Abstract
Background
The aim of this study was to investigate clinical efficacy and safety of Remifemin on peri-menopausal symptoms in endometriosis patients with a post-operative GnRH-a therapy.
Material/Methods
We treated 116 women who had endometriosis with either Remifemin (n=56) 20 mg bid po or Tibolone (n=60) 2.5 mg qd po for 12 weeks after GnRH-a injection. The efficacy was evaluated by Kupperman menopausal index (KMI), and hot flash/sweating scores. The safety parameters such as liver and renal functions, lipid profile, endometrial thickness, and serum sex hormone level, as well as the incidence of adverse events were recorded.
Results
(1) After GnRH-a therapy, KMI and hot flash/sweating scores in both groups increased significantly (P<0.05) but we found no significant difference for KMI (2.87±1.40 for Remifemin and 2.70±1.26 for Tibolone) and hot flash/sweating scores (0.94±1.72 for Remifemin and 1.06±1.78 for Tibolone) between the 2 groups (P>0.05). (2) No statistical change was observed in liver or renal functions and lipid profile in both groups before and after the treatment (P>0.05). The post-therapeutic serum FSH, LH, and E2 level and endometrial thickness decreased remarkably in both groups (P<0.05). E2 level in the Remifemin group was obviously lower than that in the Tibolone group (P<0.05), and FSH and LH levels were strongly higher (P<0.05). No significant difference in thickness were found in either group (P>0.05). The Remifemin group had far fewer adverse events than the Tibolone group (P<0. 05).
Conclusions
Compared with Tibolone, Remifemin had a similar clinical efficacy and was safer for the peri-menopausal symptoms induced by GnRH-a in endometriosis patients.
MeSH Keywords: Drug Therapy, Endometriosis, Treatment Outcome
Background
Endometriosis (EMS) is an extremely prevalent estrogen-dependent gynecological disorder, which affects quality of life and fertility of reproductive-age women [1,2]. Current treatment of EMS is mainly based on surgery and ovarian suppressive agents. A major challenge for treating women with EMS is the risk of recurrence. Symptomatic recurrence rates of EMS have been reported to range from 21.5% at 2 years to 50% at 5 years after treatment [3], which imposes a burden on patients and clinicians [4]. Various medical treatments have been suggested for EMS, such as oral contraceptive pills and gonadotropin-releasing hormone agonists (GnRH-a). GnRH-a can modulate the peritoneal microenvironment by decreasing the concentrations of angiogenic and growth factors [5] and is an important treatment modality for EMS, significantly reducing EMS-related pain [6,7].
However, the use of GnRH-a could reduce the estrogen level of the patients, leading to peri-menopausal symptoms such as hot flashes, colpoxerosis, sexual hypoactivity, and bone loss, which hinders its long-term and extensive application [8]. Because peri-menopausal symptoms cause a serious and long-lasting decrease in the quality of life, some women avoid or discontinue such therapy. These peri-menopausal symptoms could be alleviated by the hormone-based “add-back therapy” [9]. In recent years, however, the results of large epidemiological studies have lead to the rejection of hormone therapy because its long-term use might cause liver damage, venous embolism, and vascular disease, as well as increase the incidence and mortality of endometrial cancer, ovarian cancer, and breast cancer [10]. Thus, the search for alternative treatment options is of considerable scientific and public interest [11,12].
The plant black cohosh, known as Actaea racemosa or Cimicifuga racemosa, is a member of the buttercup family. It is a perennial plant native to North America. Black cohosh extract contains no phytoestrogen and has no estrogen or progestogen activities [13]. It has been studied as an effective treatment for symptoms associated with menopause, without increasing cancer risk [14,15]. It has no significant influence on follicle-stimulating hormone (FSH), luteinizing hormone (LH), 17β-estradiol (E2), or prolactin (PRL). The mechanism by which black cohosh extract alleviates menopause symptoms is not clear. It might have a direct impact on nerve centers as a neurotransmitter modulator, act on 5-HT receptor, or act on u-opiate receptor of menopausal women [16]. Remifemin, a standardized pharmaceutical preparation, is the most popular black cohosh extract in China. It should have the same efficacy in treating non-physiological menopause symptoms caused by drugs as well as suppressing pituitary gonadotropin hormone secretion by GnRH-a. The combination of Remifemin and GnRH-a therapy shows promise in EMS treatment.
In spite of the popularity of this alternative therapeutic option, adequate data is not available on the clinical efficacy and safety of Remifemin for peri-menopausal symptoms induced by post-operative GnRH-a therapy for endometriosis [17,18]. Tibolone was shown to effectively reduce menopausal symptoms [19]. The present study aimed to evaluate the efficacy and safety of Remifemin in comparison to Tibolone on peri-menopausal symptoms introduced by post-operative GnRH-a therapy for endometriosis in Chinese women.
Material and Methods
Subjects
The study was conducted as a prospective, randomized, controlled trial to compare the efficacy and safety of Remifemin with that of Tibolone. The project was approved by the relevant Ethics Committee. Written informed consent was obtained from all participants prior to the therapy and they received detailed information about the study.
A total of 125 women [20,21] with GnRH-a (gonadotropin-releasing hormone agonist) treatment after endometriosis (EMS) surgery in the Department of Gynecology, the Third Affiliated Hospital of Suzhou University (China) between July 2010 and March 2013 were recruited for the study. With the patients’ informed consent to the study, they were randomly assigned to either the Remifemin or Tibolone group. Nine women who discontinued treatment or were lost to follow-up visit were excluded from the study. Finally, 116 patients were surveyed and analyzed, with 56 women in the Remifemin group and 60 women in the Tibolone group. All women with ovarian endometriosis underwent laparoscopic surgery. According to the 1985 revised American Fertility Society (r-AFS) classification of endometriosis, the EMS patients were categorized to stage III (N=18) and IV (N=38) in the Remifemin group and to stage III (N=24) and IV (N=36) in the Tibolone group.
We excluded patients who lacked typical medical history, did not follow diagnostic norms, or who did not have surgery, as well as patients with liver damage, severe diabetes, hypertension medical history, thromboembolic disease or thrombophilia, and who used hormones or other anti-perimenopause medicine.
Methods
All patients were treated with GnRH-a (Zoladex: Goserelin injection) after laparoscopic ovarian cyst removal surgery. GnRH-a (Zoladex, 3.6 mg, hypodermic injection) was injected into the patients 1 week after the operation for the first time, followed by another injection 4 weeks later. There were 3 injections in total. The Remifemin group was given oral Remifemin tablets (20 mg bid po for 12 weeks) and the Tibolone group was given oral Tibolone tablets (2.5 mg qd po for 12 weeks). The duration of follow-up was 12 weeks, with 1 cycle of 4 weeks. Follow-up started 4 weeks after the first GnRH-a injection. Patients who discontinued therapy within the 12-week period, used hormonal drugs in the first 4 weeks, took non-hormonal drugs (including nutrition) or ate food that might interfere with symptoms, or used any hypnotic, sedative, or antidepressant drugs, were all removed from the statistical analysis.
Evaluation Index
Efficacy parameters
Kupperman menopausal index (KMI). The modified KMI is an internationally recognized and validated scale for the quantitative determination of menopausal symptoms [10]. The modified KMI consists of 11 items, including hot flashes/sweating, paresthesia, insomnia, nervousness, melancholia, vertigo, fatigue, arthralgia, headache, palpitation, and formication. KMI scores divided into 15–20, 21–35, and >35 were used to rate the degree of severity as mild, moderate, and severe, respectively [19]. The patients were monitored by means of Kupperman test, which was administered initially and the 4th, 8th, and 12th week after the GnRH-a therapy and scores used for statistical analysis.
Hot flashes/sweating scores. The hot flashes/sweating scores of the patients initially and 4, 8, and 12 weeks after the GnRH-a therapy were recorded and used for statistical analysis.
Safety parameters
Liver and renal function. Liver and renal functions were assessed initially and at the 12th week after the GnRH-a treatment. The liver function referred to glutamic-pyruvic transaminase (ALT) and glutamic oxalacetic transaminase (AST), and the renal function was based on blood urea nitrogen (BUN) and creatinine (Cr). All collected data were used for statistical analysis.
Lipid profile. Lipid profile, including total cholesterol (CHOL), triglyceride (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL), were tested before and 12 weeks after the GnRH-a therapy. All collected data were used for statistical analysis.
Serum sex hormone levels. Levels of the following hormones were measured in serum: 17β-estradiol (E2), follicle-stimulating hormone (FSH), and luteinizing hormone (LH). Hormone data based on venous blood examination were collected before and 12 weeks after GnRH-a therapy and were used for statistical analysis.
Endometrial thickness. Endometrial thickness was determined through B ultrasound (PT380 Color Doppler Ultrasound Diagnosis System) before and 12 weeks after GnRH-a treatment and was used for statistical analysis.
Adverse reaction events. The adverse reaction events of the patients during the therapy were recorded. They included anaphylaxis (rash, pruritus, and dyspnea), headache, dizziness, nausea, emesis, abdominal discomfort, weight increase, breast distending pain, and vaginal bleeding or spotting of unknown etiology.
Data analysis
Statistical analysis for the comparisons between the 2 groups (Remifemin vs. Tibolone) was done using the t-test with the statistical program SPSS version 13.0. All results are expressed as χ̄±s and were compared with the χ2 test for any difference between the 2 groups. A value of P<0.05 was considered to be statistically significant.
Results
Patient characteristic
Characteristics of patients in the 2 groups were comparable with respect to all indexes, including age, body mass index (BMI), ratio of waist/hip circumference, blood pressure, and heart rate (Table 1). No significant difference in any index was observed between the 2 groups (P>0.05).
Table 1.
Demographic data of the endometriosis patients in group Remifemin (N=56) and Tibolone (N=60).
| Index | Rmifemin (N=56) | Tibolone (N=60) | P value |
|---|---|---|---|
| Age (years) | 28.23±3.02 | 29.12±2.01 | >0.05 |
| Body mass index (BMI, kg/m2) | 23.53±2.23 | 23.02±1.78 | >0.05 |
| waist/hip circumference | 0.88±0.05 | 0. 87±0.07 | >0.05 |
| Systolic pressure (mmHg) | 113.35±9.25 | 115.12±11.09 | >0.05 |
| Diastolic pressure (mmHg) | 74.27±7.98 | 75.15±8.07 | >0.05 |
| Heart rate (times/min) | 76.56±9.87 | 78.12±8.96 | >0.05 |
Efficacy of the 2 treatments on climacteric symptoms induced by post-operative GnRH-a injection
Climacteric complaints induced by post-operative GnRH-a injection were evaluated by KMI and hot flashes/sweating scores initially and at 4, 8, and 12 weeks after therapy.
KMI scores of the 2 groups
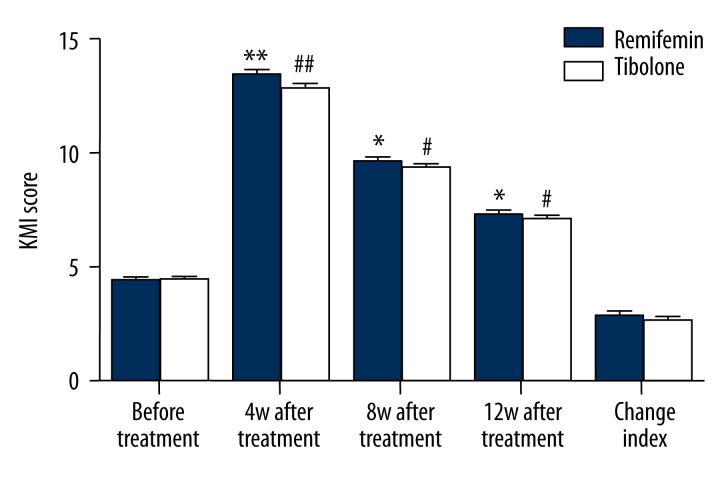
The patients were monitored by KMI administered before and at the 4th, 8th, and 12th week after GnRH-a therapy. No significant difference was observed between group the 2 groups (P>0.05) before GnRH-a therapy. For both groups, the KMI scores were found to have a significant increase after GnRH-a therapy (P<0.05), but without reaching statistical significance (P>0.05) at the same therapy period of the 4th, 8th, and 12th week between the 2 groups. After therapy the KMI score increased by 2.87±1.40 for the Remifemin group and 2.70±1.26 for the Tibolone group (P>0.05). There was no significant difference in KMI scores between the 2 groups (P>0.05) (Figure 1).
Figure 1.
Comparison of KMI scores of Remifemin and Tibolone before and after GnRH-a therapy: * p<0.05, ** p<0.01, vs. Remifemin group before GnRH-a therapy; # p<0.05, ## p<0.01, vs. the tibolone group before GnRH-a therapy.
Hot flashes/sweating scores
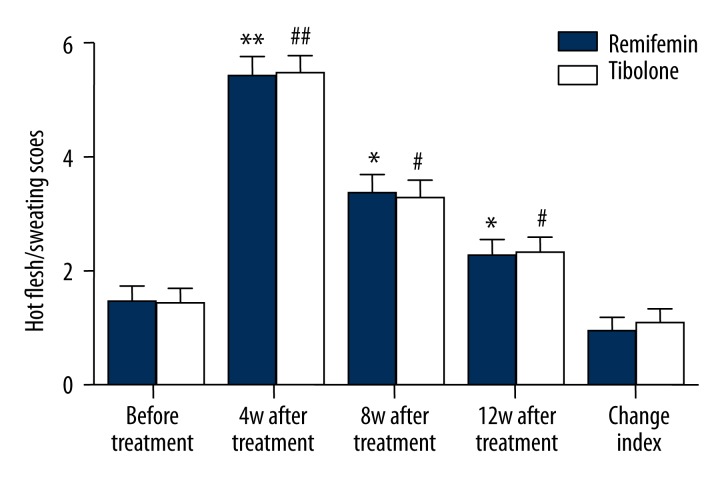
The hot flashes/sweating scores of the patients before and at the 4th, 8th, and 12th week after GnRH-a therapy were recorded. No significant difference was observed between the 2 groups (P>0.05) before GnRH-a therapy. For both groups, the hot flash/sweating scores were found to significantly increase after GnRH-a therapy (P<0.05), but without reaching statistical significance (P>0.05) at the same therapeutic period of the 4th, 8th, and 12th week between the 2 groups. After the therapy, the hot flashes/sweating score increased by 0.94±1.72 for the Remifemin group and 1.06±1.78 for the Tibolone group (P>0.05). There was no significant difference in hot flashes/sweating scores between the 2 groups (P>0.05) (Figure 2).
Figure 2.
Comparison of hot flashes/sweating scores of Remifemin and Tibolone before and after GnRH-a therapy: * p<0.05, ** p<0.01, vs. the Remifemin group before GnRH-a therapy; # p<0.05, ## p<0.01, vs. the tibolone group before GnRH-a therapy.
Safety evaluation of the 2 treatments
Parameters for evaluating the safety of the 2 treatments included liver and renal functions (ALT (U/L), AST (U/L), BUN(mmol/L), and Cr(μmol/L)), lipid profile (CHOL (mg/dl), TG (mg/dl), LDL (mg/dl), HDL (mg/dl), serum sex hormone levels (E2 (pg/ml), FSH(IU/L), LH(IU/L)), and endometrial thickness, as well as the incidence of adverse events in both groups before and after therapy.
Liver and renal function
We found no significant difference (P>0.05) between the 2 groups before treatment in factors related to liver and renal function, including ALT (U/L), AST (U/L), BUN (mmol/L), and Cr (μmol/L). There was no significant difference in liver and renal function changes before or after the therapy in each group (P>0.05). All data were shown in Table 2.
Table 2.
Comparison of liver and renal function of group Remifemin and Tibolone before and after GnRH-a therapy.
| Index | Remifemin | Tibolone | ||||
|---|---|---|---|---|---|---|
| Before | After | P value | Before | After | P value | |
| ALT | 17.23±2.14 | 18.34±2.35 | >0.05 | 18.35±2.06 | 18.08±3.37 | >0.05 |
| AST | 25.52±3.62 | 26.34±3.02 | >0.05 | 24.72±4.57 | 25.26±2.95 | >0.05 |
| BUN | 5.71±1.38 | 6.05±2.10 | >0.05 | 5.58±2.05 | 5.71±1.29 | >0.05 |
| Cr | 57.35±3.02 | 55.29±2.34 | >0.05 | 56.26±2.34 | 58.17±1.08 | >0.05 |
Lipid profile
No significant difference (P>0.05) was found between groups in lipid profile, including CHOL (mg/ml), TG (mg/ml), LDL (mg/ml), and HDL (mg/ml), before GnRH-a therapy. There was no significant difference in lipid profile changes before or after therapy in either group (P>0.05). All data are shown in Table 3.
Table 3.
Comparison of lipid profile of group Rmifemin and Tibolone before and after GnRH-a therapy.
| Index | Remifemin | Tibolone | ||||
|---|---|---|---|---|---|---|
| Before | After | P value | Before | After | P value | |
| CHOL | 233.23±32.25 | 228.34±23.45 | >0.05 | 246.14±26.17 | 237.08±21.16 | >0.05 |
| TG | 123.12±39.19 | 118.67±41.21 | >0.05 | 121.31±34.51 | 119.34±42.05 | >0.05 |
| LDL | 146.61±31.76 | 139.03±34.52 | >0.05 | 150.61±29.08 | 148.72±31.71 | >0.05 |
| HDL | 57.17±13.43 | 59.67±16.22 | >0.05 | 56.09±14.15 | 55.89±18.31 | >0.05 |
Serum sex hormone levels
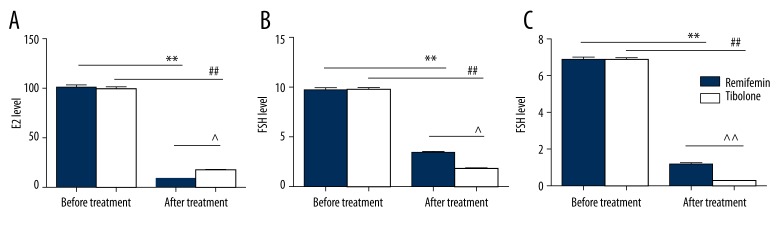
Levels of the following hormones were measured in serum: E2, FSH, and LH. No significant difference was observed for these 3 hormones between the 2 groups before GnRH-a therapy (P>0.05). However, after therapy, the 3 hormone levels decreased significantly compared with non-treatment (P<0.05). In addition, after treatment, the E2 level of the Remifemin group was lower than the Tibolone group, and FSH and LH levels were higher (P<0.05). All results are shown in Figure 3.
Figure 3.
Comparison of serum sex hormone levels of Remifemin and Tibolone before and after GnRH-a therapy: * p<0.05, ** p<0.01, vs. the Remifemin group before GnRH-a therapy; # p<0.05, ## p<0.01, vs. the tibolone group before GnRH-a therapy; ^ p<0.05, ^^ p<0.01, vs. the Remifemin group after GnRH-a therapy.
Endometrial thickness
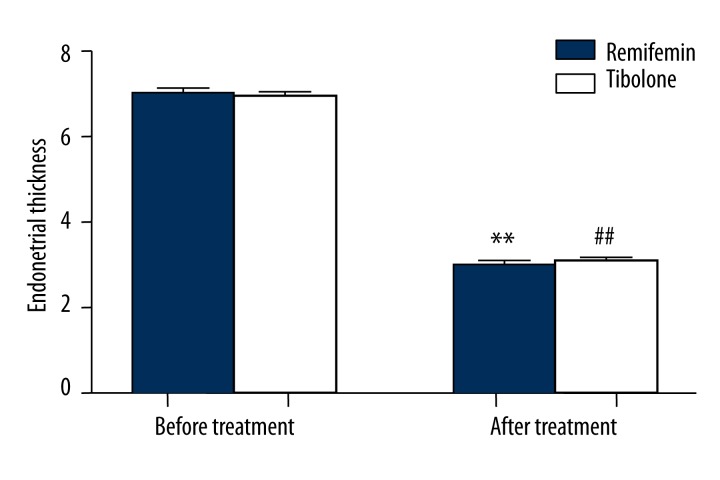
No significant difference was observed in endometrial thickness between the 2 groups before GnRH-a therapy (P>0.05). However, after therapy, thickness decreased significantly compared with non-treatment (P<0.05). After therapy there was no significant difference in thickness between the 2 groups (P>0.05). The results are shown in Figure 4.
Figure 4.
Comparison of endometrial thickness (mm) of Remifemin and tibolone before and after GnRH-a therapy: * p<0.05, ** p<0.01, vs. the Remifemin group before GnRH-a therapy; # p<0.05, ## p<0.01, vs. the tibolone group before GnRH-a therapy.
Adverse reaction events
None of the patients who underwent therapy had severe adverse reactions and they all completed the study in good situation. A few patients had slight rash and headache, which was not included in the statistical analysis. Data on common adverse events are included in Table 4. There was obviously lower incidence of vaginal bleeding or spotting, and breast distending pain in the Remifemin group than in the Tibolone group (P<0.05). Incidence of nausea, emesis, and abdominal discomfort was not significantly different between groups (P>0.05). In conclusion, the overall incidence of adverse reactions in the Remifemin group was significantly lower than in the Tibolone group (P<0.05).
Table 4.
Comparison of adverse reaction situation of group Remifemin and Tibolone.
| Index | Remifemin | Tibolone | P value | ||
|---|---|---|---|---|---|
| Number | Percent (%) | Number | Percent (%) | ||
| Vaginal bleeding or spotting | 6 | 10.71 | 19 | 31.67 | <0.05 |
| Vaginal bleeding | 2 | 3.57 | 13 | 21.37 | <0.05 |
| Spotting | 4 | 7.14 | 6 | 10.00 | >0.05 |
| Nausea and emesis | 5 | 8.93 | 7 | 11.67 | >0.05 |
| Abdominal discomfort | 3 | 5.36 | 5 | 8.33 | >0.05 |
| Breast pain | 4 | 7.14 | 12 | 20.00 | <0.05 |
| Sum | 18 | 32.14 | 43 | 71.67 | <0.05 |
Discussion
This was a prospective, randomized, controlled trial designed to compare the efficacy and safety of Remifemin with that of Tibolone. The results demonstrated that different degrees of peri-menopausal symptoms occurred in both groups after GnRH-a therapy. When treated with Remifemin and Tibolone, respectively, the scores of KMI and hot flash/sweating of both decreased obviously. However, at the same period, there was no significant difference in KMI and hot flash/sweating scores between the 2 groups. All results showed that Remifemin and Tibolone both had very good and similar efficacy in easing peri-menopausal symptoms introduced by post-operative GnRH-a therapy.
To compare the safety, as well as the toxic and adverse effects, of Remifemin and Tibolone, we investigated variations in the following factors before and after Remifemin and Tibolone therapy: liver and renal function, lipid profile, serum sex hormone levels, and endometrial thickness. There is evidence that black cohosh preparations might have some hepatotoxicity or estrogenic activity [22,23] but some researchers believe otherwise. Li et al. [24] used Remifemin to treat climacteric symptoms and reported that there was no significant difference in liver function and hormones index for the patients who took oral Remifemin tablets. Hu et al. [25] found that rats given black cohosh preparations for 4 weeks had no obvious change in uterine wet weight, uterine index, or E2 level, indicating advantages of black cohosh for peri-menopausal symptoms treatment. In our study, there were no significant differences observed in liver and renal function and lipid profile before and after therapy between the 2 groups. However, the levels of E2, FSH, and LH decreased significantly after therapy, due to the suppression of pituitary gonadotropin hormone secretion by GnRH-a. All of the above results show that short-term use of Remifemin or Tibolone does not remarkably affect liver and renal function and blood lipid metabolism. In comparison with the Tibolone group, the level of E2 was clearly lower and FSH and LH were higher in the Remifemin group after therapy, indicating that Remifemin does not have an obvious estrogen-like effect.
There was no significant difference in endometrial thickness after treatment between the 2 groups. EMS patients accepted a post-operative GnRH-a injection, which would lead to very low level of sex hormone (estrogen deficiency). As a result, the endometrial thickness in both groups decreased remarkably compared with the pre-therapeutic level. The metabolic products of Tibolone – 3α-OH and 3β-OH derivatives with estrogenic activity – might influence uterine endometrial thickness. However, the effect of the routine small dose of Tibolone (2.5 mg qd) on endometrium was very slight. In fact, compared to the shrinking effect of GnRH-a on endometrium, the influence of Tibolone on endometrium was negligible. If there is a clinical need to increase endometrial thickness (for example, after an intrauterine adhesion operation), usually a large dose of estrogen would be used because a small dose of estrogen might stimulate the endometrium only slightly. For example, the dose of Progynova (estradiol valerate tablets) required to stimulate endometrial growth is usually 3 mg tid or 5 mg bid. As a result, the effect of low-dose (add-back therapy dose) Progynova (1 mg qd) or Tibolone (2.5 mg qd) on the endometrial thickness was too small to be detected, thus the relative ‘increase’ in endometrial thickness in the Tibolone group had no statistical significance in comparison with Remifemin group. The results in this study corresponded to previous results [21,26].
Bai [27] showed that Remifemin had a similar efficacy as Tibolone, but a lower adverse event rate. Rebbeck [28] demonstrated that black cohosh remarkably reduced the incidence of breast cancer, indicating its feasibility for prophylaxis and treatment of breast cancer. In our study, the incidence of vaginal bleeding or spotting, and breast distending pain in the Remifemin group was obviously lower than in the Tibolone group. The overall incidence of adverse reaction in the Remifemin group was significantly lower, which corresponds to previous results.
Our study was limited by the absence of a placebo group. Even though the effectiveness of Remifemin over placebo was proved by Wuttke et al. [29], the need to produce well-controlled confirmatory results with black cohosh was indicated by several authors and is still necessary [21,30–35].
However, the present results add to knowledge about use of herbal remedies in add-back therapy practice, from both the clinical and the safety point of view. The positive action of Remifemin on peri-menopausal symptoms induced by GnRH-a injection was confirmed in this study. It is important to stress that the Remifemin treatment used in this study does not affect liver function, renal function, lipid profile, or hormonal levels, and was well-tolerated by almost all EMS women recruited. Our results demonstrate that Remifemin (20 mg bid) may be considered a consistent and safe ‘add-back’ option to counteract specific peri-menopausal symptoms induced by post-operative GnRH-a in clinical practice.
Conclusions
The use of GnRH-a in endometriosis patients could reduce the estrogen level, resulting in some peri-menopausal symptoms, but had a seriously negative impact on the quality of life. Short-term use of Remifemin was able to improve peri-menopausal symptoms as effectively as Tibolone, without obvious impact on patient liver and renal function, as well as blood lipid metabolism. In addition, Remifemin does not have an obvious estrogen-like effect. It could be a safe and effective choice for patients who are concerned about the cancer risk of estrogen use. In comparison with Tibolone, the incidence of adverse effects with Remifemin was lower. Therefore, Remifemin is potentially a safe option for treatment. However, the safety of long-term clinical use of Remifemin requires further research of longer duration and with larger sample sizes.
Footnotes
Source of support: This Study was supported by Changzhou Medical Innovative Youth Personnel Project (Changzhou Health & Science (2010) 368 KY201139)
Conflict of interest
None.
References
- 1.Nirgianakis K, Bersinger NA, McKinnon B, et al. Regression of the inflammatory microenvironment of the peritoneal cavity in women with endometriosis by GnRHa treatment. Eur J Obstet Gyn RB. 2013;170:550–54. doi: 10.1016/j.ejogrb.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Zeng B, Hu J, Yuan R, et al. Increased expression of importin13 in endometriosis and endometrial carcinoma. Med Sci Monit. 2012;18(6):CR361–67. doi: 10.12659/MSM.882879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almassinokiani F, Mehdizadeh A, Sariri E, et al. Effects of simvastatin in prevention of pain recurrences after surgery for endometriosis. Med Sci Monit. 2013;19:534–39. doi: 10.12659/MSM.883967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Chinese medical association branch of obstetrics and gynaecology endometriosis group. The diagnosis and treatment of endometriosis. Chin J Obstet Gynecol. 2007;42(9):645–47. [Google Scholar]
- 5.Leng JH, Lang JH, Yang JX. Progress of diagnosis and treatment of endometriosis. Chin J Obstet Gynecol. 2000;35(1):53–55. [Google Scholar]
- 6.Liu DY, Gu MJ, Shu JZ, et al. Effect of triptorelin and an extended-interval dosing regimen in the treatment of patients with endometriosis and adenomyoma. Chin J Obstet Gynecol. 2006;41(10):657–59. [PubMed] [Google Scholar]
- 7.Yang DZ, Wang MY. Proceedings of the third national meeting on endometriosis and chronic pelvic pain. Chin J Obstet Gynecol. 2010;45(4):243–45. [PubMed] [Google Scholar]
- 8.Khaleque NK, Michio K, Koichi H, et al. Cell proliferation effect of GnRH agonist on pathological lesions of women with endometriosis, adenomyosis and uterine myoma. Hum Reprod. 2010;25(11):2878–90. doi: 10.1093/humrep/deq240. [DOI] [PubMed] [Google Scholar]
- 9.Wang YQ, Zhang SF, Chen X, et al. Effects and safety of gonadotrophin-releasing hormone agonist combined with estradiol patch and oral medroxyprogesterone acetate on endometriosis. Chinese J Obstet Gynecol. 2009;44(7):7–9. [PubMed] [Google Scholar]
- 10.Liu PS, Li X, Liu Y. Ximingting tablets in the treatment of women with climacteric syndrome. J Shandong Univ Med. 2008;46(8):791–94. [Google Scholar]
- 11.Zhou L, Liu YJ. Progress of black cohosh treatment for peri-menopausal syndrome. Res Integ Tradit Chin West Med. 2011;3(3):150–52. [Google Scholar]
- 12.Kargar M, Hadjibabaie M, Gholami K. Simvastatin versustriptorelin in prevention of pain recurrences after surgery for endometriosis. Med Sci Monit. 2013;19:858. doi: 10.12659/MSM.889682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viereck V, Emons G, Wuttke W. Black cohosh. just another phytoestrogen? Trends Endocrin Met. 2005;16(5):214–12. doi: 10.1016/j.tem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Tian QJ, Xu L, Shen Q. A pilot study of Cimicifuga racemosa for the treatment of menopausal symptoms in women surviving gynecological malignant tumors. J Reprod Med. 2011;20(3):167–73. [Google Scholar]
- 15.Ross SM. Menopause: A standardized isopropanolic black cohosh extract (Remifemin) is found to be safe and effective for menopausal symptoms. Holist Nurs Pract. 2012;26(1):58–61. doi: 10.1097/HNP.0b013e31823d1f67. [DOI] [PubMed] [Google Scholar]
- 16.Powell SL, Godeck T, Nikolic D, et al. In vitro serotonergic activity of black cohosh and identification of N-methylserotonin as a potential active constituent. J Agric Food Chem. 2008;56(24):11718–26. doi: 10.1021/jf803298z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen ZL, Shi HR, Ren F. Clinical observation of Remifemin for treatment of menopausal symptoms in patients with gynecological cancers after operation. Henan Med Res. 2013;22(4):496–98. [Google Scholar]
- 18.Vermes G, Banhidy F, Acs N. The Effects of Remifemin® on Subjective Symptoms of Menopause. Adv Ther. 2005;22(2):148–54. doi: 10.1007/BF02849885. [DOI] [PubMed] [Google Scholar]
- 19.Xu YX, Zhang YZ, Wang Q. GnRHa and add-back therapy with low dose tibolone in the treatment of endometriosis. Current Adv Obstet Gyneco. 2003;12(2):120–23. [Google Scholar]
- 20.Morris EP, Rymer J, Robinson J, Fogelman I. Efficacy of tibolone as “add-back therapy” in conjunction with a gonadotropin-releasing hormone analogue in the treatment of uterine fibroids. Reprod Endocrin. 2008;89(2):421–28. doi: 10.1016/j.fertnstert.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 21.Nappi RE, Malavasi B, Brundu B, Facchinetti F. Efficacy of Cimicifuga racemosa on climacteric complaints: A randomized study versus low-dose transdermal estradiol. Gyneco Endocrin. 2005;20(1):30–35. doi: 10.1080/09513590400020922. [DOI] [PubMed] [Google Scholar]
- 22.Jin H, Zhang QX. Black cohosh hepatotoxicity report draws attention of the drug administration department. World Phytomed. 2006;21(6):249. [Google Scholar]
- 23.Liu ZP, Yang ZH, Zhu MX. Estrogenicity of black cohosh (cimicifuga racemosa) and its effect on estrogen receptor level in human breast cancer MCF-7 cells. J Hygi Res. 2001;30(2):77–79. [PubMed] [Google Scholar]
- 24.Li YL, Cui MH. Efficacy of Remifemin for controll of climacteric symptoms. Prog Obstet Gynecol. 2011;20(6):462–65. [Google Scholar]
- 25.Hu GM, Liu Y, Wang HY. Effect of black cohosh on the endometrium of rats without ovary and serum sex homone levels. J Tradit Chin Med. 2008;6(5):26–28. [Google Scholar]
- 26.Bai WP, Zepelin HH, Wang SY, et al. Efficacy and tolerability of a medicinal product containing an isopropanolic black cohosh extract in Chinese women with menopausal symptoms: A randomized, double blind, parallel-controlled study versus Tibolone. Maturitas. 2007;58:31–41. doi: 10.1016/j.maturitas.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Bai WP, Wang SY, Liu JL, et al. Efficacy and safety of remifemin compared to tibolone for controlling of perimenopausal symptoms. Chin J Obstet Gynecol. 2009;8(44):597–600. [PubMed] [Google Scholar]
- 28.Rebbeck TR, Troxel AB, Norman S, et al. A retrospective case-control study of the use of hormone-related supplements and association with breast cancer. Int J Cancer. 2007;120(7):1523–28. doi: 10.1002/ijc.22485. [DOI] [PubMed] [Google Scholar]
- 29.Wuttke W, Seidlova-Wuttke D, Gorkow C. The Cimicifuga preparation BNO 1055 vs. conjugated estrogens in a doubleblind placebo-controlled study: effects on menopause symptoms and bone markers. Maturitas. 2003;44:867–77. doi: 10.1016/s0378-5122(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 30.McKenna DJ, Jones K, Humphrey S, Hughes K. Black cohosh: efficacy, safety, and use in clinical and preclinical applications. Altern Ther Health Med. 2001;7:93–100. [PubMed] [Google Scholar]
- 31.Lieberman S. A review of the effectiveness of Cimicifuga racemosa (black cohosh) for the symptoms of menopause. J Womens Health. 1998;7:525–29. doi: 10.1089/jwh.1998.7.525. [DOI] [PubMed] [Google Scholar]
- 32.Liske E. Therapeutic efficacy and safety of Cimicifuga racemosa for gynaecologic disorders. Adv Ther. 1998;15:45–53. [PubMed] [Google Scholar]
- 33.Borrelli F, Ernst E. Cimicifuga racemosa: a systematic review of its clinical efficacy. Eur J Clin Phannacol. 2002;58:235–41. doi: 10.1007/s00228-002-0457-2. [DOI] [PubMed] [Google Scholar]
- 34.Huntley A, Ernst E. A systematic review of the safety of black cohosh. Menopause. 2003;10:58–64. doi: 10.1097/00042192-200310010-00010. [DOI] [PubMed] [Google Scholar]
- 35.Liske E, Hanggi W, Henneicke-von Zepelin HH, et al. Physiological investigation of a unique extract of black cohosh (Cimicifugae racemosae rhizoma): a 6-month clinical study demonstrates no systemic estrogenic effect. J Womens Health Gend Based Med. 2002;11:163–74. doi: 10.1089/152460902753645308. [DOI] [PubMed] [Google Scholar]