Abstract
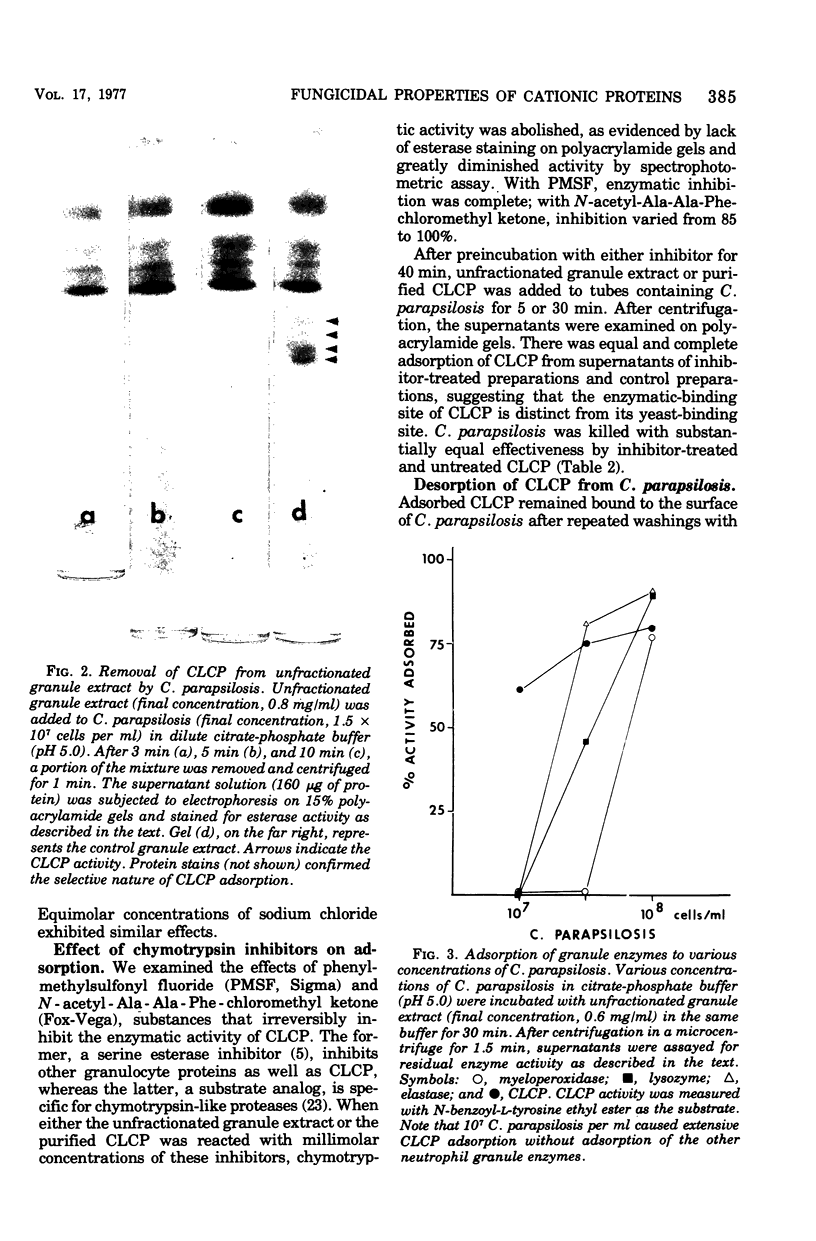
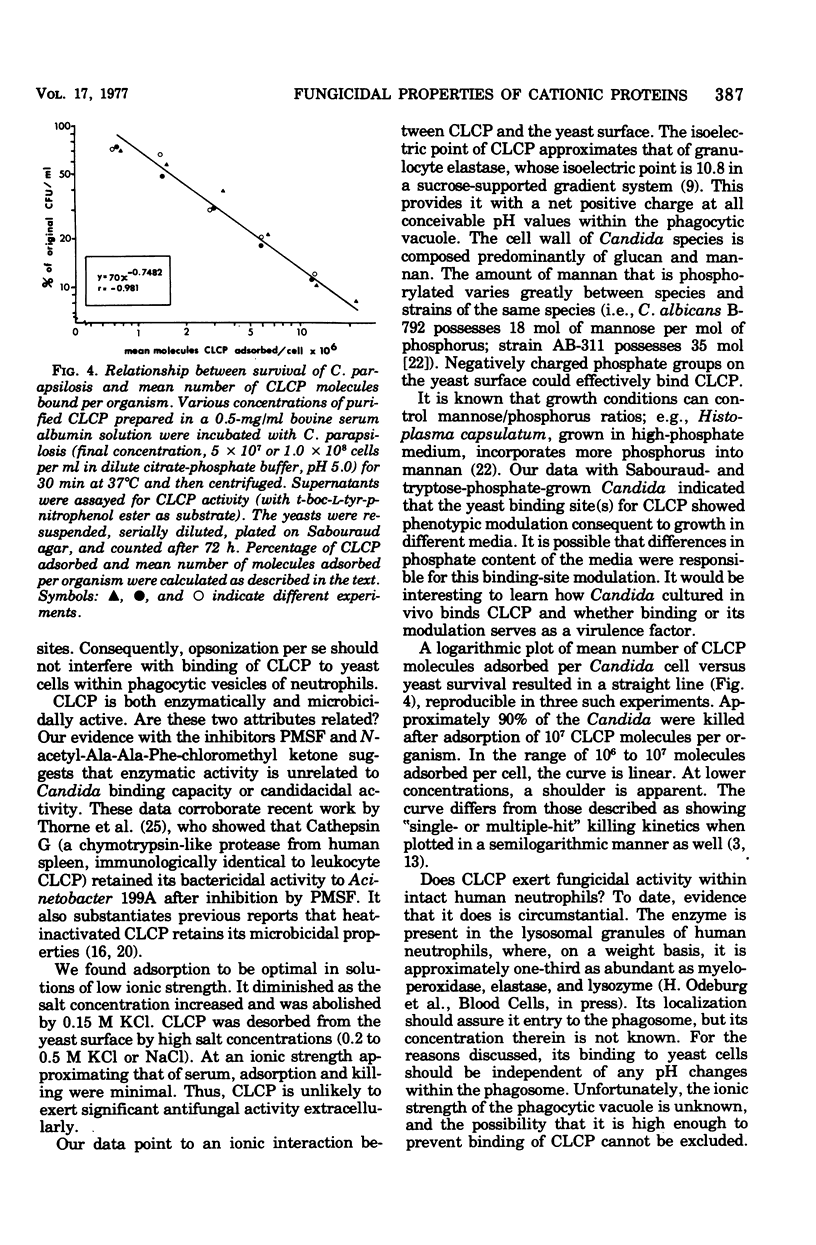
Human neutrophils contain a chymotrypsin-like cationic protein (CLCP) that binds to the surface of Candida parapsilosis and is preferentially adsorbed by yeasts mixed with unfractionated extracts of neutrophil granules. Adsorption of CLCP to opsonized or nonopsonized yeasts was rapid at pH 4 through 8. Irreversible inhibition of the enzymatic site of CLCP by phenylmethylsulfonylfluoride or N-acetyl-Ala-Ala-Phe-chloromethyl ketone did not affect its adsorption by yeast. Adsorption, highly sensitive to ionic strength, was abrogated by 0.15 M KCl. The number of CLCP molecules adsorbed per yeast cell and the loss of colony-forming units are described by an exponential relationship.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- Janoff A. Alanine p-nitrophenyl esterase activity of human leucocyte granules. Biochem J. 1969 Aug;114(1):157–159. doi: 10.1042/bj1140157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Basch R. S. Further studies on elastase-like esterases in human leukocyte granules. Proc Soc Exp Biol Med. 1971 Apr;136(4):1045–1049. doi: 10.3181/00379727-136-35424. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Role of the superoxide anion in the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1974 Jun 25;249(12):3724–3728. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Functional aspects of a second mechanism of candidacidal activity by human neutrophils. J Clin Invest. 1972 Oct;51(10):2566–2572. doi: 10.1172/JCI107073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ladra K. M., Hake R. B. Nonoxidative fungicidal mechanisms of mammalian granulocytes: demonstration of components with candidacidal activity in human, rabbit, and guinea pig leukocytes. Infect Immun. 1975 Jun;11(6):1226–1234. doi: 10.1128/iai.11.6.1226-1234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Antibacterial activity of cationic proteins from human granulocytes. J Clin Invest. 1975 Nov;56(5):1118–1124. doi: 10.1172/JCI108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Mechanisms for the microbicidal activity of cationic proteins of human granulocytes. Infect Immun. 1976 Dec;14(6):1269–1275. doi: 10.1128/iai.14.6.1269-1275.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I., Venge P. Cationic proteins of human granulocytes. II. Separation of the cationic proteins of the granules of leukemic myeloid cells. Blood. 1974 Aug;44(2):235–246. [PubMed] [Google Scholar]
- Powers J. C., Wilcox P. E. Design and synthesis of inhibitors for crystallographic studies on the active site of chymotrypsin. J Am Chem Soc. 1970 Mar 25;92(6):1782–1783. doi: 10.1021/ja00709a073. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Oliver R. C., Barrett A. J. Lysis and killing of bacteria by lysosomal proteinases. Infect Immun. 1976 Aug;14(2):555–563. doi: 10.1128/iai.14.2.555-563.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser L., Blout E. R. The use of p-nitrophenyl N-tert-butyloxycarbonyl-L-alaninate as substrate for elastase. Biochim Biophys Acta. 1972 Apr 7;268(1):257–260. doi: 10.1016/0005-2744(72)90223-9. [DOI] [PubMed] [Google Scholar]