Abstract
Objective:
The current study examined whether social phobia (SP) symptoms in early adolescence prospectively predicted alcohol use through middle adolescence in a community sample of youth.
Method:
Data from an ongoing longitudinal study (N = 277) of mechanisms of HIV-related risk behaviors in youth were used to assess the extent to which SP symptoms in early adolescence (mean [SD] age = 11.00 years [0.81]) would predict alcohol use across five annual assessment waves. Adolescents completed measures of SP symptoms, depressive symptoms, and alcohol use at each wave.
Results:
Higher SP symptoms at baseline predicted higher average odds of alcohol consumption during subsequent waves but did not significantly predict an increase in the odds of alcohol use as a function of time. Within a lagged model, SP symptoms measured at a prior assessment point (1 year earlier) predicted greater odds of drinking alcohol at the following assessment point. Importantly, alcohol use did not significantly predict SP symptoms over time. These results suggest that early SP symptoms are an important risk factor for increased odds of subsequent alcohol use.
Conclusions:
The present findings highlight that elevated SP symptoms place adolescents at risk for early alcohol use. Early interventions targeting SP symptoms may be crucial for the prevention of problematic alcohol use in early to mid-adolescence. Implications for prevention and treatment approaches are discussed.
One of the most robust and commonly cited predictors of problematic alcohol use in adulthood is early-onset alcohol use during childhood and adolescence (e.g., Brown and Tapert, 2004; Rohde et al., 2001), with earlier drinking onset (before age 14) predictive of an increased likelihood of meeting criteria for alcohol dependence in adulthood (Hingson et al., 2006). Social phobia (SP) and subthreshold SP symptoms have been implicated as risk factors for early alcohol use in childhood and adolescence (e.g., Anderson et al., 2011; Blumenthal et al., 2010; Tomlinson et al., 2013). From a developmental perspective, the transition from childhood to adolescence is an important period characterized by significant changes in school and peer relationships (Windle et al., 2008), which are potential sources of stress and anxiety for susceptible youth. Simultaneous changes in stress and emotion regulation systems associated with the onset of puberty can lead to greater sensitivity to negative affect (Spear, 2009). The confluence of these factors may contribute to an increased risk for SP symptoms and for the use of alcohol as a strategy for coping with anxiety and negative affect associated with SP (for a review, see Morris et al., 2005).
The negative reinforcement model proposed by Hussong et al. (2011) provides a clear framework for teasing apart the prospective relationship between SP symptoms and alcohol use among adolescents. The sensitivity to social rejection, interpersonal skills deficits, and social withdrawal experienced by youth with SP symptoms may place them at increased risk for initiating and maintaining alcohol use to cope with negative affect associated with these symptoms (Hussong et al., 2011). SP symptoms tend to precede the onset of alcohol use (Morris et al., 2005), and use may be maintained over time because of the functional role that alcohol consumption plays in managing symptoms of SP both during and in anticipation of social interactions or social performance situations.
Despite theory suggesting that SP symptoms place youth at increased risk for alcohol use, only a few studies exist examining this relationship. Unfortunately, these studies are largely equivocal, as support has been found for the role of SP symptoms as both a protective and a risk factor for alcohol use. Moreover, many of these studies are cross-sectional, limiting our ability to understand the potential predictive role of SP symptoms. Regarding support for SP symptoms as a protective factor, Tomlinson and Brown (2012) found that among a sample of eighth-grade students, SP symptoms were significantly associated with less frequent drinking at parties. Similarly, Myers et al. (2003) found that among a sample of high school students, those with higher SP symptoms were less likely to report substance use, including alcohol, tobacco, and marijuana use. In contrast, recent studies demonstrate more complex relationships between SP symptoms and alcohol use. For example, among seventh and eighth graders, fear of negative evaluation was associated with an increased likelihood of early drinking initiation among both boys and girls, whereas lower levels of social anxiety/distress in novel social situations was related to increased risk for drinking initiation among girls (Tomlinson et al., 2013). Similarly, Anderson et al. (2011) demonstrated that SP symptoms and need for affiliation interacted, such that greater need for affiliation and greater SP symptoms were most strongly associated with alcohol use when peers were consuming alcohol. Finally, the only longitudinal study of these relationships indicated that SP symptoms (social fears) assessed at age 18 conveyed elevated risk for heavy drinking, alcohol use, and alcohol dependence at follow-up 12–15 years later (Crum and Pratt, 2001). Taken together, although a negative reinforcement model of the developmental course of alcohol use suggests that youth with symptoms of SP may be particularly susceptible to the initiation of alcohol use to regulate affective distress, the literature to date remains largely inconclusive for determining whether SP symptoms place youth at heightened risk for alcohol use across this critical developmental period.
Although the literature examining the relationship between SP symptoms and alcohol use is somewhat sparse, there are comparatively more studies that have focused on the relationship between SP diagnosis and problematic alcohol use or alcohol use disorders (AUDs). This literature can enhance our understanding of the relationship between dimensional SP symptoms and alcohol use among youth. First, SP typically precedes the onset of alcohol dependence, with the mean age at onset for SP around age 15 (Grant et al., 2005) and the mean age at onset for alcohol dependence around age 35 (Dawson et al., 2008). For example, one study demonstrated that SP at age 16 was associated with a 4.5 times greater likelihood of meeting criteria for alcohol dependence at age 30 (Buckner et al., 2008), whereas another study indicated that among adolescents and young adults, SP at baseline (age 14–24) predicted the persistence of alcohol dependence between baseline and follow-up (4 years later) as well as the onset of regular and hazardous alcohol use at follow-up (Zimmerman et al., 2003). Importantly, only SP and externalizing disorders at baseline were associated with more rapid transition from alcohol use to alcohol dependence; bipolar disorder, panic attacks, nicotine dependence, and problematic cannabis use were not associated with this transition (Behrendt et al., 2011). Thus, there is compelling evidence to suggest that an SP diagnosis places individuals at increased risk for alcohol use, alcohol dependence, and persistence of AUDs over time. However, whether SP symptoms assessed dimensionally place individuals at risk for alcohol use is less clear.
There are important limitations to the literature examining the relationship between SP symptoms and alcohol use among youth that merit investigation. Although it is established that the onset of SP symptoms tends to precede the development of problematic alcohol use, currently it is unknown if SP symptoms increase the likelihood of earlier alcohol use initiation among youth. Moreover, to our knowledge, no studies to date have examined the utility of SP symptoms before middle school for prospectively predicting alcohol use later in adolescence. Finally, research examining the relationship between SP symptoms and alcohol use has largely been cross-sectional in nature, making it difficult to determine if early SP symptoms predict alcohol use over time. In an effort to develop efficacious prevention and intervention programs in the future, it is important to improve our understanding of the relationship between alcohol use and SP symptomatology, specifically whether SP symptoms prospectively predict early alcohol use.
The aims of the present study are twofold: (a) to examine if baseline SP symptoms in early adolescence predict increased odds of alcohol use in subsequent years through middle adolescence and (b) to examine whether increases in SP symptoms over time predict increased odds of alcohol use over time. Consistent with previous prospective studies examining if diagnostic-level SP during middle adolescence predicts later alcohol use (Behrendt et al., 2011; Buckner et al., 2008; Crum and Pratt, 2001; Zimmermann et al., 2003), we hypothesized that (a) baseline SP symptoms would predict increased odds of alcohol use throughout middle adolescence and (b) SP symptoms measured annually would prospectively predict increased odds of alcohol use over time from late childhood through middle adolescence.
Method
Participants
Permission to conduct research was obtained from the University of Maryland Institutional Review Board. This study used data from a larger, longitudinal study of behavioral, environmental, and genetic mechanisms of HIV-related risk behaviors in youth. Details of the larger longitudinal study have been reported elsewhere (e.g., Cummings et al., 2013; MacPherson et al., 2010). In brief, participants were a sample of youth and their parents recruited in the greater metropolitan Washington, D.C., area via media outreach and mailings to area schools, libraries, and Boys and Girls Clubs. Recruitment lasted approximately 2 years, with recruitment aimed toward youth in the fifth and sixth grades who were proficient in English; no other exclusion criteria were used. Interested families who met inclusion criteria were invited to come to the University of Maryland campus, accessible by public transportation. The present study sought to examine whether SP symptoms predicted increased odds of alcohol use over time; thus, we used all five waves of available data from our ongoing longitudinal study.
Procedure
Upon arrival at the baseline assessment session, a detailed description of the study procedures was provided, and the parent/guardian and youth signed informed consent/assent. The youth was then accompanied to a separate room to complete assessments. Assessments included self-report questionnaires, interviews, and computerized tasks. These procedures were repeated at all interview points, with each study session lasting approximately 1 hour.
Measures
Demographics.
The parent/guardian completed a basic demographics form for personal information as well as information about the child. The form included age, gender, race, education level of the parents, and annual family income.
Psychopathology.
The Revised Child Anxiety and Depression Scale (RCADS; Chorpita et al., 2000) was used to assess SP and major depressive disorder (MDD) symptoms in the current study. The RCADS was designed to assess symptoms of childhood disorders, including major depression, social phobia, panic disorder, separation anxiety disorder, generalized anxiety disorder, and obsessive–compulsive disorder according to criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association, 1994). Participants completed all subscales of the RCADS. Items asked about the frequency of symptoms (never, sometimes, often, always) and were scored on a 4-point Likert scale. RCADS subscale scores were obtained by summing across relevant items. The RCADS has demonstrated convergent validity with existing measures of childhood anxiety and anxiety disorders (Chorpita et al., 2000).
Alcohol use.
We used a modified version of the Youth Risk Behavior Surveillance System (Grunbaum et al., 2002) to assess past-year engagement in alcohol use at each assessment wave. Response options for past-year alcohol use were zero, once, a few times, 1–3 times per month, 1–3 times per week, and almost every day or more. The frequencies of each response option above zero were low, with endorsement of no other response option rising above 22%. Specifically, reports of zero use were 72.4%, 64.6%, 52.8%, 49.1%, and 40.6% at Waves 1 through 5, respectively. Therefore, because of the item distributions and the variable interval between response options, a dichotomous scale was constructed to identify whether the youth had engaged in alcohol use at least one time in the prior year (1) or zero times (0) in the prior year.
Analyses
All data were analyzed using SPSS version 20 (IBM Corp., Armonk, NY). We first examined zero-order correlations between alcohol use, SP symptoms, and potential covariates (age, gender, MDD symptoms) at each assessment wave and prospectively across assessment waves. For the two main study aims, we used repeated-measures analyses for binomial outcomes via generalized estimating equations (GEEs). GEE is an extension of the generalized linear model that assumes correlated observations, in this case within subjects over time, of independent and dependent variables (Hanley et al., 2003). GEE was used because it can accommodate missing time points and can handle repeated measures within subject data, which are necessarily correlated. Because GEE assumes data are missing completely at random (MCAR), before conducting subsequent GEE analyses, in order to determine whether data were MCAR, we used Little’s MCAR test for alcohol, SP, and MDD variables. For all variables, Little’s test was nonsignificant, alcohol: χ2(35) = 43.58, p = .15; SP: χ2(39) = 30.35, p = .84; MDD: χ2(35) = 20.98, p = .97. Thus, data can be assumed to be MCAR, suggesting that GEE is appropriate. For the two main study aims, GEE analyses were conducted with a binomial distribution, logit link function, and an independent correlation matrix specified. For testing the reverse model, we used GEE with a normal distribution, identity link function, and independent correlation matrix; this was to determine whether alcohol use over time predicted SP symptoms over time.
To address our first aim examining whether SP symptoms at Wave 1 predicted increased odds of drinking across the four subsequent time points, we used GEE with the dependent variable of percentage used alcohol at each of Waves 2, 3, 4, and 5. A significant effect of time in the GEE analyses provides an estimate of the change in alcohol use across the multiple assessment waves and is expressed as a single odds ratio (OR; MacPherson et al., 2010). In light of previous work establishing a relationship between youth depressive symptomatology and alcohol use (e.g., Chen et al., 1999; Stice et al., 1998), this model included baseline MDD symptoms as a covariate. Additional covariates included baseline age, gender, the linear effect of time, the interaction between baseline SP symptoms and time, and the interaction between baseline MDD symptoms and time. We included these interaction terms to determine whether baseline psychopathology predicted changes in alcohol use over the four waves of data.
To address the aim examining whether SP symptoms measured 1 year prior prospectively predicted increases in the odds of alcohol use, we used a lagged GEE model. In this model, SP symptoms measured at the prior wave were used to predict the odds of alcohol consumption at the subsequent wave (e.g., Wave 1 SP symptoms were used to predict the odds of alcohol consumption at Wave 2, Wave 2 SP symptoms were used to predict the odds of alcohol consumption at Wave 3, etc.). This model controlled for prior-wave MDD symptoms, the linear effect of time, gender, and age at baseline. In the second analysis, youth were included who attended the baseline (Wave 1) session and at least one follow-up session (Wave 2, 3, 4, or 5). Youth were excluded who missed study sessions for all of Waves 2, 3, 4, and 5 (n = 17). Follow-up rates were 88.3%, 89.5%, 84.4%, and 75.3% for Waves 2–5, respectively.
Using the same GEE procedures, we also tested the reverse model, as described above, which was a lagged model in which change in alcohol use was examined as a predictor of change in SP symptoms. We did this as a theory confirmation to ensure that SP symptoms were predicting alcohol use, rather than the reverse relationship. We included the linear effect of time, MDD symptoms assessed at Years 1–4, baseline SP symptoms, gender, and baseline age.
Results
Sample description
The current sample (N = 277) was 43.7% female, 49.3% non-Hispanic White, 35.5% African American, 2.9% Latino, 1.4% Asian American, and 10.9% mixed or other ethnicity with a median household income of U.S. $85,000. The mean (SD) age of the sample was 11.00 (0.81), 12.10 (0.91), 13.10 (0.89), 14.00 (0.90), and 15.00 (0.95) at Waves 1, 2, 3, 4, and 5, respectively. The majority of the sample was in the fifth (26.8%) or sixth (45.2%) grade at baseline.
Descriptive statistics and intercorrelations
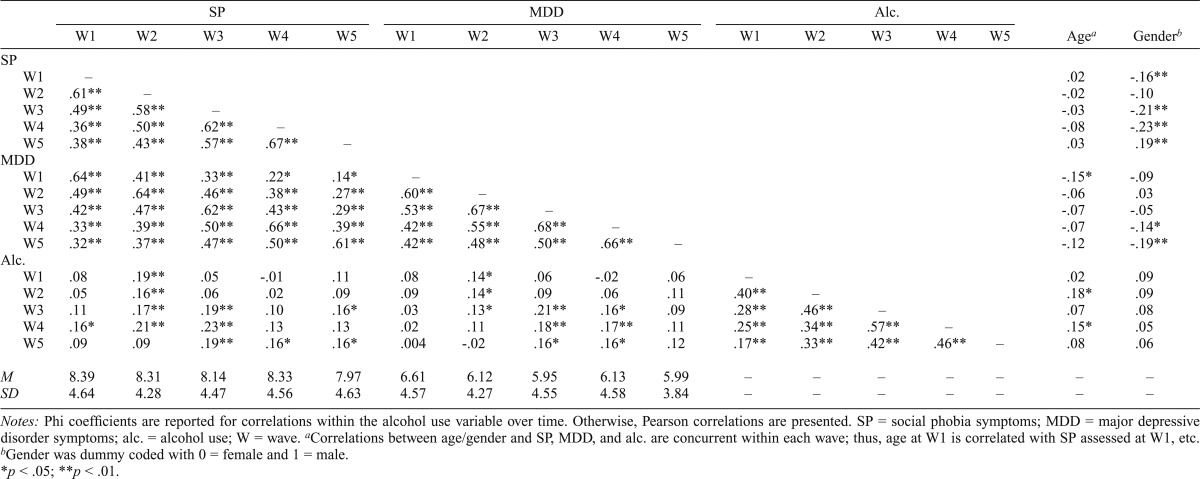
Skewness values for the SP symptoms scores at each assessment wave were all less than 0.8; thus, transformation was not necessary. Paired-sample t tests did not indicate a significant increase or decrease in average SP symptoms from Wave 1 to Wave 2, t(242) = -0.001, p = .99; Wave 2 to Wave 3, t(232) = 1.06, p = .29; Wave 3 to Wave 4, t(227) = -1.17, p = .24; or Wave 4 to Wave 5, t(199) = 1.06, p = .29. Descriptive statistics for SP symptoms across waves were [M(SD), range] Wave 1: 8.39 (4.64), 0–27; Wave 2: 8.31 (4.28), 0–23; Wave 3: 8.14 (4.47), 0–27; Wave 4: 8.33 (4.56), 0–24; and Wave 5: 7.97 (4.63), 0–21. Descriptive statistics for MDD symptoms across waves were [M (SD), range] Wave 1: 6.61 (4.57), 0–27; Wave 2: 6.12 (4.27), 0–26; Wave 3: 5.95 (4.55), 0–29; Wave 4: 6.13 (4.59), 0–22; and Wave 5: 5.99 (3.84), 0–18 (Table 1).
Table 1.
Zero-order correlations between predictor variables and alcohol use
| SP |
MDD |
Alc. |
|||||||||||||||
| W1 | W2 | W3 | W4 | W5 | W1 | W2 | W3 | W4 | W5 | W1 | W2 | W3 | W4 | W5 | Agea | Genderb | |
| SP | |||||||||||||||||
| W1 | – | .02 | -.16** | ||||||||||||||
| W2 | .61** | – | -.02 | -.10 | |||||||||||||
| W3 | .49** | .58** | – | -.03 | -.21** | ||||||||||||
| W4 | .36** | .50** | .62** | – | -.08 | -.23** | |||||||||||
| W5 | .38** | .43** | .57** | .67** | – | .03 | .19** | ||||||||||
| MDD | |||||||||||||||||
| W1 | .64** | .41** | .33** | .22* | .14* | – | -.15* | -.09 | |||||||||
| W2 | .49** | .64** | .46** | .38** | .27** | .60** | – | -.06 | .03 | ||||||||
| W3 | .42** | .47** | .62** | .43** | .29** | .53** | .67** | – | -.07 | -.05 | |||||||
| W4 | .33** | .39** | .50** | .66** | .39** | .42** | .55** | .68** | – | -.07 | -.14* | ||||||
| W5 | .32** | .37** | .47** | .50** | .61** | .42** | .48** | .50** | .66** | – | -.12 | -.19** | |||||
| Alc. | |||||||||||||||||
| W1 | .08 | .19** | .05 | -.01 | .11 | .08 | .14* | .06 | -.02 | .06 | – | .02 | .09 | ||||
| W2 | .05 | .16** | .06 | .02 | .09 | .09 | .14* | .09 | .06 | .11 | .40** | – | .18* | .09 | |||
| W3 | .11 | .17** | .19** | .10 | .16* | .03 | .13* | .21** | .16* | .09 | .28** | .46** | – | .07 | .08 | ||
| W4 | .16* | .21** | .23** | .13 | .13 | .02 | .11 | .18** | .17** | .11 | .25** | .34** | .57** | – | .15* | .05 | |
| W5 | .09 | .09 | .19** | .16* | .16* | .004 | -.02 | .16* | .16* | .12 | .17** | .33** | .42** | .46** | – | .08 | .06 |
| M | 8.39 | 8.31 | 8.14 | 8.33 | 7.97 | 6.61 | 6.12 | 5.95 | 6.13 | 5.99 | – | – | – | – | – | – | – |
| SD | 4.64 | 4.28 | 4.47 | 4.56 | 4.63 | 4.57 | 4.27 | 4.55 | 4.58 | 3.84 | – | – | – | – | – | – | – |
Notes: Phi coefficients are reported for correlations within the alcohol use variable over time. Otherwise, Pearson correlations are presented. SP = social phobia symptoms; MDD = major depressive disorder symptoms; alc. = alcohol use; W = wave.
Correlations between age/gender and SP, MDD, and alc. are concurrent within each wave; thus, age at W1 is correlated with SP assessed at W1, etc.
Gender was dummy coded with 0 = female and 1 = male.
p < .05;
p < .01.
Zero-order correlations among the independent variables (SP symptoms, age, gender, MDD symptoms) were examined (Table 1). In brief, SP symptoms and MDD symptoms were significantly correlated with age and gender at some waves, but not all, and SP symptoms were significantly correlated with MDD symptoms across all waves (rs = .14–.66, all ps < .05). We also examined correlations within the SP symptoms and MDD symptoms variables over time (Table 1). For both, the correlations were relatively robust, ranging from .36 to .67 for SP symptoms and from .42 to .68 for MDD symptoms (all ps < .01).
Zero-order correlations were examined between baseline predictors (child age, child gender, MDD symptoms, baseline alcohol use, SP symptoms) and Waves 1–5 alcohol use (Table 1). Baseline alcohol use was significantly correlated with alcohol use at all subsequent time points. Concurrent zero-order correlations were also examined between predictor variables (SP symptoms, MDD symptoms, age, gender) and alcohol use within each time point (Table 1). In general, concurrent associations were small to moderate, ranging from .08 to .21. Rates of past-year alcohol use were 27.6%, 35.4%, 47.2%, 50.9%, and 59.4% for Waves 1–5, respectively.
Generalized estimating equations
Baseline social phobia symptoms predicting alcohol use.
We hypothesized that youth with elevated SP symptoms at baseline would have increased odds of drinking at Waves 2–5. To test this, we used GEE, as described previously. When alcohol use was examined, the results indicated a significant main effect of time (OR = 1.37, 95% CI [1.24, 1.53], p < .001) indicating that youth had greater odds of using alcohol over time. There was a main effect of baseline SP symptoms predicting greater overall odds of alcohol use that approached statistical significance (OR = 1.06, 95% CI [1.00, 1.12], p = .051). There were no significant main effects for baseline MDD symptoms (OR = 1.00, 95% CI [0.94, 1.06], p = .88), gender (OR = 1.29, 95% CI [0.87, 1.91], p = .21), or baseline age (OR = 1.21, 95% CI [0.94, 1.56], p = .14) on the odds of alcohol use at the four points assessed. The interaction of time with baseline SP symptoms was not significant (OR = 1.03, 95% CI [0.99, 1.06], p = .15), nor was the interaction between baseline MDD symptoms and time (OR = 0.98, 95% CI [0.94, 1.01], p = .12), suggesting that baseline SP and MDD symptoms did not change the odds of alcohol use as a function of time. We also examined the interactive effect of gender and SP symptoms, SP symptoms and MDD symptoms, as well as gender, SP symptoms, and time in additional models. These interactions were not significant and, as such, are not included here. To calculate effect sizes for significant variables, we followed the procedures outline by Chinn (2000), such that effect sizes were calculated from model-derived odds ratios. Based on these procedures, the effect size for time was d = 0.17, and the effect size for SP symptoms was d = 0.03.
Social phobia symptoms predicting subsequent alcohol use.
We also hypothesized that elevated SP symptoms measured 1 year prior would prospectively predict increased odds of alcohol consumption at the subsequent annual assessment. To test this, we used the previously described lagged model. Results supported a significant main effect of time (OR = 1.41, 95% CI [1.27, 1.58], p < .001), indicating that the odds of alcohol use increased over time. Additionally, there was a significant effect of SP symptoms measured prospectively (OR = 1.06, 95% CI [1.01, 1.11], p = .03), indicating that elevated SP symptoms measured at a prior year predicted increased odds of alcohol consumption during the subsequent year. There were no significant main effects of prior-year MDD symptoms (OR = 1.04, 95% CI [0.99, 1.09], p = .09), gender (OR = 1.31, 95% CI [0.87, 1.97], p = .19), or age (OR = 1.26, 95% CI [0.99, 1.62], p = .07) on the odds of alcohol consumption over time. Again, we examined the interactive effect of gender and SP symptoms as well as SP symptoms and MDD symptoms in additional models. These interactions were not significant and are not included here. We calculated effect sizes for significant variables. Based on these procedures, the effect size for time was d = 0.19, and the effect size for SP symptoms was d = 0.03.
Reverse model: Alcohol use as a predictor of social phobia symptoms.
We tested the reverse model to rule out the possibility that the significant relationship between SP symptoms and odds of alcohol use over time was attributable to alcohol use predicting SP symptoms. To test this reverse model, we used the lagged model previously described. Within this model, there were no significant main effects of time (β = 0.01, SE = 0.15, p = .97), alcohol use during the prior year (β = 0.62, SE = 0.33, p = .06), or baseline age (β = -0.004, SE = 0.22, p = .99) on SP symptoms over time. There were significant main effects of gender (β = -1.30, SE = 0.37, p < .001), baseline SP symptoms (β = 0.30, SE = 0.05, p < .001), and prior-year MDD symptoms (β = 0.27, SE = 0.04, p < .001). Youth with higher MDD symptoms scores during the prior years had higher SP symptoms during the following years, and girls had higher average SP symptoms than boys. There was also a significant interaction between gender and time (β = -0.44, SE = 0.22, p = .04), indicating that boys’ SP symptoms decreased more over time as compared with girls’ SP symptoms.
Discussion
The literature on the relationship between SP symptoms and alcohol use has been largely inconclusive. Although negative reinforcement models of alcohol use suggest that SP symptoms in early adolescence may convey risk for alcohol use, the degree to which SP symptoms in early adolescence predict increased odds of alcohol use over time in middle adolescence has remained unknown. Considering that the onset of alcohol use frequently begins during adolescence and that earlier onset alcohol use is a risk factor for the development of problematic alcohol use over time, it is crucial to understand the relationship between SP symptoms and alcohol use for the future development of early targeted prevention and intervention programs. The current study is the first to our knowledge to examine whether SP symptoms in early adolescence predict and co-occur with alcohol use at subsequent years through middle adolescence. Although effect sizes are relatively small, there are a number of important key findings, which we review in the following.
First, elevated SP symptoms at baseline predicted higher overall odds of alcohol use. However, elevated SP symptoms at baseline did not significantly predict changes in the odds of alcohol use over time. More specifically, these findings indicate that youth with elevated SP symptoms at baseline were more likely on average to have used alcohol at subsequent assessment points but not to have an increased likelihood of use as a function of time. Additionally, this effect was not attributable to an interactive effect between gender and SP symptoms, SP symptoms and MDD symptoms, or gender, SP symptoms, and time. This suggests that SP symptoms in early adolescence increase youth’s odds of alcohol use and extends the literature in two important ways: (a) to SP symptomatology assessed dimensionally and at younger ages and (b) to less problematic alcohol use.
Second, SP symptoms assessed prospectively on an annual basis predicted an increased likelihood of drinking alcohol in subsequent years. Of note, there was no time-varying effect of MDD symptoms, interactive effect of gender and SP symptoms, or synergistic effect of SP symptoms and MDD symptoms, highlighting the unique role that SP symptoms may play in the risk of alcohol use. Taken together with results suggesting that baseline SP symptoms predict increased odds of alcohol use, both baseline SP symptoms and SP symptoms assessed annually may hold promise in prospectively predicting increased odds of alcohol use. Although SP symptoms were relatively stable over time in our sample, SP symptoms assessed annually conferred an increased risk for alcohol use at subsequent assessments. Of importance, we ran a third, reverse model, ruling out the possibility that the significant relationship between SP symptoms and odds of alcohol use over time was due to alcohol use predicting SP symptoms. Although alcohol use did not prospectively predict SP over time, gender and MDD symptoms did significantly predict SP symptoms over time. Taken together with the previously reviewed results, this suggests that elevated SP symptoms as early as age 10 convey a unique risk for alcohol use over time and that this effect is not attributable to the effect of alcohol use on SP symptoms.
These results are relevant for both prevention and intervention efforts. In terms of prevention, our results provide support for previously presented arguments (e.g., Hussong et al., 2011; Ialongo et al., 2006) suggesting that internalizing symptomatology, including SP symptoms, emerges early in life and necessitates targeted efforts in preventing early alcohol use and AUDs. Research along these lines has shown promise, with Kendall et al. (2004) demonstrating that early interventions for SP are associated with decreased substance use 7 years after initial treatment. In light of the present findings, elevated SP symptoms in early adolescence could be used to identify children at risk for alcohol use, who could be targeted with evidence-based treatments for SP, not only to reduce SP symptoms but also to decrease the risk of early alcohol use. Thus, targeting symptoms of SP could help to prevent the emergence of alcohol-related problems.
Previous literature supports the usefulness of early prevention efforts before alcohol use becomes problematic or ingrained (Stewart et al., 2005). For example, Conrod et al. (2006) developed a brief intervention addressing personality risk factors for early-onset alcohol use including hopelessness, anxiety sensitivity, impulsivity, and sensation seeking, which has found support (Conrod et al., 2008). Similar principles could be applied to preventative interventions targeting SP symptoms as a risk factor for alcohol use. Specifically, youth with elevated levels of SP symptoms could be provided with evidence-based strategies targeting the link between SP symptoms and alcohol use. Therapeutic strategies such as psychoeducation, relaxation, and exposure-based strategies could be provided to decrease SP symptoms and the odds of alcohol use.
Moving toward intervention, to the extent that alcohol use may develop in order to manage preexisting symptoms of SP (Robinson et al., 2009), intervention programs for alcohol use among adolescents could also directly target symptoms of SP. Although the present sample was a nonclinical sample with relatively low rates of alcohol use, existing theories suggest that one of the functions of alcohol use is to manage SP symptoms and that youth with elevated SP symptoms may be at risk for the development of more problematic levels of SP over time as well as for problematic alcohol use. Thus, addressing symptoms of SP in the context of alcohol-based interventions may also be important. An argument for this type of combined therapy has been previously presented (Morris et al., 2005). Specifically, Morris et al. (2005) suggest that traditional cognitive–behavioral strategies for addressing SP could be adapted to include a psychoeducational component that would address the interrelationship between SP and alcohol use as well as cognitive restructuring regarding the function of alcohol in social anxiety–provoking situations and graduated exposures in which social situations are approached without alcohol use as an option.
The present research has several limitations. First, we relied on self-reports of past-year alcohol use across the assessment waves, a decision we made based on the rationale that biological verification of alcohol use might have been difficult with children in this sample because alcohol use was infrequent enough to limit the effectiveness of these tests (Allen and Litten, 2001; Allen et al., 1994). Similarly, our alcohol use variable was a single-item measure regarding past-year frequency of use, with relatively low rates of use across waves. Thus, we are unable to draw conclusions regarding the potential relationship between SP and the frequency of alcohol use or alcohol-related problems over time. Second, to maximize measurement parsimony and reduce assessment burden to participants, we used the RCADS to measure both SP and MDD symptoms. Future investigations of the prospective relationship between SP, MDD, and alcohol use would benefit from a more comprehensive SP and MDD battery. Third, although our sample was sociodemographically diverse, it was a convenience community-based sample, which could limit the generalizability of findings. In addition, because our sample was a convenience sample residing across a major metropolitan area rather than a school-based sample, participants did not necessarily transition from elementary to middle school or from middle school to high school at the same grades, and assessments were not conducted at systematic intervals in relation to school transitions. Thus, we did not assess the potential effect of school transition on change in alcohol use in our models. Fourth, effect sizes were relatively small and, as such, future replication of these results will be important.
With these limitations in mind, the present study highlights the importance of SP symptoms in predicting alcohol use over time among young adolescents. A number of future directions within this line of research would be valuable, including further expanding the age range studied to both younger children and older adolescents/young adults. Expanding the age group examined would afford insight regarding when SP symptoms first begin to convey risk for alcohol use as well as the potential age limits when SP symptoms might no longer predict risk for alcohol use. Further, it would be beneficial to examine additional measures of SP symptoms, alcohol use, and motives for alcohol use in this age group, as well as mechanisms underlying the relationship between SP symptoms and alcohol use, in order to better understand the nature of the relationship between SP symptoms and alcohol use. Our findings offer a number of exciting future areas to explore to deepen our understanding of which children choose to use alcohol during early and middle adolescence.
Footnotes
Funding for this study was provided by National Institute on Drug Abuse Grants 1F31DA034999 (to Jennifer Dahne) and R01 DA018647 (to Carl W. Lejuez).
References
- Allen JP, Litten RZ. The role of laboratory tests in alcoholism treatment. Journal of Substance Abuse Treatment. 2001;20:81–85. doi: 10.1016/s0740-5472(00)00144-6. [DOI] [PubMed] [Google Scholar]
- Allen JP, Litten RZ, Anton RF, Cross GM. Carbohydrate-deficient transferrin as a measure of immoderate drinking: Remaining issues. Alcoholism: Clinical and Experimental Research. 1994;18:799–812. doi: 10.1111/j.1530-0277.1994.tb00043.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Anderson KG, Tomlinson K, Robinson JM, Brown SA. Friends or foes: social anxiety, peer affiliation, and drinking in middle school. Journal of Studies on Alcohol and Drugs. 2011;72:61–69. doi: 10.15288/jsad.2011.72.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt S, Beesdo-Baum K, Zimmermann P, Höfler M, Perkonigg A, Bühringer G, Wittchen H-U. The role of mental disorders in the risk and speed of transition to alcohol use disorders among community youth. Psychological Medicine. 2011;41:1073–1085. doi: 10.1017/S0033291710001418. [DOI] [PubMed] [Google Scholar]
- Blumenthal H, Leen-Feldner EW, Frala JL, Badour CL, Ham LS. Social anxiety and motives for alcohol use among adolescents. Psychology of Addictive Behaviors. 2010;24:529–534. doi: 10.1037/a0019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Tapert SF. Adolescence and the trajectory of alcohol use: Basic to clinical studies. Annals of the New York Academy of Sciences. 2004;1021:234–244. doi: 10.1196/annals.1308.028. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Schmidt NB, Lang AR, Small JW, Schlauch RC, Lewinsohn PM. Specificity of social anxiety disorder as a risk factor for alcohol and cannabis dependence. Journal of Psychiatric Research. 2008;42:230–239. doi: 10.1016/j.jpsychires.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L-S, Anthony JC, Crum RM. Perceived cognitive competence, depressive symptoms and the incidence of alcohol-related problems in urban school children. Journal of Child & Adolescent Substance Abuse. 1999;8:37–53. [Google Scholar]
- Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Statistics in Medicine. 2000;19:3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Yim L, Moffitt C, Umemoto LA, Francis SE. Assessment of symptoms of DSM-IV anxiety and depression in children: A revised child anxiety and depression scale. Behaviour Research and Therapy. 2000;38:835–855. doi: 10.1016/s0005-7967(99)00130-8. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Castellanos N, Mackie C. Personality-targeted interventions delay the growth of adolescent drinking and binge drinking. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2008;49:181–190. doi: 10.1111/j.1469-7610.2007.01826.x. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Stewart SH, Comeau N, Maclean AM. Efficacy of cognitive-behavioral interventions targeting personality risk factors for youth alcohol misuse. Journal of Clinical Child and Adolescent Psychology. 2006;35:550–563. doi: 10.1207/s15374424jccp3504_6. [DOI] [PubMed] [Google Scholar]
- Crum RM, Pratt LA. Risk of heavy drinking and alcohol use disorders in social phobia: A prospective analysis. American Journal of Psychiatry. 2001;158:1693–1700. doi: 10.1176/appi.ajp.158.10.1693. [DOI] [PubMed] [Google Scholar]
- Cummings JR, Bornovalova MA, Ojanen T, Hunt E, MacPherson L, Lejuez C. Time doesn’t change everything: The longitudinal course of distress tolerance and its relationship with externalizing and internalizing symptoms during early adolescence. Journal of Abnormal Child Psychology. 2013;41:735–748. doi: 10.1007/s10802-012-9704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Blanco C, Stinson FS, Chou SP, Goldstein RB, Huang B. The epidemiology of social anxiety disorder in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2005;66:1351–1361. doi: 10.4088/jcp.v66n1102. [DOI] [PubMed] [Google Scholar]
- Grunbaum J, Kann L, Kinchen SA, Williams B, Ross JG, Lowry R, Kolbe L. Youth Risk Behavior Surveillance—United States, 2001. Morbidity and Mortality Weekly Report. 2002;51:1–64. [PubMed] [Google Scholar]
- Hanley JA, Negassa A, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. American Journal of Epidemiology. 2003;157:364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: Age at onset, duration, and severity. Archives of Pediatrics & Adolescent Medicine. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Hussong AM, Jones DJ, Stein GL, Baucom DH, Boeding S. An internalizing pathway to alcohol use and disorder. Psychology of Addictive Behaviors. 2011;25:390–404. doi: 10.1037/a0024519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ialongo NS, Rogosch FA, Cicchetti D, Toth SL, Buckley J, Petras H, Neiderhiser J. A developmental psychopathology approach to the prevention of mental health disorders. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, Vol. 1: Theory and method. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2006. pp. 968–1018. [Google Scholar]
- Kendall PC, Safford S, Flannery-Schroeder E, Webb A. Child anxiety treatment: Outcomes in adolescence and impact on substance use and depression at 7.4-year follow-up. Journal of Consulting and Clinical Psychology. 2004;72:276–287. doi: 10.1037/0022-006X.72.2.276. [DOI] [PubMed] [Google Scholar]
- MacPherson L, Magidson JF, Reynolds EK, Kahler CW, Lejuez CW. Changes in sensation seeking and risk-taking propensity predict increases in alcohol use among early adolescents. Alcoholism: Clinical and Experimental Research. 2010;34:1400–1408. doi: 10.1111/j.1530-0277.2010.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EP, Stewart SH, Ham LS. The relationship between social anxiety disorder and alcohol use disorders: A critical review. Clinical Psychology Review. 2005;25:734–760. doi: 10.1016/j.cpr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Myers MG, Aarons GA, Tomlinson K, Stein MB. Social anxiety, negative affectivity, and substance use among high school students. Psychology of Addictive Behaviors. 2003;17:277–283. doi: 10.1037/0893-164X.17.4.277. [DOI] [PubMed] [Google Scholar]
- Robinson J, Sareen J, Cox BJ, Bolton J. Self-medication of anxiety disorders with alcohol and drugs: Results from a nationally representative sample. Journal of Anxiety Disorders. 2009;23:38–45. doi: 10.1016/j.janxdis.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Kahler CW, Seeley JR, Brown RA. Natural course of alcohol use disorders from adolescence to young adulthood. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:83–90. doi: 10.1097/00004583-200101000-00020. [DOI] [PubMed] [Google Scholar]
- Spear LP. Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology. 2009;21:87–97. doi: 10.1017/S0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SH, Conrod PJ, Marlatt GA, Comeau MN, Thush C, Krank M. New developments in prevention and early intervention for alcohol abuse in youths. Alcoholism: Clinical and Experimental Research. 2005;29:278–286. doi: 10.1097/01.alc.0000153547.34399.e8. [DOI] [PubMed] [Google Scholar]
- Stice E, Barrera M, Jr, Chassin L. Prospective differential prediction of adolescent alcohol use and problem use: Examining the mechanisms of effect. Journal of Abnormal Psychology. 1998;107:616–628. doi: 10.1037//0021-843x.107.4.616. [DOI] [PubMed] [Google Scholar]
- Tomlinson KL, Brown SA. Self-medication or social learning? A comparison of models to predict early adolescent drinking. Addictive Behaviors. 2012;37:179–186. doi: 10.1016/j.addbeh.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Tomlinson KL, Cummins KM, Brown SA. Social anxiety and onset of drinking in early adolescence. Journal of Child & Adolescent Substance Abuse. 2013;22:163–177. doi: 10.1080/1067828X.2012.747994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, Dahl RE. Transitions into underage and problem drinking: Developmental processes and mechanisms between 10 and 15 years of age. Pediatrics. 2008;121:S273–S289. doi: 10.1542/peds.2007-2243C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Wittchen HU, Höfler M, Pfister H, Kessler RC, Lieb R. Primary anxiety disorders and the development of subsequent alcohol use disorders: A 4-year community study of adolescents and young adults. Psychological Medicine. 2003;33:1211–1222. doi: 10.1017/s0033291703008158. [DOI] [PubMed] [Google Scholar]


