Voriconazole is an important antifungal agent used to treat invasive fungal infections; however, its administration can be difficult because of the narrow range between the level required for therapeutic efficacy and the level at which there is risk for hepatic and neurological toxicity. The purpose of this study was to elucidate the relationships among oral dosage, voriconazole levels and liver enzyme levels among leukemia patients.
Keywords: Antifungals, Drug levels, Liver toxicity, Voriconazole
Abstract
INTRODUCTION:
Voriconazole plasma concentrations have been correlated with oral dosing in healthy subjects, but have been poorly characterized in ill patients with hematological malignancies receiving intensive chemotherapy.
METHODS:
The relationship between orally administered voriconazole, plasma concentrations and liver toxicity was examined in a cohort of 69 primarily acute leukemia patients undergoing intensive chemotherapy.
RESULTS:
Oral administration of voriconazole was associated with significant interpatient variability, with voriconazole steady-state concentrations ranging from 0 μg/mL to 16.6 μg/mL. Approximately 20% of patients achieved steady-state concentrations <1 μg/mL. When adjusted for weight, patients receiving higher voriconazole doses tended toward higher plasma concentrations; however, there was no significant relationship between the plasma concentration and genotype, age, sex or use of concomitant proton pump inhibitors. Voriconazole concentrations were correlated with higher serum alkaline phosphatase levels at day 6 to 8, and with higher bilirubin and aspartate aminotransferase levels at day 14 to 16, but not with other liver enzyme levels.
CONCLUSION:
In ill patients with acute leukemia and related disorders undergoing treatment with oral voriconazole, there is a poor correlation between the voriconazole dose and plasma concentrations, and many patients achieve levels that are considered to be subtherapeutic. The findings support the routine use of therapeutic drug monitoring in these patients.
Abstract
INTRODUCTION :
Les concentrations plasmatiques de voriconazole sont corrélées avec les doses orales chez les sujets en santé, mais sont mal caractérisées chez les patients malades atteints d’une hémopathie maligne sous chimiothérapie intensive.
MÉTHODOLOGIE :
Les chercheurs ont examiné le lien entre le voriconazole administré par voie orale et la toxicité hépatique dans une cohorte de 69 patients atteints surtout de leucémie aiguë sous chimiothérapie intensive.
RÉSULTATS :
L’administration de voriconazole par voie orale s’associait à une importante variabilité interpatient, les concentrations à l’état stable oscillant entre 0 μg/mL et 16,6 μg/mL. Environ 20 % des patients ont obtenu des concentrations à l’état stable de moins de 1 μg/mL. Après rajustement selon le poids, les patients qui receviaent des doses plus élevées de voriconazole avaient tendance à présenter des concentrations plasmatiques plus élevées. Cependant, on ne constatait aucun lien significatif entre la concentration plasmatique et le géno-type, l’âge, le sexe ou l’utilisation concomitante d’inhibiteurs de la pompe à protons. Les concentrations de voriconazole étaient corrélées avec des taux de phosphatase alcaline sérique plus élevés les jours 6 à 8 et à des taux de bilirubine et d’aspartate aminotransférase plus élevés les jours 14 à 16, mais pas à d’autres taux d’enzymes hépatiques.
CONCLUSION :
Chez les patients malades atteints d’une leucémie aiguë et de troubles connexes qui suivent un traitement au voriconazole par voie orale, la corrélation entre la dose de voriconazole et les concentrations plasmatiques est faible, et de nombreux patients obtiennent des taux considérés comme subthérapeutiques. Les observations soutiennent une pharmacovigilance systématique chez ces patients.
Invasive fungal infections (IFI) are a significant source of morbidity and mortality in acute leukemia patients receiving intensive chemotherapy and hematopoietic stem cell transplant (HSCT) (1–4). Voriconazole is a second-generation triazole antifungal that has demonstrated activity in the treatment of a variety of invasive fungal pathogens and has been approved as a first-line treatment for invasive aspergillosis (5–9).
After oral administration, voriconazole readily achieves plasma concentrations above the minimum inhibitory concentration for major pathogens in cancer patients as well as in healthy subjects (10,11). Due to the possible association between low voriconazole plasma concentrations and poor patient outcomes, and between elevated levels and hepatic and neurological toxicity, therapeutic drug monitoring (TDM) has been suggested to enhance efficacy and minimize toxicity (3,4,12–18). Previous pharmacokinetic studies have primarily involved healthy volunteers and intravenous dosing in patients. In severely ill patients undergoing intensive chemotherapy or HSCT, and receiving oral voriconazole, several factors may influence levels including treatment-related gastrointestinal and hepatic toxicity, genetic polymorphisms of the cytochrome P450 (CYP) isoenzyme system, altered protein binding, drug interactions and variable food intake (19–25). These may lead to large and unpredictable variations in voriconazole plasma concentrations (14,16,23,24).
To shed light on these uncertainties, we prospectively investigated the kinetics of voriconazole levels, as well as the relationship between steady-state levels and hepatotoxicity, in a cohort of severely neutropenic acute leukemia patients receiving oral voriconazole as therapy for suspected IFI.
METHODS
Patient population
All adult malignant hematology patients being treated with oral voriconazole from June 2007 to September 2010 were eligible for enrollment. All patients had conditions associated with severe neutropenia and could be at any stage of the treatment regimen including, but not limited to, induction/reinduction chemotherapy, consolidation chemotherapy or supportive care/palliative chemotherapy. Patients with grade ≥2 liver enzyme or bilirubin elevations at baseline, as defined by Common Terminology Criteria for Adverse Events version 4.03 (26), or those who had received voriconazole in the previous 15 days were excluded. Ethics approval for the present study was obtained from the local institutional review board. All patients provided written informed consent for voriconazole level testing and CYP2C19 isoenzyme genotyping.
All eligible patients were treated with oral voriconazole with doses ranging from 200 mg to 300 mg every 12 h as per the discretion of the treating physician. Patients were advised to take voriconazole on an empty stomach, and doses were administered at 10:00 and 22:00. Some patients received loading doses of voriconazole – either 6 mg/kg intravenously or 400 mg orally – every 12 h for two doses. At the time of patient consent, education/counselling was provided to ensure that patients were receiving voriconazole appropriately. For the determination of toxicity, all patients underwent liver enzyme testing for alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and total bilirubin levels at least once weekly. Liver enzyme data from the day of voriconazole initiation (day 1), day 6 to 8 and day 14 to 16 of therapy were used for correlation analysis but, if not available, the earliest available subsequent liver function test was used.
Determination of voriconazole level
Voriconazole levels of inpatients were determined at day 2 to 3 (level 1) following initiation of voriconazole, and subsequently at day 4 to 5 (level 2) and day 6 to 8 (level 3). Each level was obtained within 1 h before administration of the next dose. Ambulatory patients had a one-time steady-state level drawn at day 6 to 8 (level 3) in relation to their outpatient clinic visit. If level 3 was not available, level 2 was used for further analysis. As a noninterventional study, the results of the voriconazole levels were not released to the treating physicians and were not used to guide subsequent therapy.
Voriconazole level determination was performed using liquid chromatography tandem mass spectrometry (LC-MS/MS) at the Mass Spectrometry Specialty Laboratory (Laboratory Medicine Program, University Health Network, Toronto, Ontario), similar to previously published work (27–29). LC-MS/MS was chosen over high-performance liquid chromatography with ultraviolet detection or a bioassay due to its greater sensitivity and specificity, and a shorter run time (29).
Sample preparation:
50 μL aliquots of plasma were mixed with 50 μL of d3-voriconazole internal standard. Deuterated isotopic compound was chosen rather than a compound with a similar structure or another antifungal drug because it is a first-choice standard for selective quantification by mass spectrometry (30). After the addition of zinc chloride (0.2 M), voriconazole and internal standard were extracted with ethyl acetate. After centrifugation, the supernatant was evaporated. The residue was then dissolved in 1 mL of methanol solution containing 2 mM ammonium formate with 0.1% of formic acid and analyzed by LC-MS/MS.
LC-MS/MS system:
This consisted of a High-Pressure Liquid Chromatography 1200 system (Agilent Technologies, USA) coupled to an API 5000 (AB SCIEX, Canada) triple quadrupole mass spectrometer. Chromatographic separation was performed in isocratic elution mode at a flow rate of 0.70 mL/min on a Waters Xterra Phenyl (3.0 mm × 50 mm, 3.5 μm) column. The mobile phase was a mixture of acetonitrile (buffer A) containing 2 mM ammonium acetate and 0.1% formic acid, and methanol (buffer B) containing 2 mM ammonium formate and 0.1% formic acid. The 50:50 ratio of buffers A and B was maintained for 1.5 min. Typical detection time for both voriconazole and d3-voriconazole was 0.48 min.
Assay specifications:
Precision studies for voriconazole assay showed that coefficients of variation within and between assays ranged from 1.3% to 5.5%. Functional sensitivity was 0.1 mg/L. To evaluate analytical measurement range, a calibration curve was made to cover the therapeutic range (0 mg/L to 6 mg/L) and extended to 20 mg/L. Regression analysis showed that the response was linear within this range of concentrations, with R2 = 0.9968. The mean recovery was 99.2% (coefficient of variation = 8.5%). Accuracy of the voriconazole assay was evaluated by participating in the external quality assessment program (31) and also by performing method comparison studies using ultra-performance liquid chromatography with ultraviolet detection and LC-MS/MS methods currently in clinical use at two Canadian hospitals.
Genotyping
Genomic regions containing CYP2C19 *2, *3, *4, *5 and *7 polymorphisms were amplified in a multiplexed polymerase chain reaction as previously described (32). Briefly, the purified polymerase chain reaction products were then used as templates in the SNaPshot reaction (Life Technologies, USA), in which extension primers were designed to be of different lengths and each anneal adjacent to a targeted single-nucleotide polymorphism. The extension primers were extended by one nucleotide using fluorescently labelled dideoxynucleoside triphosphate. The cleaned extended products were separated by capillary electrophoresis on the ABI Prism 3100 Avant Genetic Analyzer (Applied Biosystems, USA) and analyzed using GeneMapper version 4.0 (Life Technologies).
Statistical analysis
Patients’ treatment, baseline characteristics and clinical outcomes were reported using descriptive statistics. Categorical variables, such as patient sex, genotyping, inpatient/outpatient, diagnosis, IFI, loading dose, pretransplant tyrosine kinase inhibitor use, frequency of transplantations in the first chronic phase, matched sibling donor, stem cell source and conditioning regimen, were summarized using counts and percentages. Continuous variables, such as age, voriconazole levels and liver enzyme levels, were summarized using medians and ranges. χ2 test/Fisher’s exact tests (as appropriate) were used to assess the association between categorical variables. Student’s t test/Wilcoxon rank-sum test (as appropriate) was used to compare continuous outcome variables for two factors, while ANOVAs/Kruskal-Wallis tests (as appropriate) were used to compare continuous outcomes among categorical covariates having >2 levels. Spearman’s correlation coefficient was used to investigate the relationship of voriconazole levels with continuous covariates (33). A two-tailed P≤0.05 was considered to be statistically significant. All analyses were performed using SAS version 9.2 (SAS Institute Inc, USA).
RESULTS
Patient and treatment characteristics
Sixty-nine patients received 71 courses of voriconazole, with most courses (86%) administered on an inpatient basis. Loading doses were administered during 38% of voriconazole courses, with most patients receiving oral loading. Patients receiving intravenous loading doses were switched to oral voriconazole after 24 h. Most patients received 200 mg twice daily (BID) following loading doses, with a median voriconazole dose of 2.95 mg/kg BID (range 1.7 mg/kg to 5.0 mg/kg) (Table 1).
TABLE 1.
Patient characteristics and voriconazole dosing
| Characteristic | |
|---|---|
| Patients, n | 69 |
| Median age, years (range) | 58 (19–80) |
| Male sex, % | 56.5 |
| Voriconazole courses, n | 71 |
| Inpatients | 61 (85.9) |
| Outpatients | 9 (12.7) |
| Unknown | 1 (1.4) |
| Diagnosis (% voriconazole courses) | |
| Acute myeloid leukemia | 63 (88.7) |
| Acute lymphoblastic leukemia | 4 (5.6) |
| Acute promyelocytic leukemia | 2 (2.8) |
| Aplastic anemia | 1 (1.4) |
| Myelodysplasia | 1 (1.4) |
| Invasive fungal infection (% voriconazole courses) | 42 (59.2) |
| Proven | 3 (4.2) |
| Probable | 9 (12.7) |
| Possible | 30 (42.3) |
| Voriconazole dosing | |
|
| |
| Loading dose | |
| Intravenous | 2 (2.8) |
| Oral | 25 (35.2) |
| No loading dose | 22 (31) |
| Unknown | 22 (31) |
| Oral maintenance dose | |
| 200 mg BID | 61 (85.9) |
| 250 mg BID | 1 (1.4) |
| 300 mg BID | 6 (8.5) |
| Unknown | 3 (4.2) |
| Maintenance dose, mg/kg | |
| 1–≤2 | 4 (5.9) |
| 2–≤3 | 33 (48.5) |
| 3–≤4 | 25 (35.2) |
| 4–≤5 | 6 (8.8) |
Data presented as n (%) unless otherwise indicated. BID Twice per day
Voriconazole levels
A total of 111 voriconazole levels were measured: 28 at the first time point between day 2 to 3 (median day 2, level 1); 20 at the second time point between day 4 to 5 (median day 4, level 2); and 63 at the third time point between day 5 to 8 (median day 6, level 3). Median voriconazole levels were not significantly different among the three different time points. There was a statistically significant correlation between the voriconazole level at the three different time points (P=0.04, r=0.598 comparing levels 1 and 2; P=0.001, r=0.647 comparing levels 1 and 3; P<0.001, r=0.912 comparing levels 2 and 3). The steady-state level (level 3) was used for subsequent toxicity assessment. However, because there was a very good correlation between voriconazole levels at the level 3 and level 2 time points, the level 2 time point was used in eight cases for which level 3 was not available (Table 2).
TABLE 2.
Voriconazole levels at measurement time points as well as in steady state
| Time point | Median level (range), μg/mL |
|---|---|
| Level 1 (day 2–3, n=28) | 2.55 (0.10–7.40) |
| Level 2 (day 4–5, n=20) | 2.29 (0.65–8.30) |
| Level 3 (day 6–8, n=63) | 2.61 (0–16.6) |
| Steady-state level*, μg/mL | n (%) |
|
| |
| ≤0.5 | 11 (15.5) |
| ≤1.0 | 14 (19.7) |
| ≤2.0 | 26 (36.6) |
| ≥5.0 | 15 (21.2) |
Steady state level is defined as level 3
There was significant interpatient variability in the steady-state plasma concentration of voriconazole. Patients with a daily voriconazole dose of 200 mg BID had voriconazole levels ranging from 0 μg/mL to 16.6 μg/mL with a coefficient of variation of 89.7%. Fourteen voriconazole courses (19.7%) were associated with a peak steady state level of ≤1.0 μg/mL. In contrast, levels ≥5 μg/mL were found during 15 (21.2%) of the voriconazole courses (Figure 1A); all of the latter occurred at the 200 mg BID dose (Table 2). Although patients receiving voriconazole doses of 300 mg BID had a higher median voriconazole level compared with those receiving 200 mg BID, this was not statistically significant (4.5 μg/mL versus 2.5 μg/mL; P=0.051, Figure 1B). As shown in Figure 1C, patients receiving a voriconazole dose of 3 mg/kg to 4 mg/kg had significantly higher median voriconazole levels then those receiving a 2 mg/kg to 3 mg/kg dose (2.0 μg/mL versus 3.3 μg/mL; P=0.046). The median voriconazole level was also higher among patients receiving a dose of 4 mg/kg to 5 mg/kg compared with those receiving a 3 mg/kg to 4 mg/kg dose, but this difference was not statistically significant (P=0.66). Coefficients of variation for patients receiving 1 mg/kg to 2 mg/kg, 2 mg/kg to 3 mg/kg, 3 mg/kg to 4 mg/kg and 4 mg/kg to 5 mg/kg were 6.8%, 79.8%, 88.2% and 57.2%, respectively.
Figure 1).
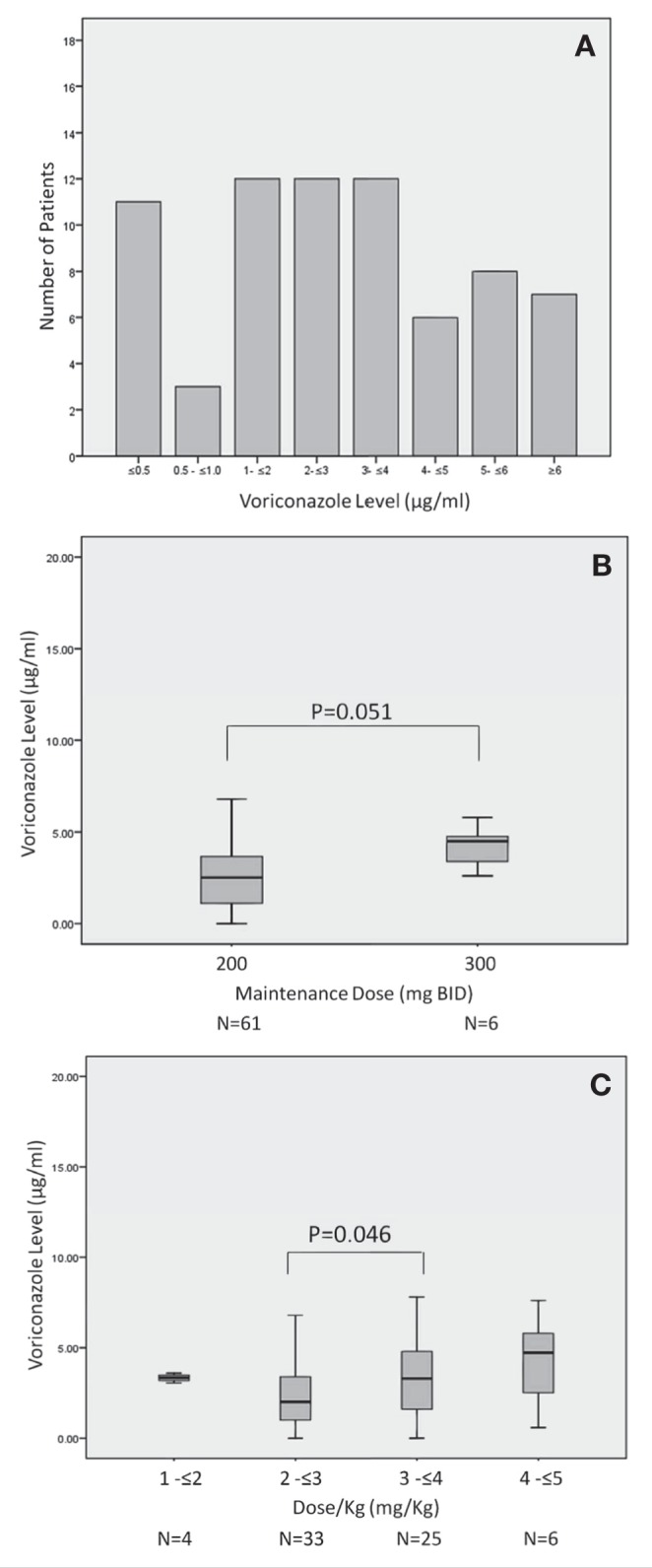
A Distribution of steady-state voriconazole concentrations. B Variability between voriconazole maintenance dose and voriconazole steady-state levels. C Variability between the voriconazole maintenance dose/kg and voriconazole steady-state levels. BID Twice per day
Correlative factors for voriconazole levels
There was no correlation between patient age and voriconazole levels measured either as a continuous variable or as a discrete variable using 2 μg/mL as a cut-off (P=0.98). There was also no significant association between sex and the likelihood of achieving a steady-state level of >2 μg/mL (P=0.094).
Fourteen patients were receiving a concomitant proton pump inhibitor (PPI) during the study period – 10 patients were taking esomeprazole, two patients omeprazole and two patients lansoprazole. There was no significant association between PPI use and achievement of a voriconazole steady-state level of >2 μg/mL (P=0.65).
Correlation with genotype:
CYP2C19 genotyping results were available for 29 patients and characterized as either slow metabolizers (presence of either *2 or *3 genotype), rapid metabolizers (*17 genotype) or neither (*1/*1 genotype). As shown in Table 3, there was no significant difference in the median steady state voriconazole levels among different genotypes (P=0.2609).
TABLE 3.
Results of CYP2C19 genotyping studies
| Genotype | n | Steady-state voriconazole level, median (range), μg/mL |
|---|---|---|
| *1/*1 | 13 | 3.16 (0.40–7.80) |
| *1/*2 or *1/*3 | 8 | 2.38 (0.00–5.90) |
| *1/*17 | 5 | 1.14 (0.43–3.60) |
| *2/*17 | 3 | 1.10 (1.00–3.00) |
| P | Not significant |
CYP Cytochrome P450
Correlation with liver enzymes:
At day 6 to 8 of therapy, 69 patients were still on voriconazole; of these, one patient each had bilirubin, AST and ALT levels >3× the upper limit of normal (ULN). Only the ALP was significantly correlated with the voriconazole level (P=0.003, r=0.37), with bilirubin only trending toward significance (P=0.06, r=0.242). At day 14 to 16, 49 patients were receiving voriconazole and four had bilirubin levels >3× ULN, while one patient had an elevated AST level (3.2× ULN) and two had an elevated ALP level (3.2× and 4× ULN). Both the bilirubin (r=0.436; P=0.003) and the AST (r=0.337; P=0.02) at day 14 to 16 were significantly correlated with the steady-state voriconazole concentration. Relative to patients with a normal bilirubin, those with an abnormal bilirubin (>ULN) had significantly higher median voriconazole levels at both the day 6 to 8 liver enzyme assessment (2.4 μg/mL versus 3.5 μg/mL; P=0.03) and the day 14 to 16 liver enzyme assessment (2.1 μg/mL versus 3.5 μg/mL; P=0.026). In contrast, there was no significant difference in plasma voriconazole levels for those with an abnormal versus normal AST, ALT or ALP levels (data not shown). Of the 15 patients with steady-state voriconazole levels >5 μg/mL, four had an elevated bilirubin level >ULN at day 6 to 8 and at day 14 to 16.
DISCUSSION
Voriconazole TDM has been conducted in both prophylactic and therapeutic settings, with variable results (3,9,12–14,16,17,23,24,34–37). However, many of these studies evaluated intravenous dosing and included a small number of patients. Many patients receive oral voriconazole therapy for practical reasons (eg, ambulatory care therapy) and cost savings. Our study was designed to determine voriconazole levels in the setting of ill patients, primarily with acute leukemia, receiving oral dosing and to evaluate factors associated with the variability in levels.
Because the minimum inhibitory concentration that inhibits the growth of 90% of organisms is ≤1 μg/mL for most organisms targeted by voriconazole and neurological toxicities generally occur at serum levels >5.5 μg/mL, several authors have suggested using a minimal voriconazole target blood concentration of 1 μg/mL to 2 μg/mL and maximal concentrations between 5.0 μg/mL and 6.0 μg/mL (4,12,15,19,38–41). However, attainment of the minimum suggested voriconazole concentration does not ensure therapeutic success, as noted by Pascual et al (4), who observed that two of five IFI associated deaths occurred among those with levels >1.0 μg/mL. Although most patients in our cohort received a maintenance voriconazole dose of 200 mg orally BID, the steady-state concentration showed considerable variability among patients, ranging from 0 μg/mL to 16.6 μg/mL. Therefore, a substantial proportion of patients achieved steady-state levels generally regarded as subtherapeutic – approximately 20% of patients achieved levels <1.0 μg/mL and 37% <2.0 μg/mL; conversely, 15% had levels >5.0 μg/mL (3,4,15,21). Such variability has been previously described. In a study of voriconazole for the treatment of invasive aspergillosis, plasma voriconazole levels ranged from <0.1 μg/mL to 9.7 μg/mL, while Trifilio et al (16) noted the dose of those with voriconazole levels <0.5 μg/mL (2.9 mg/kg to 8.9 mg/kg; median 5.1 mg/kg) was not significantly different from the dose at which levels were higher (2.7 mg/kg to 10.2 mg/kg; P=0.35) (3). Miyakis et al (12) noted 32% of patients had levels <0.5 μg/mL, while Racil et al (37) found that, for patients treated with voriconazole 200 mg BID, inter- and intrapatient variability coefficients were 81.9% and 50.5%, respectively.
The reasons for the observed variability in levels are likely multi-factorial. Lipid-rich foods can decrease the absorption by as much as 20% (34). Chemotherapy-induced gastrointestinal damage, concomitant use of PPIs affecting absorption, and hepatotoxicity due to chemotherapy and other drugs, causing altered metabolism, may affect steady-state levels. Age and sex may also influence drug levels. In a small sample of healthy volunteers receiving voriconazole, the maximum concentration and area under the curve were higher in elderly male subjects and in women compared with younger men (42). However, we were not able to determine any correlation among age, sex or concomitant PPI exposure and steady-state levels in our cohort.
Polymorphisms in the CYP2C19 enzyme involved in the metabolism of voriconazole may also contribute to the variability in voriconazole levels. Poor metabolizers, characterized by *2 or *3 alleles, are most commonly observed among non-Indian Asians; these subjects may have voriconazole serum concentrations up to four times higher than those observed in extensive metabolizers (21,43). Voriconazole is both a substrate and an inhibitor of CYP2C19, CYP2C9 and CYP3A4. In contrast, heterozygous ultrarapid metabolizer alleles (CYP2C19*17), have been reported with a frequency of 18% in both Swedes and Ethiopians, and a low rate of 4% in Chinese individuals (44). Our inability to detect any correlation between genotype and steady-state voriconazole levels may be related to the small sample size, but could also indicate that other clinical factors may override the influence of genotype.
Despite the lack of predictors, our data indicate that patients receiving a higher dose (300 mg BID) or higher dose per kilogram tend to have higher voriconazole levels. This suggests that using either a higher fixed dose, or weight-based dosing, would be preferable in the absence of TDM. However, the majority of patients achieved adequate serum levels with 200 mg BID, suggesting that TDM may reduce the risk of overdoing with higher doses. Patients with subtherapeutic levels could then be dose-escalated. Our data also demonstrated that voriconazole levels measured as early as day 2 to 3 could provide a useful indicator of this because levels were not significantly different among the three time points tested. This would permit early dose escalation as needed.
Abnormal liver function test results have been reported in 1% to 10% of patients receiving voriconazole (10,45). Pascual et al (4) found no correlation between voriconazole levels and ALP or gamma glutamyltransferase levels, and logistic regression analysis failed to demonstrate a significant association between voriconazole levels and hepatotoxicity. Both Racil et al (37) and Miyakis et al (12) further suggest that there is no statistical association between hepatotoxicity and any particular voriconazole concentration. In contrast, a group of Japanese researchers reported that eight of 11 (74%) hematology patients developed hepatic damage with threshold serum concentrations >6 μg/mL (46). Denning et al (3) noted deterioration in liver function in six of 22 patients with plasma concentrations >6 μg/mL, and Trifilio et al (16) found that increases in AST and ALP levels, but not ALT or bilirubin levels, correlated with voriconazole levels in adult patients undergoing HSCT. Within our cohort, while the AST, ALT and ALP levels showed no consistent relationship with voriconazole levels, the ALP level at day 6 to 8, and both the AST and total bilirubin levels at day 14 to 16, were significantly associated with steady-state voriconazole levels. However, other enzymes were not correlated, and elevated liver enzyme and bilirubin levels were observed in patients with voriconazole levels in the optimal range. It is unclear whether the abnormal liver tests resulted from voriconazole administration, or whether the higher voriconazole levels were a result of chemotherapy-induced hepatic toxicity resulting in abnormal voriconazole metabolism (47). Nevertheless, based on our data, the value of TDM as a predictor of liver toxicity appears weak.
Our study had several limitations. It was conducted at a single centre with a limited number of patients, and our study design did not include assessment of chemotherapy-induced gastrointestinal damage, which may affect daily nutritional intake and, subsequently, voriconazole plasma concentrations. Additionally, the majority of our patients were treated as inpatients and received additional medications concurrently with voriconazole including cardiac medications, antibiotics, PPIs and antiemetics. We were unable to fully evaluate the effects of concomitant medications because a limited number of patients received each medication. Furthermore, most concurrent medications were administered only intermittently. Although both inpatients and outpatients received extensive education regarding the necessity of taking voriconazole on an empty stomach and at designated times, we did not formally evaluate patient compliance, particularly in the case of outpatients.
In contrast to several other studies, we evaluated CYP2C19 polymorphisms; however, these were conducted after marrow reconstitution following induction, reinduction or consolidation chemotherapy. Unfortunately, some patients were not available for genetic testing during this period. Thus, results of CYP2C19 polymorphism genetic testing were available for a limited number of patients (28 of 69 [41%]). Additionally, because metropolitan Toronto is ethnically diverse, the number of patients within each subcategory (ie, slow metabolizers, intermediate metabolizers and rapid metabolizers) was further reduced. This may partially explain our findings of no significant relationship between voriconazole plasma concentrations and genotype. We found no consistent relationship between day 14 to 16 liver enzyme abnormalities and steady-state voriconazole concentrations. Because our hepatotoxicity analysis was conducted approximately one week following voriconazole steady-state measurements, it is possible that further variations in levels may have occurred in the interim. An additional limitation to our study was that we could not reliably evaluate the clinical efficacy of treatment because most infections were only considered to be possible IFIs; confirmatory cultures were not generally available and galactomannan testing was not being routinely performed. Finally, although our population consisted primarily of acute leukemia patients receiving chemotherapy, some patients with myelodysplasia and aplastic anemia were also included; however, due to the small number of these patients, we were unable to evaluate the influence of the underlying condition on plasma voriconazole concentrations.
CONCLUSION
Our study demonstrates that, in ill patients with acute leukemia and related disorders undergoing treatment with oral voriconazole, there is substantial interpatient variability in voriconazole concentrations, with poor correlations between the administered dose, clinical predictive factors, genotype and serum levels. A substantial proportion of patients receiving doses of 200 mg orally BID had steady-state levels which many consider suboptimal in treating invasive aspergillosis. The unpredictability of these levels supports the argument in favour of routine voriconazole TDM in these ill patients.
Acknowledgments
The authors acknowledge the contributions of Kathryn Quinton for collecting and processing the samples, Natalie Freeman for assistance in performing the genotyping studies and Triyu Vather for formatting of the manuscript.
REFERENCES
- 1.Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 2.Pagano L, Caira M, Candoni A, et al. Invasive aspergillosis in patients with acute myeloid leukemia: A SEIFEM-2008 registry study. Haematologica. 2010;95:644–50. doi: 10.3324/haematol.2009.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denning DW, Ribaud P, Milpied N, et al. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002;34:563–71. doi: 10.1086/324620. [DOI] [PubMed] [Google Scholar]
- 4.Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46:201–11. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 5.Troke P, Aguirrebengoa K, Arteaga C. Treatment of scedosporiosis with voriconazole: Clinical experience with 107 patients. Antimicrob Agents Chemother. 2008;52:1743–50. doi: 10.1128/AAC.01388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanzani M, Tumietto F, Vianelli N, Baccarani M. Update on the treatment of disseminated fusariosis: Focus on voriconazole. Ther Clin Risk Manag. 2007;3:1165–73. [PMC free article] [PubMed] [Google Scholar]
- 7.Herbrecht R, Denning DW, Patterson TF. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–15. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 8.Perfect JR, Marr KA, Walsh TJ, et al. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin Infect Dis. 2003;36:1122–31. doi: 10.1086/374557. [DOI] [PubMed] [Google Scholar]
- 9.Trifilio SM, Yarnold PR, Scheetz MH, Pi J, Pennick G, Mehta J. Serial plasma voriconazole concentrations after allogeneic hematopoietic stem cell transplantation. Antimicrob Agents Chemother. 2009;53:1793–6. doi: 10.1128/AAC.01316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh TJ, Pappas P, Winston DJ, et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med. 2002;346:225–34. doi: 10.1056/NEJM200201243460403. [DOI] [PubMed] [Google Scholar]
- 11.Purkins L, Wood N, Greenhalgh K, Allen MJ, Oliver SD. Voriconazole, a novel wide-spectrum triazole: Oral pharmacokinetics and safety. Br J Clin Pharmacol. 2003;56(Suppl 1):10–6. doi: 10.1046/j.1365-2125.2003.01993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyakis S, van Hal SJ, Ray J, Marriott D. Voriconazole concentrations and outcome of invasive fungal infections. Clin Microbiol Infect. 2010;16:927–33. doi: 10.1111/j.1469-0691.2009.02990.x. [DOI] [PubMed] [Google Scholar]
- 13.Okuda T, Okuda A, Watanabe N, Takao M, Takayanagi K. Retrospective serological tests for determining the optimal blood concentration of voriconazole for treating fungal infection. Yakugaku Zasshi. 2008;128:1811–8. doi: 10.1248/yakushi.128.1811. [DOI] [PubMed] [Google Scholar]
- 14.Ruping MJ, Müller C, Vehreschild JJ, et al. Voriconazole serum concentrations in prophylactically treated acute myelogenous leukaemia patients. Mycoses. 2011;54:230–3. doi: 10.1111/j.1439-0507.2009.01803.x. [DOI] [PubMed] [Google Scholar]
- 15.Smith J, Safdar N, Knasinski V, et al. Voriconazole therapeutic drug monitoring. Antimicrob Agents Chemother. 2006;50:1570–2. doi: 10.1128/AAC.50.4.1570-1572.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trifilio S, Ortiz R, Pennick G, et al. Voriconazole therapeutic drug monitoring in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2005;35:509–13. doi: 10.1038/sj.bmt.1704828. [DOI] [PubMed] [Google Scholar]
- 17.Troke PF, Hockey HP, Hope WW. Observational study of the clinical efficacy of voriconazole and its relationship to plasma concentrations in patients. Antimicrob Agents Chemother. 2011;55:4782–8. doi: 10.1128/AAC.01083-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan K, Brayshaw N, Tomaszewski K, Troke P, Wood N. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J Clin Pharmacol. 2006;46:235–43. doi: 10.1177/0091270005283837. [DOI] [PubMed] [Google Scholar]
- 19.Goodwin ML, Drew RH. Antifungal serum concentration monitoring: An update. J Antimicrob Chemother. 2008;61:17–25. doi: 10.1093/jac/dkm389. [DOI] [PubMed] [Google Scholar]
- 20.Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos. 2003;31:540–7. doi: 10.1124/dmd.31.5.540. [DOI] [PubMed] [Google Scholar]
- 21.Johnson LB, Kauffman CA. Voriconazole: A new triazole antifungal agent. Clin Infect Dis. 2003;36:630–7. doi: 10.1086/367933. [DOI] [PubMed] [Google Scholar]
- 22.Smith J, Andes D. Therapeutic drug monitoring of antifungals: Pharmacokinetic and pharmacodynamic considerations. Ther Drug Monit. 2008;30:167–72. doi: 10.1097/FTD.0b013e318167d0e0. [DOI] [PubMed] [Google Scholar]
- 23.Trifilio S, Pennick G, Pi J, et al. Monitoring plasma voriconazole levels may be necessary to avoid subtherapeutic levels in hematopoietic stem cell transplant recipients. Cancer. 2007;109:1532–5. doi: 10.1002/cncr.22568. [DOI] [PubMed] [Google Scholar]
- 24.Lazarus HM, Blumer JL, Yanovich S, Schlamm H, Romero A. Safety and pharmacokinetics of oral voriconazole in patients at risk of fungal infection: A dose escalation study. J Clin Pharmacol. 2002;42:395–402. [PubMed] [Google Scholar]
- 25.Purkins L, Wood N, Kleinermans D, Greenhalgh K, Nichols D. Effect of food on the pharmacokinetics of multiple-dose oral voriconazole. Br J Clin Pharmacol. 2003;56(Suppl 1):17–23. doi: 10.1046/j.1365-2125.2003.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Department of Health and Human Services, National Institute of Health, National Cancer Institute, Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. 2010. pp. 1–80. [Google Scholar]
- 27.Vogeser M, Schiel X, Spohrer U. Quantification of voriconazole in plasma by liquid chromatography-tandem mass spectrometry. Clin Chem Lab Med. 2005;43:730–4. doi: 10.1515/CCLM.2005.124. [DOI] [PubMed] [Google Scholar]
- 28.Keevil BG, Newman S, Lockhart S, Howard SJ, Moore CB, Denning DW. Validation of an assay for voriconazole in serum samples using liquid chromatography-tandem mass spectrometry. Ther Drug Monit. 2004;26:650–7. doi: 10.1097/00007691-200412000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Pauwels S, Vermeersch P, Van Eldere J, Desmet K. Fast and simple LC-MS/MS method for quantifying plasma voriconazole. Clin Chim Acta. 2012;413:740–3. doi: 10.1016/j.cca.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Decosterd LA, Rochat B, Pesse B, et al. Multiplex ultra-performance liquid chromatography-tandem mass spectrometry method for simultaneous quantification in human plasma of fluconazole, itraconazole, hydroxyitraconazole, posaconazole, voriconazole, voriconazole-N-oxide, anidulafungin, and caspofungin. Antimicrob Agents Chemother. 2010;54:5303–15. doi: 10.1128/AAC.00404-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brüggemann RJ, Touw DJ, Aarnoutse RE, Verweij PE, Burger DM. International interlaboratory proficiency testing program for measurement of azole antifungal plasma concentrations. Antimicrob Agents Chemother. 2009;53:303–5. doi: 10.1128/AAC.00901-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salazar-Flores J, Torres-Reyes LA, Martínez-Cortés G, et al. Distribution of CYP2D6 and CYP2C19 polymorphisms associated with poor metabolizer phenotype in five Amerindian groups and western Mestizos from Mexico. Genet Test Mol Biomarkers. 2012;16:1098–104. doi: 10.1089/gtmb.2012.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pagano M, Gauvreau K. Principles of Biostatistics. 2nd edn. Pacific Grove: Duxbury Press; 2000. p. 592. [Google Scholar]
- 34.Boyd AE, Modi S, Howard SJ, Moore CB, Keevil BG, Denning DW. Adverse reactions to voriconazole. Clin Infect Dis. 2004;39:1241–4. doi: 10.1086/424662. [DOI] [PubMed] [Google Scholar]
- 35.Husain S, Paterson DL, Studer S, et al. Voriconazole prophylaxis in lung transplant recipients. Am J Transplant. 2006;6:3008–16. doi: 10.1111/j.1600-6143.2006.01548.x. [DOI] [PubMed] [Google Scholar]
- 36.Cordonnier C, Rovira M, Maertens J, et al. Voriconazole for secondary prophylaxis of invasive fungal infections in allogeneic stem cell transplant recipients: Results of the VOSIFI study. Haematologica. 2010;95:1762–8. doi: 10.3324/haematol.2009.020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Racil Z, Winterova J, Kouba M, et al. Monitoring trough voriconazole plasma concentrations in haematological patients: Real life multicentre experience. Mycoses. 2012;55:483–92. doi: 10.1111/j.1439-0507.2012.02186.x. [DOI] [PubMed] [Google Scholar]
- 38.Cendejas-Bueno E, Cuenca-Estrella M, Gomez-Lopez A. Determination of voriconazole serum concentration by bioassay, a valid method for therapeutic drug monitoring for clinical laboratories. Antimicrob Agents Chemother. 2013;57:3437–40. doi: 10.1128/AAC.00323-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andes D, Pascual A, Marchetti O. Antifungal therapeutic drug monitoring: Established and emerging indications. Antimicrob Agents Chemother. 2009;53:24–34. doi: 10.1128/AAC.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brüggemann RJ, Antonius T, Heijst AV, Hoogerbrugge PM, Burger DM, Warris A. Therapeutic drug monitoring of voriconazole in a child with invasive aspergillosis requiring extracorporeal membrane oxygenation. Ther Drug Monit. 2008;30:643–6. doi: 10.1097/FTD.0b013e3181898b0c. [DOI] [PubMed] [Google Scholar]
- 41.Lewis RE. What is the “therapeutic range” for voriconazole? Clin Infect Dis. 2008;46:212–4. doi: 10.1086/524670. [DOI] [PubMed] [Google Scholar]
- 42.2001. pp. 1–56. Food and Drug Administration, Background document for the Antiviral Drug Products Advisory Committee Meeting. Voriconazole AC briefing document. Center for Drug Evaluation and Research, Division of Special Pathogen and Immunologic Drug Products.
- 43.Desta Z, Zhao X, Shin JG, Flockhart DA. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet. 2002;41:913–58. doi: 10.2165/00003088-200241120-00002. [DOI] [PubMed] [Google Scholar]
- 44.Sim SC, Risinger C, Dahl ML. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther. 2006;79:103–13. doi: 10.1016/j.clpt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 45.den Hollander JG, van Arkel C, Rijnders BJ, Lugtenburg PJ, de Marie S, Levin MD. Incidence of voriconazole hepatotoxicity during intravenous and oral treatment for invasive fungal infections. J Antimicrob Chemother. 2006;57:1248–50. doi: 10.1093/jac/dkl108. [DOI] [PubMed] [Google Scholar]
- 46.Ueda K, Nannya Y, Kumano K, et al. Monitoring trough concentration of voriconazole is important to ensure successful antifungal therapy and to avoid hepatic damage in patients with hematological disorders. Int J Hematol. 2009;89:592–9. doi: 10.1007/s12185-009-0296-3. [DOI] [PubMed] [Google Scholar]
- 47.Brown J, Freeman BB. Rethinking the use of voriconazole therapeutic drug monitoring in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2005;36:177. doi: 10.1038/sj.bmt.1705012. [DOI] [PubMed] [Google Scholar]


