Abstract
Objective. Negative results of recent randomized clinical trials testing the hypothesis of target therapy for patients with high on-treatment platelet reactivity (HOPR) have questioned its independent impact on clinical outcomes. 26 studies with 28.178 patients were included, with a median age of 66.8 (64–68) and 22.7% (22.4–27.8), of female gender. After a median follow-up of 1 year (0.1–1), cardiac adverse events occurred in 8.3% (3–11; all results are reported as median and interquartile range) of patients. Pooling all studies together, on-treatment platelet reactivity significantly increased the risk of adverse events (OR 1.33 [1.09, 1.64], I 2 = 0%). However, a sensitivity analysis showed that HOPR did not increase the risk of adverse events for patients with ACS, AMI, or stable angina as well as patients resistant to aspirin, ADP antagonists, or both. For all studies, publication bias was formally evident; after adjusting for this, HOPR did not significantly increase adverse cardiac events (OR 1.1 : 0.89–1.22, I 2 0%). Conclusions. After adjusting for clinical confounders (like risk factors and clinical presentation) and for relevant publication bias, HOPR was not an independent prognostic indicator in unselected patients with both stable and unstable coronary disease for an adverse cardiac event. The clinical importance of HOPR for high-risk populations remains to be assessed.
1. Introduction
Aspirin and ADP receptor antagonists represent an unquestionable strategy for patients undergoing percutaneous coronary intervention (PCI), both for stable and unstable coronary disease [1]. High on-treatment platelet reactivity (HOPR), variously defined and analyzed, has been reported in up to 30% of these patients [2] and has been linked to adverse cardiac events at follow-up [3–6].
Due to the high prevalence of HOPR and the assumption that HOPR increases the risk of adverse cardiac events, randomized clinical trials were performed to test the safety and efficacy of a tailored strategy (defined as an increase in dose or a switch to another ADP receptor antagonist) in patients undergoing PCI. When appraised separately, most of these studies were negative, without achieving the expected reduction in recurrent thrombotic events [7–9].
Prognostic impact of HOPR was assessed by at least two meta-analyses, although limited from methodological flaws [3, 4], due to lack of adjustement for baseline differences in burden of traditional risk factors and clinical presentation, which may explain themselves the increased risk of adverse cardiac events in selected patients. These two studies, however, have not tested the independent clinical effect of inadequate platelet inhibition on outcomes; moreover they evaluated patients with different risk profiles (ACS and stable angina) and different treatments (aspirin together with ADP antagonists or periprocedural glycoprotein inhibitors [10, 11]).
Randomisation of patients to HOPR and non-HOPR groups is obviously not feasible; consequently a bias analysis may help to elucidate the impact of HOPR on clinical prognosis independently from cardiovascular risk factors and clinical presentations.
2. Methods
The recent Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) amendment to the Quality of Reporting of Meta-analyses (QUOROM) statement, and recommendations from The Cochrane Collaboration and Meta-analysis of Observational Studies in Epidemiology (MOOSE) were followed during the development of the present systematic review [11–16].
2.1. Search Strategy and Study Selection
Pertinent articles were searched in Medline, Cochrane Library, Biomed Central, and Google Scholar in keeping with established methods with MESH strategy and with the following terms: (Prognosis/Broad[filter]) AND (platelet * AND (reactivity OR aggregation OR activation OR response *) AND (death OR (myocardial AND infarction))). Three independent reviewers (Fabrizio D'Ascenzo, Umberto Barbero, and Marta Bisi) screened the retrieved citations via the title and/or abstract; divergences were resolved via consensus. If potentially pertinent, studies were then appraised as complete reports according to the following explicit selection criteria. Studies were included if (i) reporting more than 50 patients (ii) independent prognostic impact of HOPR evaluated through multivariate analysis, while exclusion criteria were (i) nonhuman setting, (ii) duplicate reporting (in which case the manuscript reporting the largest sample of patients was selected), and (iii) interventional studies.
2.2. Data Extraction, End Points, and Sensitivity Analysis
Three unblinded independent reviewers (Fabrizio D'Ascenzo, Umberto Barbero, and Marta Bisi) abstracted the following data on prespecified forms: authors, journal, year of publication, location of the study group, and baseline clinical and interventional features. Data extraction was conducted by mutual agreement and all potential disagreement was solved by consensus. Incidence of adverse cardiac events (all-cause mortality and cardiovascular mortality, nonfatal myocardial infarction and stroke, and revascularization and stent thrombosis) was the primary end point. Sensitivity analyses were performed appraising aspirin and ADP receptor antagonists separately. Similarly we appraise indications for PCI in stable and unstable disease (i.e., either unstable angina, ST and non-ST segment elevation myocardial infarction). Finally, we analyze all-cause death, stent thrombosis and major bleedings.
2.3. Internal Validity and Quality Appraisal
Unblinded independent reviewers (Fabrizio D'Ascenzo, Umberto Barbero, and Marta Bisi) evaluated quality of included studies on prespecified forms. Modifying the MOOSE items to take into account the specific features of included studies [11], we separately abstracted and appraised study design, setting, and data source, as well as risk of analytical, selection, adjudication, detection, and attrition bias (expressed as low, moderate, or high risk of bias, as well as incomplete reporting leading to inability to ascertain the underlying risk of bias).
2.4. Data Analysis and Synthesis
Continuous variables are reported as mean (standard deviation) or median (interquartile). Categorical variables are expressed as n/N (%). Statistical pooling was performed according to a random-effect model with generic inverse-variance weighting, computing risk estimates with 95% confidence intervals, using RevMan 5 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark), and Comprehensive Meta-Analysis. Metaregression analysis was performed to identify impact of length of follow-up on results. Small study bias was appraised by graphical inspection of funnel plots and formally through Begg and Mazumdar rank correlation, Egger's regression intercept, and Duval and Tweedie trim and fill [14].
3. Results
2189 records were identified through database searching, and 38 were appraised at text level and finally twenty-six studies (see Appendix) were included (Figure 1) including 28.178 patients. The median age was 66.8 (64–68), with 22.7% (22.4–27.8) being female. Diabetes mellitus, hypertension, hyperlipidemia, and a history of previous MI were reported in 29% (24.2–34), 84% (58.9–89), 70% (54.4–71), and 30% (18–39), respectively. Stable angina was the admission diagnosis for 45% (37–100) of patients, ACS for 45% (33–100), and AMI for 12% (0–34). HOPR on aspirin was reported in 25% (22–26) of population, 29% (25–37) for patients on ADP receptor antagonists, and 26% (22–39) for both (Tables 1, 2, and 3). After a median follow-up of 1 year (0.1–1), adverse cardiac events occurred in 8.3% (3–11) of patients. Pooling all studies together, HOPR significantly increased the risk of adverse cardiac events (OR 1.33 [95% CI: 1.09, 1.64], I 2 0%, Figure 2). At metaregression analysis, length of follow up did not influence these results (Beta −0.001, P 0.58). HOPR did not increase risk of death (OR 1.13 [0.96, 1.33], I 2 0%), of stent thrombosis (OR 1.25 [0.87, 1.78], I 2 0%), and of major bleedings (1.20 [0.93, 1.56], I 2 21%, Figure 3).
Figure 1.
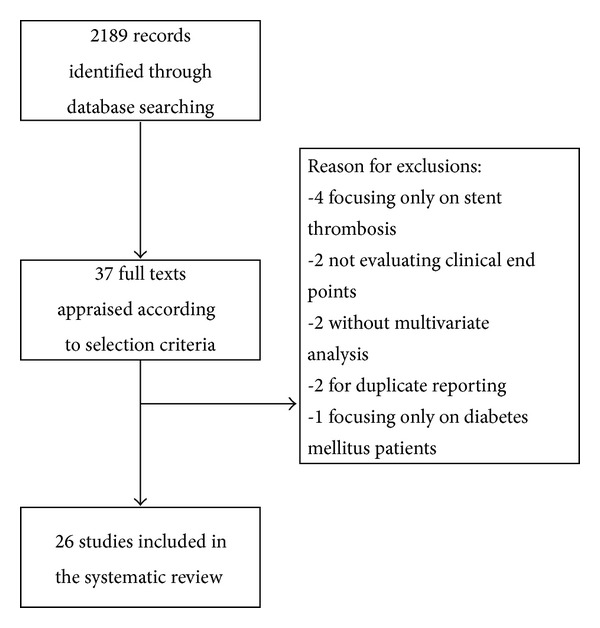
Review's profile.
Table 1.
Baseline features of included studies.
| Number of patients | Age | Female patients (%) | Diabetes mellitus (%) | Hypertension (%) | Hyperlipidemia (%) | Previous myocardial infarction (%) | Stable angina (%) | Acute coronary syndromes (%) | Myocardial infarction (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Angiolillo et al., 2007 [17] | 173 | 67 ± 9 | 35 | 100 | 65 | 68 | 53 | 100 | 0 | 0 |
| Bliden et al., 2007 [18] | 100 | 66 ± 11 | 28 | 44 | 74 | 83 | 40 | 75 | 13 | 12 |
| Breet et al., 2010 [19] | 410 | 64 ± 11.3 | 26.8 | 17.3 | 72.4 | 77.6 | 58.3 | 100 | 0 | 0 |
| Breet et al., 2010 [19] | 920 | 64 ± 10.6 | 24.6 | 18.4 | 77.5 | 80.9 | 54.6 | 100 | 0 | 0 |
| Buonamici et al., 2007 [2] | 804 | 69 ± 11 | 25 | 21 | 62 | 50 | 26 | 34 | 39 | 27 |
| Campo et al., 2010 [20] | 826 | 68 ± 12 | 25.2 | 24 | 72.3 | 59.4 | 38.6 | 64.4 | 35.6 (low risk UA) | — |
| Chiu et al., 2011 [21] | 144 | 65 ± 10 | 24 | 46.5 | 68.8 | 50 | 18 | 55 | 45 | — |
| Collet et al., 2012 [7] | 106 | 64 ± 10 | 23 | 25 | 58 | 56 | 46 (previous ACS) | 0 | 100 | 0 |
| Cuisset et al., 2006; 300 mg [22] | 146 | 64.2 ± 10.3 | 21 | 29 | 58 | 56 | 44 (previous ACS) | 0 | 100 | 100 |
| Cuisset et al., 2006; 600 mg [22] | 146 | 65.2 ± 12 | 27 | 33 | 56 | 55 | 45 (previous ACS) | 0 | 100 | 100 |
| Geisler et al., 2006 [23] | 379 | 67.5 ± 10 | 26.9 | 34.7 | 79.6 | 60.6 | 45.5 (previous ACS) | 54 | 45 | — |
| Hochholzer et al., 2006 [24] | 802 | 66.4 ± 9.1 | 21.8 | 24.8 | 82.3 | nd | 22.9 | 100 | 0 | 0 |
| Jin et al., 2013 [25] | 181 | 61.3 ± 12.1 | 16.6 | 24.9 | 39.5 | 95.5 | 3.9 | 0 | 100 | 100 |
| D.-W. Park et al., 2011 [26] | 809 | 64 | 33.2 | 30.5 | 66.3 | 45.4 | 7.2 | 100 | — | — |
| Ko et al., 2011 [27] | 222 | 63.3 | 31.5 | 32.0 | 72.1 | 46.8 | 5.9 | 100 | — | — |
| Marcucci et al., 2012 [28] | 1187 | 69 | 25.2 | 24.0 | 65.4 | 54.4 | x | — | 100 | 35 |
| Motoda et al., 2012 [29] | 450 | 71.1 | 31.5 | 42.8 | 74.0 | 60.2 | 31.1 | 100 | — | — |
| K. W. Park et al., 2011 [30] | 2546 | 61.7 | 29.9 | 28.5 | 58.9 | 61.0 | 5.9 | 55.6 | 44.4 | — |
| Park et al. (ACS), 2013 [31] | 1095 | 62 | 21 | 26 | 60 | 58 | 5 | — | 100 | |
| Park et al. (Stable Angina), 2013 [31] | 1329 | 63 | 22 | 27 | 57 | 63 | 4 | 100 | 0 | 0 |
| Parodi et al., 2011 [32] | 1789 | 69 | 20 | 19.8 | 57.0 | 44.7 | 18.1 | — | 100 | 46 |
| Patti et al., 2008 [33] | 160 | 66 | 19.3 | 34.3 | nd | 74.3 | 28.1 | 45.7 | 54.3 | — |
| Pettersen et al., 2012 [34] | 1001 | 62.3 | 21.8 | 20.0 | 55.4 | 98.3 | 43.7 | 100 | — | — |
| Price et al., 2011 [9] | 380 | 68 | 23.2 | 28.9 | 88.2 | 35.5 | 31.6 | 100 | — | — |
| Saia et al., 2013 [35] | 833 | 67.6 | 25 | 28.7 | 69.3 | 67.5 | 32.0 | 0 | 0 | 100 |
| Sibbing et al., 2009 [36] | 1608 | 67.5 | 23.0 | 29.0 | 91.6 | 70.0 | 32.0 | 66.9 | 33 | 20 |
| Sibbing et al., 2012 [37] | 564 | 67.7 | 22.3 | 31.2 | 89.3 | 70.5 | 19.1 | — | 100 | 100 |
| Siller-Matula et al., 2013 [38] | 403 | 64.2 | 24.1 | 32.0 | 84.6 | 76.4 | 32.0 | 67 | 33.0 | 33.0 |
| Stone et al., 2013 [39] | 8665 | 63.6 | 26 | 32.4 | 79.6 | 74.3 | 48.3 | 27.6 | 24.1 | |
Table 2.
Incidence of reactivity on aspirin, clopidogrel or both and kind of assays used.
| Reactivity on aspirin and ADP receptor antagonists (%) | Reactivity on aspirin (%) | Reactivity on ADP receptor antagonists (%) | Assays used | |
|---|---|---|---|---|
| Angiolillo et al., 2007 [17] | — | — | 25 | Light Transmittance Aggregometry (ADP 20 mmol/L-upper quartile) |
| Bliden et al., 2007 [18] | — | — | 22 (LTA) 30 (TEG) |
Light Transmittance Aggregometry (ADP 5 mmol/L) Thromboelastography |
| Breet et al., 2010 [19] | 14.7 | 8.5 (aspirin only) | 25.1 (clopidogrel only) | Verify Now aspirin/Verify Now P2Y12 |
| Breet et al., 2010 [19] | 26.9 (LTA 5) 23.3 (LTA 20) |
21.1 (LTA 5) 24.7 (LTA 20) |
14.9 (LTA 5) 13.0 (LTA 20) |
Light Transmittance Aggregometry (ADP 5 mmol/L-LTA 5, and 20 mmol/L-LTA 20) |
| Buonamici et al., 2007 [2] | — | — | 13 | Light Transmittance Aggregometry (ADP 10 mmol/L) |
| Campo et al., 2010 [20] | 3 | 15 | 21.6 | Verify Now aspirin/Verify Now P2Y12 |
| Chiu et al., 2011 [21] | — | — | 33 | Platelet Function Analyzer-100 |
| Collet et al., 2012 [7] | — | 27 | 26 | Both ADP and arachidonic acid (AA) as agonists to explore the responses to clopidogrel and aspirin, respectively |
| Cuisset et al., 2006; 300 mg [22] | — | — | 25 | Light Transmittance Aggregometry (ADP 10 mmol/L) |
| Cuisset et al., 2006; 600 mg [22] | — | — | 15 | Light Transmittance Aggregometry (ADP 10 mmol/L) |
| Geisler et al., 2006 [23] | — | — | 5.8 | Light Transmittance Aggregometry (ADP 20 mmol/L) |
| Hochholzer et al., 2006 [24] | — | — | 50 | Verify Now P2Y 12 |
| Jin et al., 2013 [25] | — | nd | 55 | Multiple electrode aggregometry, Verify Now P2Y 12, Verify Now Aspirin |
| D.-W. Park et al., 2011 [26] | — | — | 40.9 | Light Transmittance Aggregometry (ADP 10 mmol/L) |
| Ko et al., 2011 [27] | 52 | — | — | ADP-induced platelet aggregation using a whole blood analyzer |
| Marcucci et al., 2012 [28] | 11 | 17 | 44 | Multiple electrode aggregometry |
| Motoda et al., 2012 [29] | — | — | 50 | Multiple electrode aggregometry |
| K. W. Park et al., 2011 [30] | — | — | 25 | Verify Now P2Y 12 |
| Park et al. (ACS), 2013 [31] | 63 | Verify Now P2Y 12 | ||
| Park et al. (Stable Angina), 2013 [31] | 61 | Verify Now P2Y 12 | ||
| Parodi et al., 2011 [32] | — | 26 | — | PFA 100 |
| Patti et al., 2008 [33] | — | — | 32.1 | Verify Now P2Y 12 |
| Pettersen et al., 2012 [34] | — | — | 20 | Multiple electrode aggregometry (ADP) |
| Price et al., 2011 [9] | — | — | 36 | Multiple electrode aggregometry (ADP) |
| Saia et al., 2013 [35] | — | — | 67 | Verify Now P2Y12 |
| Sibbing et al., 2009 [36] | 8 | 27 | 19 | Multiple electrode aggregometry (AA and ADP) |
| Sibbing et al., 2012 [37] | ||||
| Siller-Matula et al., 2013 [38] | ||||
| Stone et al., 2013 [39] | 42.7 | Verify Now aspirin/Verify Now P2Y12 |
Table 3.
Incidence and definition of outcome appraised in the multivariate model.
| Follow-up (months) | Definition of outcome | Incidence of outcome | |
|---|---|---|---|
| Angiolillo et al., 2007 [17] | 24 | Cardiovascular death, ACS, and stroke | 15.2 1st quartile |
| 12.2 2nd quartile | |||
| 12.2 3rd quartile | |||
| 37.7 4th quartile | |||
|
| |||
| Bliden et al., 2007 [18] | 1 12 |
Death secondary to any cardiovascular cause, stroke, myocardial infarction (ami), and target/nontarget vessel revascularization |
23 (1 month FU) 50 (12 months FU) |
|
| |||
| Breet et al., 2010 [19] | 12 | All-cause death, nonfatal ami, stent thrombosis, and stroke | LTA 5 11.3 (DHPR) |
| 8.8 (HAPR) | |||
| 10.9 (HCPR) | |||
| 4.1 (NPR) | |||
| LTA 20 10.7 (DHPR) | |||
| 9.6 (HAPR) | |||
| 11.7 (HCPR) | |||
| 4.2 (NPR) | |||
|
| |||
| Buonamici et al., 2007 [2] | 6 | Stent thrombosis | 3.1 |
|
| |||
| Campo et al., 2010 [20] | 12 | All-cause death, nonfatal ami, and stroke | Full Responder (FR) 8.6 |
| Poor Responder (PR) 15.8 | |||
| ASA FR 10 PR 13 | |||
| Clop FR 5.9 PR 17.3 | |||
|
| |||
| Chiu et al., 2011 [21] | 24 | Cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke | 10 |
|
| |||
| Collet et al., 2012 [7] | 1 | Stent thrombosis | 2 |
|
| |||
| Cuisset et al., 2006; 300 mg [22] | 1 | Cardiovascular death, nonfatal ami, stent thrombosis, and stroke | 12 |
| 33.3 HPR 0.5 NPR | |||
|
| |||
| Cuisset et al., 2006; 600 mg [22] | 1 | Cardiovascular death, nonfatal ami, stent thrombosis, and stroke | 4.1 |
| 27.2 HPR 0.008 NPR | |||
|
| |||
| Geisler et al., 2006 [23] | 3 | Cardiovascular death, nonfatal ami, and nonfatal stroke | 6.6 |
| 5.6 Adequate clopidogrel response | |||
| 22.7 Low clopidogrel response | |||
|
| |||
| Hochholzer et al., 2006 [24] | 1 | All-cause death, nonfatal ami, and percutaneous revascularization | 1.9 |
| 3.5 in upper quartile | |||
|
| |||
| Jin et al., 2013 [25] | 12 | Cardiovascular death, nonfatal ami, and nonfatal stroke | 11 |
|
| |||
| D.-W. Park et al., 2011 [26] | 12 | Cardiac death and nonfatal ami | 1.4 |
| 0.9 Adequate clopidogrel response | |||
| 2.8 Low clopidogrel response | |||
|
| |||
| Ko et al., 2011 [27] | 1 | All-cause death, nonfatal ami, nonfatal stroke, and percutaneous revascularization | 8.6 |
|
| |||
| Marcucci et al., 2012 [28] | 12 | Cardiac death and nonfatal ami | 9.6 |
|
| |||
| Motoda et al., 2012 [29] | 12 | Cardiac death, nonfatal ami, stent thrombosis, and target vessel revascularization | 12 |
| 19 in HPR | |||
| 5.1 in NPR | |||
|
| |||
| K. W. Park et al., 2011 [30] | 24 | Cardiac death, nonfatal ami, nonfatal stroke, and urgent percutaneous revascularization | 14.6 HPR |
| 8.7 LPR | |||
|
| |||
| Park et al. (ACS), 2013 [31] | 72 | Cardiac death, nonfatal ami, nonfatal stroke, urgent percutaneous revascularization, and stent thrombosis | |
|
| |||
| Park et al. (Stable Angina), 2013 [31] | 72 | Cardiac death, nonfatal ami, nonfatal stroke, urgent percutaneous revascularization, and stent thrombosis | |
|
| |||
| Parodi et al., 2011 [32] | 1 | Cardiac death, nonfatal ami and percutaneous revascularization | 3 1st quartile |
| 5 2nd quartile | |||
| 10 3rd quartile | |||
| 20 4th quartile | |||
|
| |||
| Patti et al., 2008 [33] | 24 | All-cause death, nonfatal ami, unstable angina, and stroke | 13.3 HAPR |
| 9.9 LAPR | |||
|
| |||
| Pettersen et al., 2012 [34] | 6 | Cardiovascular death, nonfatal myocardial infarction, and stent thrombosis | 6.5 HPR |
| 1 LPR | |||
|
| |||
| Price et al., 2011 [9] | 1 | Stent thrombosis | 2.2 HPR |
| 0.2 LPR | |||
|
| |||
| Saia et al., 2013 [35] | 12 | All-cause death, ami, and urgent target vessel revascularization | |
|
| |||
| Sibbing et al., 2009 [36] | 1 | All-cause death, ami, and urgent target vessel revascularization | Abciximab/UFH: 9.4 HPR 6.7 LPR |
| Bivalirudin: 22.0 HPR 5.0 LPR | |||
|
| |||
| Sibbing et al., 2012 [37] | 12 | Acute coronary syndrome, stent thrombosis, stroke, death, and revascularization | 37.5 DHPR |
| 33.3 HCPR | |||
| 25.6 HAPR | |||
| 18.6 LPR | |||
|
| |||
| Siller-Matula et al., 2013 [38] | Acute coronary syndrome, stent thrombosis, stroke, death, and revascularization | ||
|
| |||
| Stone et al., 2013 [39] | 24 | All-cause death and myocardial infarction and stent thrombosis | 2.4 death |
| 3.9 mi | |||
| 1.3 ST | |||
Figure 2.
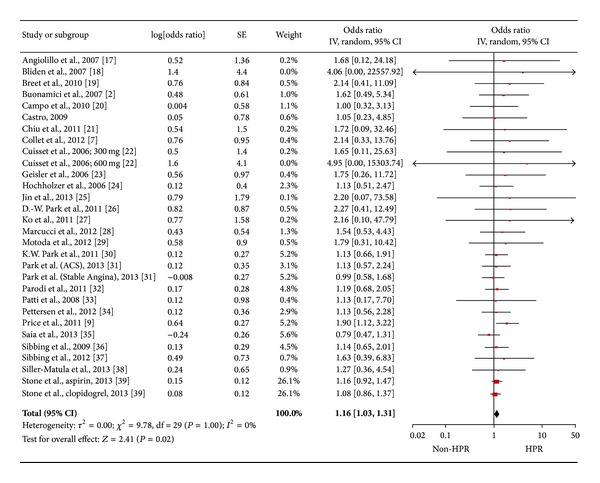
Pooled analysis of odds ratio for platelet reactivity for all studies [28.178 patients].
Figure 3.
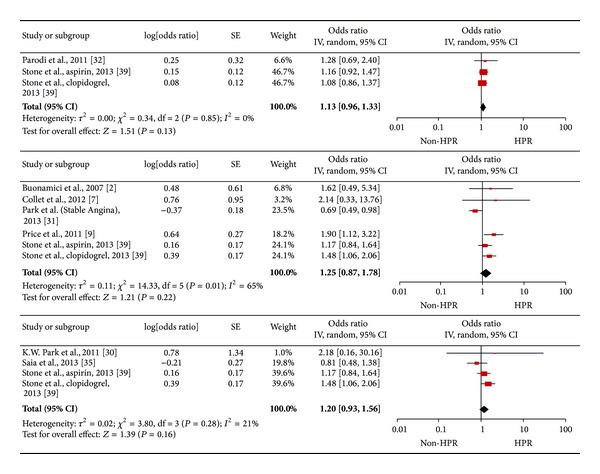
Pooled analysis of odds ratio according to end point (all-cause death [19099 patients], stent thrombosis [25848 patients] and clinically relevant bleeding [19472 patients] from above to below).
Sensitivity analysis for diagnosis showed that HOPR did not increase the risk of adverse cardiac events for patients with ACS (1.06 [0.79, 1.43], I 2 = 0%), AMI (0.95 [0.61, 1.46], I 2 = 0%), or stable angina (1.16 [0.82, 1.63], I 2 = 0%, Figure 4).
Figure 4.
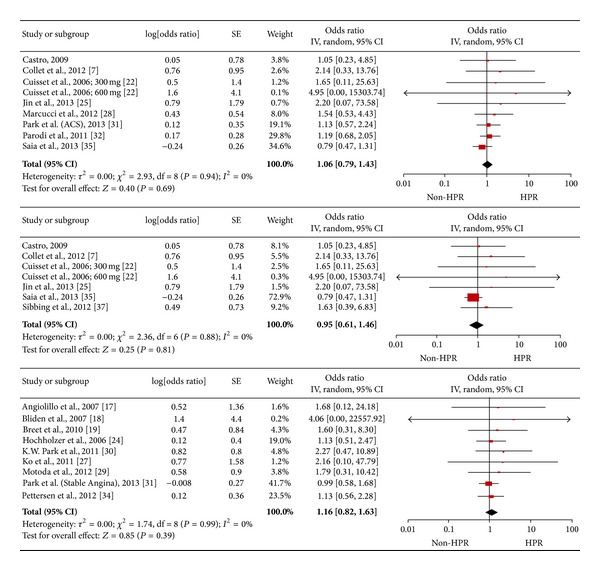
Pooled analysis of odds ratio for platelet reactivity according to diagnosis (ACS [3103 patients], acute myocardial infarction [2189 patients], stable angina [4487 patients] from above to below).
Sensitivity analysis according to type of antiplatelet medication indicated that neither was HOPR an independent predictor of adverse cardiac events, nor did this show if patients were resistant to aspirin, ADP antagonists (clopidogrel in all studies), or both (1.16 [0.93, 1.45], I 2 = 0%; 1.09 [0.93, 1.28], I 2 = 0%; and 1.26 [0.70, 2.27], I 2 = 0%, Figure 5).
Figure 5.
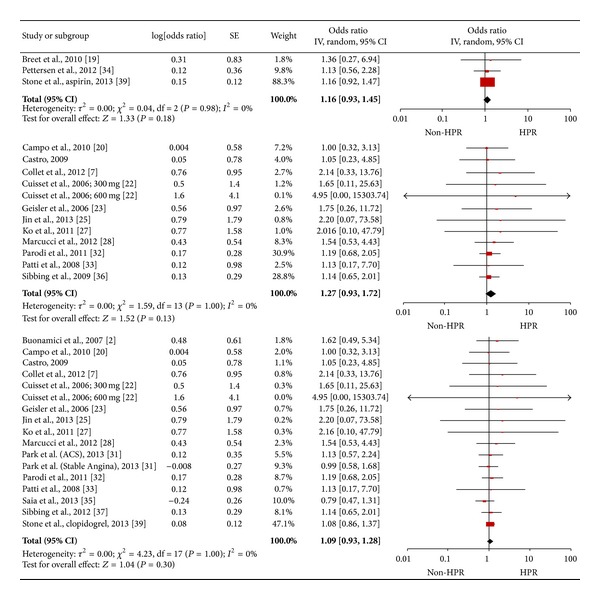
Pooled analysis of odds ratio according to reactivity (aspirin: 10066 patients; ADR receptor antagonists: 6750 patients; both: 17436 patients, from above to below in Figure 5).
For all studies, publication bias was graphically evident (Figure 6) and formally assessed with Begg and Mazumdar rank correlation (with a positive Tau of 0.31) and with Egger's regression intercept (Intercept 0.42 : 0.11–0.69; t-value 2.81). After adjusting for this bias with Duval and Tweedie trim and fill, HOPR was not a significant prognostic indicator for all studies (OR 1.1 : 0.89–1.22, I 2 0%; trim and fill methods evaluate publication bias by evaluating number of “asymmetric” trials on the right side, removing and replacing them with missing counterparts at the pooled estimate, and evaluating the adjusted confidence interval [14]).
Figure 6.
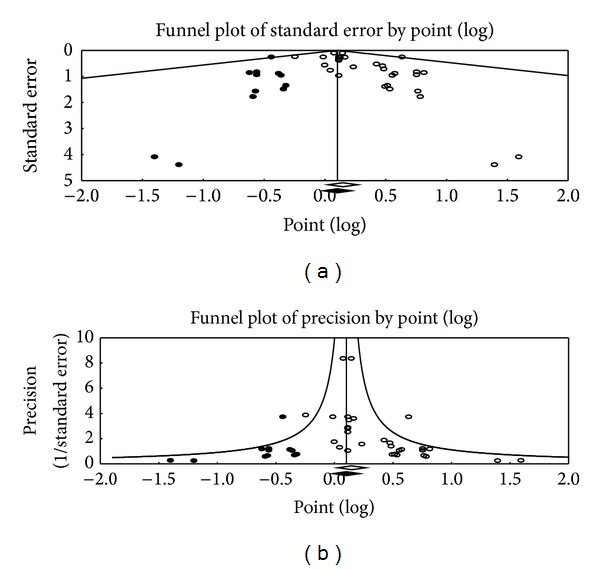
Funnel plot of standard error (a) and of precision (b). White box: observed studies. Black box: imputed study (trim and fill methods evaluates publication bias by evaluating number of “asymmetric” trials on the right side, removing and replacing them with missing counterparts at the pooled estimate and evaluating the adjusted confidence interval).
4. Discussion
The main results of the present meta-analysis, investigating incidence and impact of HOPR on prognosis, are as follows: (a) HOPR represents a frequent finding for patients with coronary artery disease, both in chronic and acute settings; (b) current evidence is limited from relevant publication bias; (c) after adjustment for clinical and methodological confounders HOPR appraised for “all comers” with CAD does not significantly increase the hazard of adverse cardiac events; and (d) usefulness in high-risk patients may not be excluded and remains to be assessed.
Many reasons can explain nonresponsiveness to antiplatelet medications, such as interindividual variability in the metabolism of clopidogrel (which is a prodrug activated by CYP-3A4, CYP-2C19, and CYP1A2), drug-drug interactions (i.e., interaction on the same metabolic pathway for clopidogrel, but also competition for binding sites on COX-1 by nonsteroidal anti-inflammatory medications and aspirin), P2Y12 receptor polymorphisms and increased platelet turnover during inflammation, acute coronary events, and diabetes mellitus. Interestingly, conventional cardiovascular risk factors themselves (smoking, diabetes, and hyperlipidemia) and also the same clinical pattern of unstable angina, increasing macrophage's thromboxane synthesis, enhance resistance to aspirin [40].
Previously, numerous observational studies have demonstrated the causal relationship between laboratory evidence of nonresponsiveness to aspirin or clopidogrel and an increase hazard of death, myocardial reinfarction, and stent thrombosis during secondary prevention for coronary disease [18, 19, 23, 41–43]. The obvious induction was that individualization of antiplatelet therapy based on laboratory tests should improve outcomes, even if most of these studies were limited by absence of multivariate adjustments, that is, without a global assessment of potential clinical confounders [19], for example, the presence of diabetes, which increases both HOPR and recurrent cardiac events after ACS.
However, subsequent randomized controlled trials questioned this hypothesis. In the ARMYDA-2 study, pretreatment with a 600 mg loading dose of clopidogrel given before PCI was demonstrated to be safe and, as compared with the 300-mg dose, reduced periprocedural MI without increased bleeding [44]. On the other hand, the GRAVITAS and the ARCTIC trials, which randomized patients with HOPR after PCI with drug eluting stents to high-dose clopidogrel compared with standard-dose, did not showe significant improvements in clinical outcomes [22, 33]. Later, new evidence suggested that a more tailored therapy could be attained by switching to newer drugs [9, 45, 46]. Similarly, randomized evidence failed to demonstrate a clinical impact. The TRIGGER-PCI study showed that HOPR after elective PCI with DES implantation, if detected, can be reliably corrected by switching from clopidogrel to prasugrel but again failed to demonstrate an improvement in clinical outcomes [47]. A similar result emerged from the TRILOGY-ACS trial, randomizing patients with NSTE-ACS who were medically managed [48]. More recently, switching to ticagrelor seems to be associated to an effective reduction in HOPR but studies about the effective clinical impact are still lacking [47, 49].
This meta-analysis indicates that HOPR does not seem to be a useful predictor of outcomes in an “all comers” CAD population. These results hold true both for overall studies, and, after appraisal for diagnosis, types of antiplatelet medication analysed and assays were exploited. These findings may be explained because they derive from data drawn from multivariate analysis, with a critical adjustment (even though limited by absence of randomization itself) for clinical features both increasing platelet resistance and risk of adverse events (like diabetes mellitus, smoking, or renal disease).
While HOPR should not totally be disregarded, a focus on high-risk patients seems more appropriate [49–53], for example, those with recurrent stent thrombosis in the absence of periprocedural or adherence problems or in diabetic or in HIV populations who have a well-known increased risk of recurrent events.
Current evidence remains burdened from relevant publication bias, which deeply affects clinical interpretation of HOPR. This phenomenon was described by psychologist Robert Rosenthal as the “file drawer problem"; he wrote that “journals are filled with the 5% of the studies that show Type I errors, while the file drawers are filled with the 95% of the studies that show nonsignificant results” [54]. In the cardiovascular field, this problem was recently demonstrated by Ioannidis and colleagues [55], who stated that, among 56 meta-analyses reporting relationships between biomarkers and cardiovascular events, only 13 were not affected by selection bias. However, most of current guidelines do not include this kind of evaluation, which may deeply influence every day clinical decisions.
Our analysis has some limitations, including a great number of observational studies, which brings incomplete data around follow-up and about the correct reporting of adverse effects, different definitions, and outcomes. Moreover, for each sensitivity analysis, the number of patients was inferior to that of overall population, although superior or similar to that of previous meta-analysis on this topic [3, 4]. Again, just a small number of studies could reliably monitor compliance. Platelet reactivity tests differed in each study, which also limits the HOPR definition. Because of the selection criteria, no studies selected use the Platelet Vasodilator-Stimulated Phosphorylation test (PLT-VASP test), a flow cytometry test that is today the most specific test to assess the effect of the platelet P2Y12 antagonists (clopidogrel, ticlopidine, and prasugrel) [51]. Thus, the included studies' quality was evaluated according to standardized criteria and we separately abstracted and appraised study design, setting, and data source, as well as risk of analytical, selection, adjudication, detection, and attrition bias. For all studies, publication bias was formally assessed. After adjusting for this bias, HOPR did not significantly increase adverse cardiac events for all studies.
We therefore conclude that routine assessment of HOPR is not useful, but high-risk subsets of patients (i.e., diabetics, multiple cardiovascular risk factors, and important comorbidities, especially if they need therapies potentially interacting with antiplatelet drugs) may potentially benefit from its assessment and interpretations remain to be assessed.
Abbreviations
- ACS:
Acute coronary syndrome
- AMI:
Acute myocardial infarction
- HOPR:
High on-treatment platelet reactivity
- PCI:
Percutaneous coronary intervention.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Fabrizio D'Ascenzo conceived the project and Giuseppe Biondi Zoccai performed the analysis. All the other authors were involved in the writing and the preparation of the paper.
References
- 1.EAPCI, Wijns W, Kolh P, et al. Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) European Heart Journal. 2010;31:2501–2555. [Google Scholar]
- 2.Buonamici P, Marcucci R, Migliorini A, et al. Impact of platelet reactivity after clopidogrel administration on drug-eluting stent thrombosis. Journal of the American College of Cardiology. 2007;49:2312–2317. doi: 10.1016/j.jacc.2007.01.094. [DOI] [PubMed] [Google Scholar]
- 3.D'Ascenzo F, Colombo F, Barbero U, et al. Discontinuation of dual antiplatelet therapy over 12 months after acute coronary syndromes increases risk for adverse events in patients treated with percutaneous coronary intervention: systematic review and meta-analysis. Journal of Interventional Cardiology. 2014;27(3):233–241. doi: 10.1111/joic.12107. [DOI] [PubMed] [Google Scholar]
- 4.Quadri G, D'Ascenzo F, Bollati M, et al. Diffuse coronary disease: short- and long-term outcome after percutaneous coronary intervention. Acta Cardiologica. 2013;68(2):151–160. doi: 10.1080/ac.68.2.2967272. [DOI] [PubMed] [Google Scholar]
- 5.Aradi D, Komócsi A, Vorobcsuk A, et al. Prognostic significance of high on-clopidogrel platelet reactivity after percutaneous coronary intervention: systematic review and meta-analysis. American Heart Journal. 2010;160(3):543–551. doi: 10.1016/j.ahj.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Brar SS, ten Berg J, Marcucci R, et al. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. A collaborative meta-analysis of individual participant data. Journal of the American College of Cardiology. 2011;58(19):1945–1954. doi: 10.1016/j.jacc.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 7.Collet JP, Cuisset T, Rangé G, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. The New England Journal of Medicine. 2012;367:2100–2109. doi: 10.1056/NEJMoa1209979. [DOI] [PubMed] [Google Scholar]
- 8.Trenk D, Stone GW, Gawaz M, et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. Journal of the American College of Cardiology. 2012;59(24):2159–2164. doi: 10.1016/j.jacc.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. Journal of the American Medical Association. 2011;305:1097–1105. doi: 10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- 10.Aradi D, Komócsi A, Price MJ, et al. Efficacy and safety of intensified antiplatelet therapy on the basis of platelet reactivity testing in patients after percutaneous coronary intervention: systematic review and meta-analysis. International Journal of Cardiology. 2012;167(5):2140–2148. doi: 10.1016/j.ijcard.2012.05.100. [DOI] [PubMed] [Google Scholar]
- 11.Biondi-Zoccai G, Agostoni P, Abbate A, D'Ascenzo F, Modena MG. Potential pitfalls of meta-analyses of observational studies in cardiovascular research. Journal of the American College of Cardiology. 2012;59(3):292–293. doi: 10.1016/j.jacc.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. The Lancet. 1999;354(9193):1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-Analysis of Observational Studies in Epidemiology (MOOSE) group. Journal of the American Medical Association. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.Bertrand ME, Simoons ML, Fox KA, et al. Management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. European Heart Journal. 2002;23(23):1809–1840. doi: 10.1053/euhj.2002.3385. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. The Cochrane Collaboration; 2009. http://handbook.cochrane.org/ [Google Scholar]
- 16.Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. The British Medical Journal. 2000;320(7249):1574–1577. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angiolillo DJ, Bernardo E, Sabaté M, et al. Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. Journal of the American College of Cardiology. 2007;50(16):1541–1547. doi: 10.1016/j.jacc.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 18.Bliden KP, DiChiara J, Tantry US, Bassi AK, Chaganti SK, Gurbel PA. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention: is the current antiplatelet therapy adequate? Journal of the American College of Cardiology. 2007;49(6):657–666. doi: 10.1016/j.jacc.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 19.Breet NJ, van Werkum JW, Bouman HJ, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. Journal of the American Medical Association. 2010;303:754–762. doi: 10.1001/jama.2010.181. [DOI] [PubMed] [Google Scholar]
- 20.Campo G, Fileti L, de Cesare N, et al. Long-term clinical outcome based on aspirin and clopidogrel responsiveness status after elective percutaneous coronary intervention: a 3T/2R (Tailoring Treatment with Tirofiban in Patients Showing Resistance to Aspirin and/or resistance to Clopidogrel) trial substudy. Journal of the American College of Cardiology. 2010;56(18):1447–1455. doi: 10.1016/j.jacc.2010.03.103. [DOI] [PubMed] [Google Scholar]
- 21.Chiu FC, Wang TD, Lee JK, et al. Residual platelet reactivity after aspirin and clopidogrel treatment predicts 2-year major cardiovascular events in patients undergoing percutaneous coronary intervention. European Journal of Internal Medicine. 2011;22(5):471–477. doi: 10.1016/j.ejim.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Cuisset T, Frere C, Quilici J, et al. Benefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing coronary stenting. Journal of the American College of Cardiology. 2006;48(7):1339–1345. doi: 10.1016/j.jacc.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 23.Geisler T, Langer H, Wydymus M, et al. Low response to clopidogrel is associated with cardiovascular outcome after coronary stent implantation. European Heart Journal. 2006;27:2420–2425. doi: 10.1093/eurheartj/ehl275. [DOI] [PubMed] [Google Scholar]
- 24.Hochholzer W, Trenk D, Bestehorn H-P, et al. Impact of the degree of peri-interventional platelet inhibition after loading with clopidogrel on early clinical outcome of elective coronary stent placement. Journal of the American College of Cardiology. 2006;48(9):1742–1750. doi: 10.1016/j.jacc.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 25.Jin H-Y, Yang T-H, Kim D-I, et al. High post-clopidogrel platelet reactivity assessed by a point-of-care assay predicts long-term clinical outcomes in patients with ST-segment elevation myocardial infarction who underwent primary coronary stenting. International Journal of Cardiology. 2013;167(5):1877–1881. doi: 10.1016/j.ijcard.2012.04.154. [DOI] [PubMed] [Google Scholar]
- 26.Park D-W, Lee S-W, Yun S-C, et al. A point-of-care platelet function assay and C-reactive protein for prediction of major cardiovascular events after drug-eluting stent implantation. Journal of the American College of Cardiology. 2011;58(25):2630–2639. doi: 10.1016/j.jacc.2011.08.057. [DOI] [PubMed] [Google Scholar]
- 27.Ko Y-G, Suh J-W, Kim BH, et al. Comparison of 2 point-of-care platelet function tests, VerifyNow Assay and Multiple Electrode Platelet Aggregometry, for predicting early clinical outcomes in patients undergoing percutaneous coronary intervention. American Heart Journal. 2011;161(2):383–390. doi: 10.1016/j.ahj.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 28.Marcucci R, Giusti B, Paniccia R, Gori AM, Saracini C. High on-treatment platelet reactivity by ADP and increased risk of MACE in good clopidogrel metabolizers. Platelets. 2012;23(8):586–593. doi: 10.3109/09537104.2012.658106. [DOI] [PubMed] [Google Scholar]
- 29.Motoda C, Ueda H, Hayashi Y, et al. Impact of platelet reactivity to adenosine diphosphate before implantation of drug-eluting stents on subsequent adverse cardiac events in patients with stable angina. Circulation Journal. 2012;76(3):641–649. doi: 10.1253/circj.cj-11-0435. [DOI] [PubMed] [Google Scholar]
- 30.Park KW, Jeon KH, Kang SH, et al. Clinical outcomes of high on-treatment platelet reactivity in Koreans receiving elective percutaneous coronary intervention (from results of the CROSS VERIFY study) American Journal of Cardiology. 2011;108(11):1556–1563. doi: 10.1016/j.amjcard.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Park D-W, Ahn J-M, Song H-G, et al. Differential prognostic impact of high on-treatment platelet reactivity among patients with acute coronary syndromes versus stable coronary artery disease undergoing percutaneous coronary intervention. American Heart Journal. 2013;165(1):34.e1–42.e1. doi: 10.1016/j.ahj.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Parodi G, Marcucci R, Valenti R, Gori AM, Migliorini A. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA-Journal of the American Medical Association. 2011;306(11):1215–1223. doi: 10.1001/jama.2011.1332. [DOI] [PubMed] [Google Scholar]
- 33.Patti G, Nusca A, Mangiacapra F, Gatto L, D'Ambrosio A, Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention: results of the ARMYDA-PRO (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity Predicts Outcome) study. Journal of the American College of Cardiology. 2008;52(14):1128–1133. doi: 10.1016/j.jacc.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 34.Pettersen AÅ, Seljeflot I, Abdelnoor M, Arnesen H. High on-Aspirin platelet reactivity and clinical outcome in patients with stable coronary artery disease: results from ASCET (Aspirin Nonresponsiveness and Clopidogrel Endpoint Trial) The American Heart Association. 2012;1(3) doi: 10.1161/JAHA.112.000703.e000703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saia F, Marino M, Campo G, et al. Incidence and outcome of high on-treatment platelet reactivity in patients with non-st elevation acute coronary syndromes undergoing percutaneous coronary intervention (from the VIP [verify now and inhibition of platelet reactivity] study) American Journal of Cardiology. 2013;112(6):792–798. doi: 10.1016/j.amjcard.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Sibbing D, Braun S, Morath T, et al. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. Journal of the American College of Cardiology. 2009;53(10):849–856. doi: 10.1016/j.jacc.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 37.Sibbing D, Bernlochner I, Schulz S, et al. Prognostic value of a high on-clopidogrel treatment platelet reactivity in bivalirudin versus abciximab treated non-ST-segment elevation myocardial infarction patients: ISAR-REACT 4 (Intracoronary stenting and antithrombotic regimen: rapid early action for coronary treatment-4) platelet substudy. Journal of the American College of Cardiology. 2012;60(5):369–377. doi: 10.1016/j.jacc.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 38.Siller-Matula JM, Delle-Karth G, Christ G, et al. Dual non-responsiveness to antiplatelet treatment is a stronger predictor of cardiac adverse events than isolated non-responsiveness to clopidogrel or aspirin. International Journal of Cardiology. 2013;167(2):430–435. doi: 10.1016/j.ijcard.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 39.Stone GW, Witzenbichler B, Weisz G, et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. The Lancet. 2013;382(9892):614–623. doi: 10.1016/S0140-6736(13)61170-8. [DOI] [PubMed] [Google Scholar]
- 40.Tourmousoglou CE, Rokkas CK. Clopidogrel and aspirin in cardiovascular medicine: Responders or not—current best available evidence. Cardiovascular & Hematological Agents in Medicinal Chemistry. 2008;6(4):312–322. doi: 10.2174/187152508785909483. [DOI] [PubMed] [Google Scholar]
- 41.Price MJ, Endemann S, Gollapudi RR, et al. Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. European Heart Journal. 2008;29(8):992–1000. doi: 10.1093/eurheartj/ehn046. [DOI] [PubMed] [Google Scholar]
- 42.Cuisset T, Frere C, Quilici J, et al. High post-treatment platelet reactivity identified low-responders to dual antiplatelet therapy at increased risk of recurrent cardiovascular events after stenting for acute coronary syndrome. Journal of Thrombosis and Haemostasis. 2006;4:542–549. doi: 10.1111/j.1538-7836.2005.01751.x. [DOI] [PubMed] [Google Scholar]
- 43.Marcucci R, Gori AM, Paniccia R, et al. Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up. Circulation. 2009;119(2):237–242. doi: 10.1161/CIRCULATIONAHA.108.812636. [DOI] [PubMed] [Google Scholar]
- 44.Biondi-Zoccai G, Agostoni P, Abbate A, D'Ascenzo F, Modena MG. Potential pitfalls of meta-analyses of observational studies in cardiovascular research. Journal of the American College of Cardiology. 2012;59(3):292–293. doi: 10.1016/j.jacc.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 45.D'Ascenzo F, Barbero U, Moretti C. Percutaneous coronary intervention versus coronary artery bypass graft for stable angina: meta-regression of randomized trials. Contemporary Clinical Trials. 2014;38(1):51–58. doi: 10.1016/j.cct.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Alexopoulos D, Theodoropoulos KC, Stavrou EF, et al. Prasugrel versus high dose clopidogrel to overcome early high on clopidogrel platelet reactivity in patients with ST elevation myocardial infarction. Cardiovascular Drugs and Therapy. 2012;26:393–400. doi: 10.1007/s10557-012-6407-z. [DOI] [PubMed] [Google Scholar]
- 47.Trenk D, Stone GW, Gawaz M, et al. A randomized trial of prasugrel versus clopidogrel in patients withHigh on-treatment Platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. Journal of the American College of Cardiology. 2012;59(24):2159–2164. doi: 10.1016/j.jacc.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 48.Roe MT, Armstrong PW, Fox KA, et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. The New England Journal of Medicine. 2012;367:1297–1309. doi: 10.1056/NEJMoa1205512. [DOI] [PubMed] [Google Scholar]
- 49.Almalla M, Schröder J, Hennings V, Marx N, Hoffmann R. Long-term outcome after angiographically proven coronary stent thrombosis. The American Journal of Cardiology. 2013;111(9):1289–1294. doi: 10.1016/j.amjcard.2013.01.268. [DOI] [PubMed] [Google Scholar]
- 50.D’Ascenzo F, Cerrato E, Biondi-Zoccai G, et al. Acute coronary syndromes in human immunodeficiency virus patients: a meta-analysis investigating adverse event rates and the role of antiretroviral therapy. European Heart Journal. 2012;33(7):875–880. doi: 10.1093/eurheartj/ehr456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D'Ascenzo F, Quadri G, Cerrato E, et al. A meta-analysis investigating incidence and features of stroke in HIV-infected patients in the highly active antiretroviral therapy era. Journal of Cardiovascular Medicine. 2014 doi: 10.2459/JCM.0b013e328365ca31. [DOI] [PubMed] [Google Scholar]
- 52.D’Ascenzo F, Bollati M, Clementi F, et al. Incidence and predictors of coronary stent thrombosis: evidence from an international collaborative meta-analysis including 30 studies, 221,066 patients, and 4276 thromboses. International Journal of Cardiology. 2013;167(2):575–584. doi: 10.1016/j.ijcard.2012.01.080. [DOI] [PubMed] [Google Scholar]
- 53.Angiolillo DJ. Antiplatelet therapy in type 2 diabetes mellitus. Current Opinion in Endocrinology, Diabetes and Obesity. 2007;14:124–131. doi: 10.1097/MED.0b013e32807f2ad9. [DOI] [PubMed] [Google Scholar]
- 54.Rosenthal R. The file drawer problem and tolerance for null results. Psychological Bulletin. 1979;86(3):638–641. [Google Scholar]
- 55.Tzoulaki I, Siontis KC, Evangelou E, Ioannidis JPA. Bias in associations of emerging biomarkers with cardiovascular disease. The JAMA Internal Medicine. 2013;173(8):664–671. doi: 10.1001/jamainternmed.2013.3018. [DOI] [PubMed] [Google Scholar]


