRTR waiting list deaths+SRTR posttransplant deaths)]. LWR represented the wait-listing ratio over each ascribed time interval. This stochastic model postulates that new end-stage liver disease cases enter the population according to a Poisson process with a constant rate; a proportion p of these are wait-listed for transplant and a portion 1-p go on to death without wait-listing. We estimated the binomial proportion p with LWR for the given year and subgroup. Wald-type confidence intervals were calculated based on standard errors arising from the Poisson assumptions but were essentially those of the binomial SD=LWR(1-LWR)/N, where N was the number of liver deaths observed in the year.
Subsequent comparisons were based on demographic characteristics, era, diagnosis category, and geography. Demographic characteristics included age, sex, and race/ethnicity. Era effects were measured by calculating LWR by year, overall, and by allocation rule initiation. The pre-MELD era was defined as 1999–2001 and the MELD era as 2002–2006. Geographic variation was measured based on differences in LWR by state but could not be measured at the donor service area (DSA)-level because of the lack of granular data on the location of death.
This study was approved by the SRTR Project Officer at the U.S. Health Resources and Services Administration (HRSA). HRSA has determined that this study satisfies the criteria for the IRB exemption described in the “Public Benefit and Service Program” provisions of 45 CFR 46.101(b) (5) and HRSA Circular 03. All statistical analyses were performed using SAS v9.2 (SAS Institute, Cary, NC). Statistical significance was defined as P<0.05.
Acknowledgments
Supported by University of Michigan Center for Integrated Approaches to Health Disparities Pilot Project grant and NIH/NCMHD Loan Repayment Program.
The authors thank Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, for providing editorial assistance.
Footnotes
The authors declare no conflict of interest.
A.K.M. participated in research design, performance of the research, data analysis, and writing of the paper. V.B.A. participated in research design, performance of the research, data analysis, and writing of the paper. D.S.F. participated in performance of the research, data analysis, and writing of the paper. M.Z. participated in research design, data analysis, and writing of the paper. R.M.M. participated in research design, performance of the research, data analysis, and writing of the paper. A.L. participated in research design. performance of the research, data analysis, writing of the paper.
J.K. participated in research design, performance of the research, data analysis, and writing of the paper.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
No direct research support for this project.
References
- 1.Thuluvath PJ, Guidinger MK, Fung JJ, et al. Liver transplantation in the United States, 1999–2008. Am J Transplant. 2010;10(4 Pt 2):1003. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 2.Mathur AK, Sonnenday CJ, Merion RM. Race and ethnicity in access to and outcomes of liver transplantation: a critical literature review. Am J Transplant. 2009;9:2662. doi: 10.1111/j.1600-6143.2009.02857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen GC, Thuluvath PJ. Racial disparity in liver disease: biological, cultural, or socioeconomic factors. Hepatology. 2008;47:1058. doi: 10.1002/hep.22223. [DOI] [PubMed] [Google Scholar]
- 4.Kemmer N, Neff GW. Liver transplantation in the ethnic minority population: challenges and prospects. Dig Dis Sci. 2010;55:883. doi: 10.1007/s10620-009-0803-7. [DOI] [PubMed] [Google Scholar]
- 5.Eckhoff DE, McGuire BM, Young CJ, et al. Race: a critical factor in organ donation, patient referral and selection, and orthotopic liver transplantation? Liver Transpl Surg. 1998;4:499. doi: 10.1002/lt.500040606. [DOI] [PubMed] [Google Scholar]
- 6.Eckhoff DE, McGuire BM, Young CC, et al. Race is not a critical factor in orthotopic liver transplantation. Transplant Proc. 1997;29:3729. doi: 10.1016/s0041-1345(97)01089-0. [DOI] [PubMed] [Google Scholar]
- 7.Julapalli VR, Kramer JR, El-Serag HB. Evaluation for liver transplantation: adherence to AASLD referral guidelines in a large Veterans Affairs center. Liver Transpl. 2005;11:1370. doi: 10.1002/lt.20434. [DOI] [PubMed] [Google Scholar]
- 8.Bryce CL, Angus DC, Arnold RM, et al. Sociodemographic differences in early access to liver transplantation services. Am J Transplant. 2009;9:2092. doi: 10.1111/j.1600-6143.2009.02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan G, Taqi A, Marotta P, et al. Long-term outcomes of emergency liver transplantation for acute liver failure. Liver Transpl. 2009;15:1696. doi: 10.1002/lt.21931. [DOI] [PubMed] [Google Scholar]
- 10.Schiodt FV, Chung RT, Schilsky ML, et al. Outcome of acute liver failure in the elderly. Liver Transpl. 2009;15:1481. doi: 10.1002/lt.21865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Grady JG, Alexander GJ, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 12.Women’s Health USA 2012. U.S. Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Bureau; Rockville, MD: 2013. [Google Scholar]
- 13.Moylan CA, Brady CW, Johnson JL, et al. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300:2371. doi: 10.1001/jama.2008.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volk ML, Choi H, Warren GJ, et al. Geographic variation in organ availability is responsible for disparities in liver transplantation between Hispanics and Caucasians. Am J Transplant. 2009;9:2113. doi: 10.1111/j.1600-6143.2009.02744.x. [DOI] [PubMed] [Google Scholar]
- 15.Mathur A, Schaubel DE, Gong Q, et al. Sex-based disparities in liver transplant rates in the United States. Am J Transplant. 2011;11:1435. doi: 10.1111/j.1600-6143.2011.03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathur AK, Schaubel DE, Gong Q, et al. Racial and ethnic disparities in access to liver transplantation. Liver Transpl. 2010;16:1033. doi: 10.1002/lt.22108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathur AK, Ashby VB, Sands RL, et al. Geographic variation in end-stage renal disease incidence and access to deceased donor kidney transplantation. Am J Transplant. 2010;10(4 Pt 2):1069. doi: 10.1111/j.1600-6143.2010.03043.x. [DOI] [PubMed] [Google Scholar]
- 18.Volk ML, Biggins SW, Huang MA, et al. Decision making in liver transplant selection committees: a multicenter study. Ann Intern Med. 155:503. doi: 10.1059/0003-4819-155-8-201110180-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickle LW, Mungiole M, Jones GK, et al. Atlas of United States Mortality. Hyattsville, MD: National Center for Health Statistics; 1996. [Google Scholar]
- 20.Ladner D, Davis A, Friedewald J, et al. US transplant centers are closer to those who don’t need them. Am J Transplant. 2010;10 Abstract. [Google Scholar]
- 21.Kemmer N, Safdar K, Kaiser T, et al. Impact of geographic location on access to liver transplantation among ethnic minorities. Transplantation. 2008;85:166. doi: 10.1097/TP.0b013e31816223f8. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen GC, Segev DL, Thuluvath PJ. Racial disparities in the management of hospitalized patients with cirrhosis and complications of portal hypertension: a national study. Hepatology. 2007;45:1282. doi: 10.1002/hep.21580. [DOI] [PubMed] [Google Scholar]
- 23.Boulware LE, Cooper LA, Ratner LE, et al. Race and trust in the health care system. Public Health Rep. 2003;118:358. doi: 10.1016/S0033-3549(04)50262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bach PB, Pham HH, Schrag D, et al. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351:575. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 25.Trooskin SB, Navarro VJ, Winn RJ, et al. Hepatitis C risk assessment, testing and referral for treatment in urban primary care: role of race and ethnicity. World J Gastroenterol. 2007;13:1074. doi: 10.3748/wjg.v13.i7.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White RM. Misinformation and misbeliefs in the Tuskegee Study of Untreated Syphilis fuel mistrust in the healthcare system. J Natl Med Assoc. 2005;97:1566. [PMC free article] [PubMed] [Google Scholar]
- 27.Smith AK, Ladner D, McCarthy EP. Racial/ethnic disparities in liver transplant surgery and hospice use: parallels, differences, and unanswered questions. Am J Hosp Palliat Care. 2008;25:285. doi: 10.1177/1049909108315914. [DOI] [PubMed] [Google Scholar]
- 28.Pritt BS, Hardin NJ, Richmond JA, et al. Death certification errors at an academic institution. Arch Pathol Lab Med. 2005;129:1476. doi: 10.5858/2005-129-1476-DCEAAA. [DOI] [PubMed] [Google Scholar]
- 29.Myers KA, Farquhar DR. Improving the accuracy of death certification. CMAJ. 1998;158:1317. [PMC free article] [PubMed] [Google Scholar]
- 30.Jordan JM, Bass MJ. Errors in death certificate completion in a teaching hospital. Clin Invest Med. 1993;16:249. [PubMed] [Google Scholar]
- 31.Levine GN, McCullough KP, Rodgers AM, et al. Analytical methods and database design: implications for transplant researchers, 2005. Am J Transplant. 2006;6(5 Pt 2):1228. doi: 10.1111/j.1600-6143.2006.01277.x. [DOI] [PubMed] [Google Scholar]
- 32.Dickinson DM, Dykstra DM, Levine GN, et al. Transplant data: sources, collection and research considerations, 2004. Am J Transplant. 2005;5(4 Pt 2):850. doi: 10.1111/j.1600-6135.2005.00840.x. [DOI] [PubMed] [Google Scholar]