Abstract
Hepatocellular carcinoma (HCC) usually develops in the context of chronic hepatitis triggered by viruses or toxic substances causing hepatocyte death, inflammation and compensatory proliferation of liver cells. Death receptors of the TNFR superfamily regulate cell death and inflammation and are implicated in liver disease and cancer. Liver parenchymal cell-specific ablation of NEMO/IKKγ, a subunit of the IκB kinase (IKK) complex that is essential for the activation of canonical NF-κB signalling, sensitized hepatocytes to apoptosis and caused the spontaneous development of chronic hepatitis and HCC in mice. Here we show that hepatitis and HCC development in NEMOLPC-KO mice is triggered by death receptor-independent FADD-mediated hepatocyte apoptosis. TNF deficiency in all cells or conditional LPC-specific ablation of TNFR1, Fas or TRAIL-R did not prevent hepatocyte apoptosis, hepatitis and HCC development in NEMOLPC-KO mice. To address potential functional redundancies between death receptors we generated and analysed NEMOLPC-KO mice with combined LPC-specific deficiency of TNFR1, Fas and TRAIL-R and found that also simultaneous lack of all three death receptors did not prevent hepatocyte apoptosis, chronic hepatitis and HCC development. However, LPC-specific combined deficiency in TNFR1, Fas and TRAIL-R protected the NEMO-deficient liver from LPS-induced liver failure, showing that different mechanisms trigger spontaneous and LPS-induced hepatocyte apoptosis in NEMOLPC-KO mice. In addition, NK cell depletion did not prevent liver damage and hepatitis. Moreover, NEMOLPC-KO mice crossed into a RAG-1-deficient genetic background-developed hepatitis and HCC. Collectively, these results show that the spontaneous development of hepatocyte apoptosis, chronic hepatitis and HCC in NEMOLPC-KO mice occurs independently of death receptor signalling, NK cells and B and T lymphocytes, arguing against an immunological trigger as the critical stimulus driving hepatocarcinogenesis in this model.
Liver cancer is one of the most common malignancies and the third leading cause of cancer-related deaths worldwide.1, 2 Liver cancer predominantly arises in the context of chronic inflammatory conditions, most notably in virus hepatitis (HBV and HCV).1, 2 Although infectious agents are the primary cause of liver cancer worldwide, the incidence in western countries is rising due to the increase in obesity and non-alcoholic steatohepatitis.3 The pathogenesis of hepatocellular carcinoma (HCC) is incompletely understood and it is plausible that the different underlying aetiologies determine a distinct context for liver carcinogenesis. However, the prevailing universal concept is that continuous liver parenchymal damage and hepatocyte cell death drive compensatory proliferation and within the context of a chronically inflamed liver tissue mutations and epigenetic changes accumulate eventually transforming hepatocytes into malignant cells. Therefore, understanding the tissue-intrinsic processes that determine cell death and chronic inflammation resulting in hepatocarcinogenesis is a critical need in order to design more effective therapeutic strategies.
The nuclear factor κB (NF-κB) pathway is implicated in cancer development in particular in the context of chronic inflammation.4, 5 In relation to liver cancer, NF-κB signalling has been implicated in the pathogenesis of hepatitis, liver fibrosis, cirrhosis and HCC.6, 7 The IKK complex, composed of two catalytic subunits, IKK1/IKKα and IKK2/IKKβ, and a regulatory subunit termed NEMO/IKKγ, activates NF-κB by phosphorylating inhibitor of NF-κB (IκB) proteins targeting them for degradation by the proteasome and thus allowing the nuclear accumulation of NF-κB dimers.5 IKK2 is primarily responsible for targeting and degrading IκBα thus inducing canonical NF-κB activation, although the two kinases show some degree of functional redundancy in controlling canonical NF-κB signalling.5, 8 NEMO/IKKγ is indispensable for activation of canonical NF-κB signalling.9, 10, 11
NF-κB signalling was proposed to exhibit tumour promoter or tumour suppressor properties in different models of liver cancer. In the Mdr2−/− mouse model of inflammation-driven liver carcinogenesis, NF-κB inhibition caused by transgenic IκBα super–repressor expression in hepatocytes inhibited HCC progression.12 Moreover, hepatocyte-restricted ablation of IKK2 prevented hepatitis and liver tumorigenesis induced by overexpression of lymphotoxins α and β in hepatocytes.13 However, mice with hepatocyte-specific IKK2 ablation developed more tumours induced by a single injection of the chemical carcinogen diethylnitrosamine,14 revealing a tumour suppressor role of NF-κB in this context.
Studies in mice lacking NEMO specifically in liver parenchymal cells (LPCs) further supported a tumour suppressor function of IKK/NF-κB signalling in liver cancer. NEMOLPC-KO mice showed spontaneous hepatocyte apoptosis resulting in chronic steatohepatitis and the development of HCC by the age of 1 year.15 LPC-specific ablation of Fas-Associated with Death Domain (FADD or MORT1), an adapter protein essential for the recruitment of caspase-8 to the Death Inducing Signalling Complex and the induction of death receptor-mediated apoptosis,16 prevented both spontaneous and LPS-induced apoptosis of NEMO-deficient hepatocytes and the development of steatohepatitis.15 In addition, LPC-specific knockout of caspase-8 inhibited spontaneous hepatocyte apoptosis and HCC development in NEMOLPC-KO mice, although it caused non-apoptotic hepatocyte death and cholestasis.17 Given the essential role of FADD and caspase-8 in mediating apoptosis downstream of death receptors,16 we hypothesized that death receptor-mediated apoptosis of NEMO-deficient hepatocytes drives the development of hepatitis and HCC in NEMOLPC-KO mice. The three main death receptors of the TNF receptor superfamily that are capable of inducing caspase-8-mediated apoptosis are TNFR1, Fas/CD95 and TRAIL-R/DR5.16 To address the role of death receptor-induced apoptosis in triggering the spontaneous death of NEMO-deficient hepatocytes and the development of steatohepatitis and HCC, we generated and analysed NEMOLPC-KO mice lacking TNFR1, Fas or TRAIL-R specifically in LPCs. Surprisingly, we found that LPC-specific knockout of each of the death receptors alone but also combined deficiency of TNFR1, Fas and TRAIL-R in LPCs did not prevent spontaneous hepatocyte apoptosis, hepatitis and HCC development in NEMOLPC-KO mice. In addition, knockout of TNF in all cells also did not protect NEMOLPC-KO mice from hepatocyte death, hepatitis and HCC. Collectively, these results demonstrate that TNFR1, Fas and TRAIL-R are not required for the development of chronic liver damage and HCC in NEMOLPC-KO mice.
Results
LPC-specific FADD ablation prevents liver carcinogenesis in NEMOLPC-KO mice
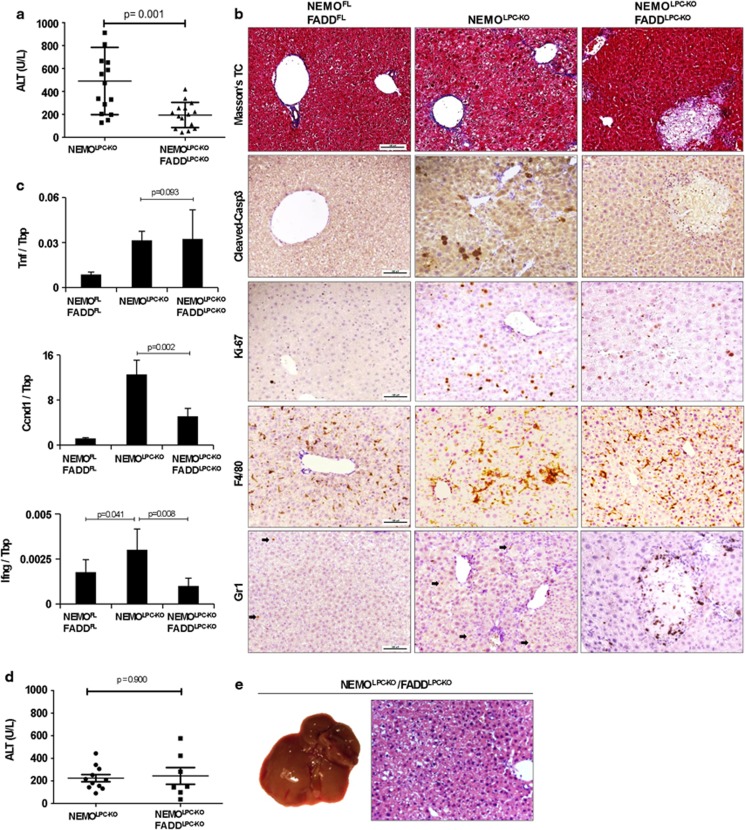
FADD is essential for the recruitment and activation of caspase-8 and the induction of apoptosis downstream of death receptors including TNFR1, Fas and TRAIL-R. We showed previously that LPC-specific FADD deficiency strongly reduced early hepatitis in NEMOLPC-KO mice as indicated by significantly lower serum alanine aminotransferase (ALT) levels and reduced hepatocyte apoptosis and compensatory proliferation.15 To further characterize the role of FADD in the spontaneous development of hepatitis and HCC in NEMOLPC-KO mice, we analysed groups of NEMOLPC-KO/FADDLPC-KO mice at the age of 8 weeks or 1 year. NEMOLPC-KO/FADDLPC-KO mice had average serum ALT levels of about 200 U/l, which were significantly reduced compared with NEMOLPC-KO mice but higher than the background levels of wild-type control mice, indicating that FADD deficiency did not fully prevent liver damage in NEMOLPC-KO mice (Figure 1a). Histological analysis showed that FADD deficiency prevented hepatocyte apoptosis as indicated by the lack of active caspase-3-positive cells in the liver of NEMOLPC-KO/FADDLPC-KO mice (Figure 1b and Supplementary Figure S2C). However, the liver of 8-week-old NEMOLPC-KO/FADDLPC-KO mice displayed some focal necrotic lesions that were surrounded by granulocytes (Figure 1b), indicating that FADD ablation protected NEMO-deficient hepatocytes from apoptosis but triggered focal necrotic hepatocyte death resulting in mildly elevated serum ALT levels. Given the important role of FADD in preventing RIPK3-mediated necroptosis,18, 19, 20 it is likely that the necrotic foci are caused by necroptotic hepatocyte death that occurs focally in the livers of NEMOLPC-KO/FADDLPC-KO mice. Hepatocyte proliferation was reduced in the livers of NEMOLPC-KO/FADDLPC-KO compared with NEMOLPC-KO mice as indicated by less Ki67-positive hepatocytes and lower mRNA expression of CyclinD1 (Figures 1b and c and Supplementary Figure S2D). In addition, immunostaining for cytokeratin 19 (CK19) revealed that FADD deficiency prevented oval cell expansion in NEMOLPC-KO/FADDLPC-KO mice (Supplementary Figure S2B). Moreover, although TNF expression was not altered the levels of IFNγ mRNA were reduced in the liver of NEMOLPC-KO/FADDLPC-KO mice indicating overall reduced inflammation, consistent with the histological analysis showing reduced numbers of F4/80-positive macrophages (Figures 1b and c).
Figure 1.
FADD deletion decreases liver damage and inflammation and prevents HCC in NEMOLPC-KO mice. (a) Serum ALT levels in 8-week-old mice. (b) Liver sections of 8-week-old mice stained with Masson's Trichrome and for cleaved caspase-3, Ki-67, F4/80 and Lys6G/Gr1. Arrows indicate GR1-positive cells (granulocytes) (c) qRT-PCR analysis of mRNA isolated from total liver tissue of 8-week-old mice (n=5 per genotype, expression normalized to Tbp). (d) Serum ALT levels from 1-year-old mice. (e) Representative macroscopic picture and H&E-stained liver section of 1-year-old NEMOLPC-KO/FADDLPC-KO mice
To address the role of FADD in HCC development in NEMOLPC-KO mice, we examined the livers of NEMOLPC-KO/FADDLPC-KO mice at the age of 1 year or more for spontaneous tumour development. Although their serum ALT levels were similar to those of NEMOLPC-KO mice, we found that none of the NEMOLPC-KO/FADDLPC-KO mice examined at the age of 1–1.5 years (n=18) showed macroscopically visible liver tumours (Figures 1d and e and Supplementary Figure S1). Histological examination also confirmed the lack of tumours or dysplastic premalignant precursor lesions (Figure 1e). Therefore, FADD deficiency fully protected NEMOLPC-KO mice from the development of HCC, demonstrating that FADD is essential for spontaneous hepatocarcinogenesis in this model.
TNF is dispensable for spontaneous hepatocyte death, hepatitis and HCC development in NEMOLPC-KO mice
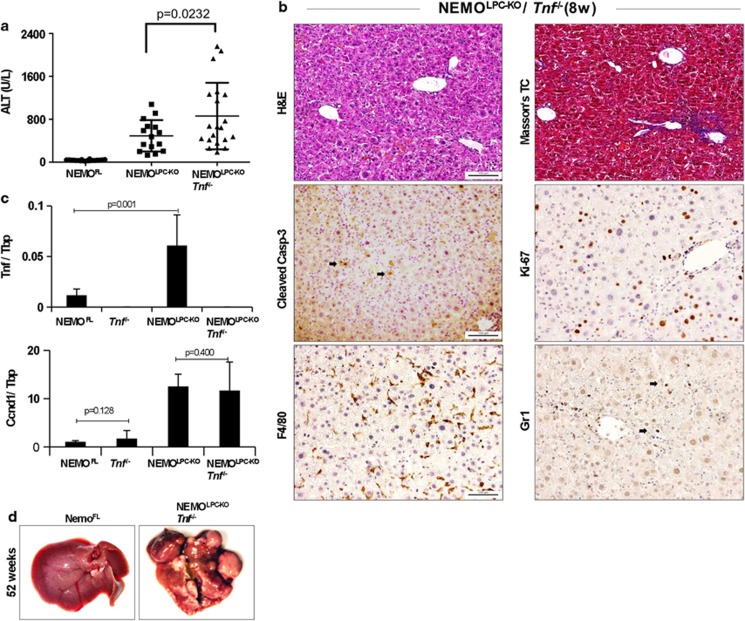
The finding that FADD deficiency prevented spontaneous hepatocyte apoptosis and HCC development in NEMOLPC-KO mice suggested that hepatitis and liver cancer in these mice are driven by death receptor-induced apoptosis. NF-κB inhibition sensitizes cells to TNF-induced apoptosis and p65 or IKK2 knockout mice die during embryogenesis due to TNF-mediated liver degeneration.21, 22 The expression of TNF is upregulated in the NEMO-deficient livers (Figure 2c); therefore, we hypothesized that TNF-mediated hepatocyte apoptosis drives hepatitis and HCC development in NEMOLPC-KO mice. To address the role of TNF, we crossed NEMOLPC-KO mice with mice lacking TNF23 to generate NEMOLPC-KO/Tnf−/− mice. Surprisingly, 8-week-old NEMOLPC-KO/Tnf−/− mice exhibited similarly increased serum ALT levels compared with NEMOLPC-KO mice, demonstrating that TNF deficiency did not prevent spontaneous liver damage in NEMOLPC-KO mice (Figure 2a). Histological analysis revealed that the livers of NEMOLPC-KO/Tnf−/− mice had increased numbers of active caspase-3-positive apoptotic hepatocytes, increased hepatocyte proliferation, oval cell expansion, fibrosis and increased infiltration of macrophages and neutrophils (Figure 2b and Supplementary Figure S2). Consistent with the increased presence of proliferating hepatocytes, livers from NEMOLPC-KO/Tnf−/− mice showed increased CyclinD1 expression (Figure 2c). These results showed that TNF deficiency could not prevent hepatocyte apoptosis and the development of spontaneous hepatitis in young NEMOLPC-KO mice. To assess whether the lack of TNF could prevent the spontaneous development of HCC, we analysed the livers of NEMOLPC-KO/Tnf−/− mice at the age of 1 year or more. Macroscopic and histological analysis of livers from NEMOLPC-KO/Tnf−/− mice at the age between 50 and 63 weeks of age revealed that all mice (n=12) developed HCC (Figure 2d). Collectively, these results demonstrated that TNF is not an essential trigger for spontaneous hepatocyte apoptosis and the development of hepatitis and HCC in NEMOLPC-KO mice.
Figure 2.
TNF deficiency does not prevent hepatocyte death, liver damage and HCC development in NEMOLPC-KO mice. (a) Serum ALT levels in 8-week-old mice. (b) Liver sections of 8-week-old NEMOLPC-KO/Tnf−/− mice stained with H&E, Masson's Trichrome and for cleaved caspase-3 (indicated by arrows), Ki-67, F4/80 and Lys6G/ Gr1 (indicated by arrows). (c) qRT-PCR analysis of mRNA isolated from total liver tissue of 8-week-old mice (n=5 per genotype, expression normalized to Tbp). (d) Macroscopic liver pictures of 1-year-old NEMOFL and NEMOLPC-KO/Tnf−/−mice
LPC-specific ablation of TNFR1, TRAIL-R or FAS does not prevent spontaneous hepatocyte death and liver inflammation in NEMOLPC-KO mice
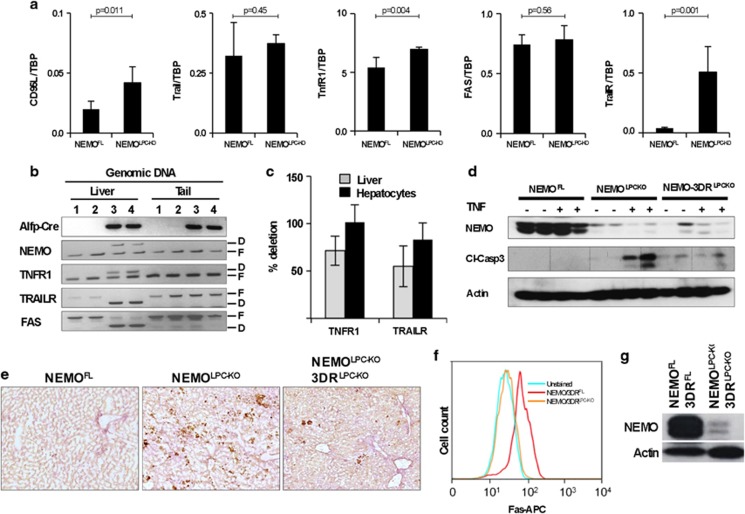
In addition to TNF, Fas ligand (FasL) and TRAIL can also induce FADD/caspase-8-mediated apoptosis. FasL and TRAIL and their receptors are expressed in NEMO-deficient livers, with TRAIL-R being strongly upregulated, suggesting that they might be involved in mediating apoptosis in hepatocytes lacking NEMO (Figure 3a). To address the role of individual death receptors, but also potential functional redundancies, we used mice carrying loxP-flanked alleles of TNFR1, Fas and TRAIL-R to generate NEMOLPC-KO mice that lack each of these receptors alone or with combined deficiency of all three death receptors specifically in LPCs (the triple TNFR1, Fas, TRAIL-R LPC-specific knockout is denoted hereafter as 3DRLPC-KO for simplicity). Liver-specific deletion was confirmed by PCR genotyping (Figure 3b). As we could not detect TNFR1 or TRAIL-R expression in wild-type or NEMO-deficient hepatocytes using FACS or immunoblotting, we performed quantitative real-time PCR to confirm the deletion efficiency for these receptors in genomic DNA from total livers or from isolated hepatocytes. We found efficient deletion of both TNFR1 and TRAIL-R in isolated hepatocytes, whereas in the whole liver the deletion efficiency was lower due to the presence of non-parenchymal cells that are not targeted by the Alfp-Cre transgene (Figure 3c). Consistent with efficient ablation of TNFR1, hepatocytes from NEMOLPC-KO/3DRLPC-KO mice were protected from TNF-induced apoptosis in contrast to hepatocytes from NEMOLPC-KO mice (Figures 3d and e). Fas deletion efficiency was confirmed using FACS analysis of hepatocytes (Figure 3f), whereas deletion of NEMO was demonstrated by immunoblotting in hepatocyte lysates (Figure 3g). Collectively, these data demonstrate that Alfp-Cre expression efficiently deleted all four alleles in the livers of NEMOLPC-KO/3DRLPC-KO mice (Figure 3e).
Figure 3.
Validation and characterization of NEMOLPC-KO/3DRLPC-KO mice. (a) qRT-PCR analysis was performed using total liver RNA (n=5 mice per genotype, expression normalized to Tbp). (b) PCR genotyping on genomic DNA isolated from total liver or tail tissue. F, floxed allele; D, deleted allele. (c) Graph showing deletion efficiency of TNFR1 and TRAIL-R measured using quantitative PCR analysis of genomic DNA from total liver tissue or isolated hepatocytes. (d and e) Immunoblot analysis of liver lysates with the indicated antibodies (d) and immunostainings of liver sections with antibodies recognizing cleaved caspase-3 (e) from NEMOLPC-KO and NEMO-3DRLPC-KO mice 5 h after TNF or PBS administration. (f) FACS analysis of surface Fas expression in hepatocytes isolated from mice with the indicated genotypes. (g) Immunoblot analysis of hepatocyte protein extracts from NEMOLPC-KO/3DRLPC-KO and control showing deletion of NEMO in hepatocytes isolated from mice
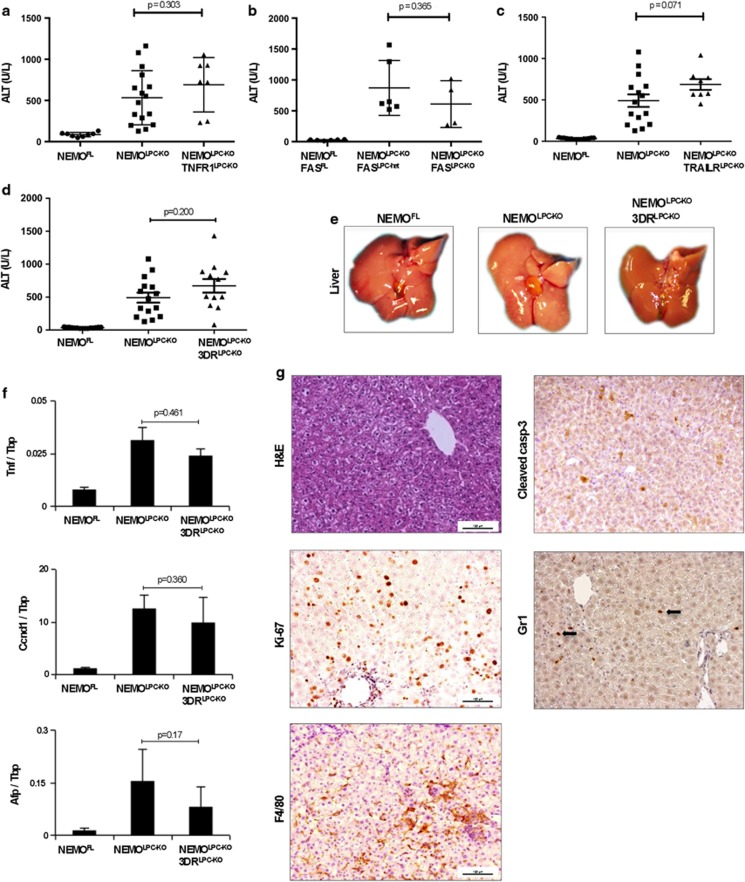
To assess whether TNFR1-, Fas- or TRAIL-R-mediated apoptosis induces the spontaneous death of NEMO-deficient hepatocytes, we measured serum ALT levels in 8-week-old NEMOLPC-KO/TNFR1LPC-KO, NEMOLPC-KO/TRAILRLPC-KO and NEMOLPC-KO/FASLPC-KO mice. These mice displayed elevated serum ALT levels that were comparable to those measured in NEMOLPC-KO mice at this age, demonstrating that LPC-specific ablation of TNFR1 or Fas or TRAIL-R failed to prevent spontaneous liver damage in NEMOLPC-KO mice (Figures 4a–c). To address potential functional redundancies between the three death receptors, we analysed NEMOLPC-KO/3DRLPC-KO mice for liver damage at the age of 8 weeks and found that they exhibited elevated serum ALT levels comparable to NEMOLPC-KO mice, demonstrating that even the lack of all three death receptors could not prevent spontaneous liver damage in NEMOLPC-KO mice (Figure 4d). Macroscopically, the livers of 8-week-old NEMOLPC-KO and NEMOLPC-KO/3DRLPC-KO mice looked indistinguishable (Figure 4e). Histological analysis of liver sections from 8-week-old mice confirmed that LPC-specific ablation of the three death receptors could not prevent spontaneous hepatocyte apoptosis in NEMOLPC-KO mice (Figure 4g and Supplementary Figure S2C). Moreover, Ki-67 staining of liver sections and quantification of Ki-67-positive cells revealed similarly increased hepatocyte proliferation in the livers of NEMOLPC-KO/3DRLPC-KO compared with NEMOLPC-KO mice (Figure 4g and Supplementary Figure S2D). Consistent with these findings, CyclinD1 mRNA expression was similarly increased in the liver of NEMOLPC-KO/3DRLPC-KO and NEMOLPC-KO mice (Figure 4f). Furthermore, immunostaining for macrophages and granulocytes revealed similar numbers and distribution of myeloid cells in the liver of NEMOLPC-KO/3DRLPC-KO and NEMOLPC-KO mice (Figure 4g). In addition, TNF mRNA expression did not differ significantly between NEMOLPC-KO and NEMO/3DRLPC-KO mice (Figure 4f). Expression of AFP, a molecular marker of fetal hepatoblasts and adult hepatic stem cells, was comparable between NEMOLPC-KO/3DRLPC-KO and NEMOLPC-KO mice (Figure 4f). However, NEMOLPC-KO/3DRLPC-KO mice showed reduced numbers of CK19-positive cells in the liver compared with NEMOLPC-KO suggesting that death receptor deficiency ameliorated oval cell expansion (Supplementary Figure S2). Collectively, these results demonstrated that LPC-specific ablation of the three death receptors, TNFR1, Fas and TRAIL-R, could not prevent spontaneous hepatocyte apoptosis, compensatory proliferation and inflammation in the liver of NEMOLPC-KO mice, showing that death receptors are not the inducers of hepatocyte apoptosis and hepatitis in these mice. These findings are surprising and show that death receptor-independent pathways induce spontaneous FADD-dependent apoptosis of NEMO-deficient hepatocytes.
Figure 4.
Individual or combined LPC-specific ablation of TNFR1, FAS and TRAIL-R does not prevent hepatocyte death or inflammation in NEMOLPC-KO mice. (a–d) Serum ALT levels in 8-week-old mice with the indicated genotypes. (e) Representative pictures of livers from 8-week-old mice with the indicated genotypes. (f) qRT-PCR analysis of mRNA isolated from total liver tissue of 8-week-old mice (n=5 mice per genotype, expression normalized to Tbp). (g) Liver sections from 8-week-old NEMOLPC-KO/3DRLPC-KO mice were stained with H&E or for cleaved caspase-3, Ki-67, F4/80, Lys6G/Gr1 (indicated by arrows)
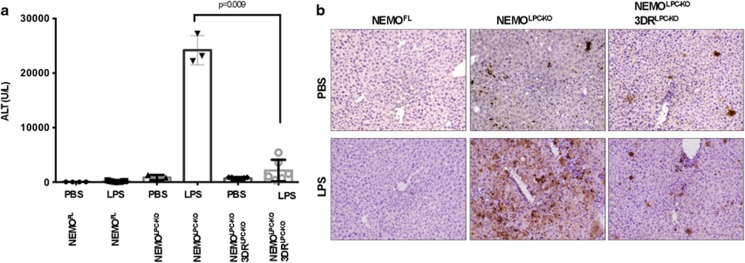
NEMO deficiency strongly sensitizes hepatocytes to death induced by LPS administration.15 Similarly to the spontaneous apoptosis also LPS-induced apoptosis of NEMO-deficient hepatocytes depends on FADD expression.15 Prompted by the surprising failure of the triple death receptor knockout to prevent spontaneous hepatocyte apoptosis in NEMOLPC-KO mice, we wanted to assess whether death receptor expression is required for LPS-induced liver damage in these mice. We therefore injected NEMOLPC-KO/3DRLPC-KO, NEMOLPC-KO and control mice with a sublethal dose of LPS and examined liver damage after 10 h by measurement of serum ALT levels and histological analysis of liver sections. As shown previously, LPS administration induced massive liver damage in NEMOLPC-KO mice, as illustrated by ALT levels above 20 000 U/l and strongly increased numbers of apoptotic hepatocytes (Figures 5a and b). Importantly, NEMOLPC-KO/3DRLPC-KO mice were protected from LPS-induced liver damage demonstrating that LPS-mediated apoptosis of NEMO-deficient hepatocytes is induced by death receptors, in contrast to the spontaneous hepatocyte death observed in NEMOLPC-KO mice that is death receptor-independent.
Figure 5.
NEMOLPC-KO/3DRLPC-KO mice are protected from LPS-mediated toxicity. (a) Serum ALT levels measured 10 h after LPS or PBS administration in mice with the indicated genotypes. (b) Representative images of liver sections from PBS- or LPS-injected mice with the indicated genotypes immunostained for cleaved caspase-3
TNFR1-, TRAIL-R- and FAS-independent liver carcinogenesis in NEMOLPC-KO mice
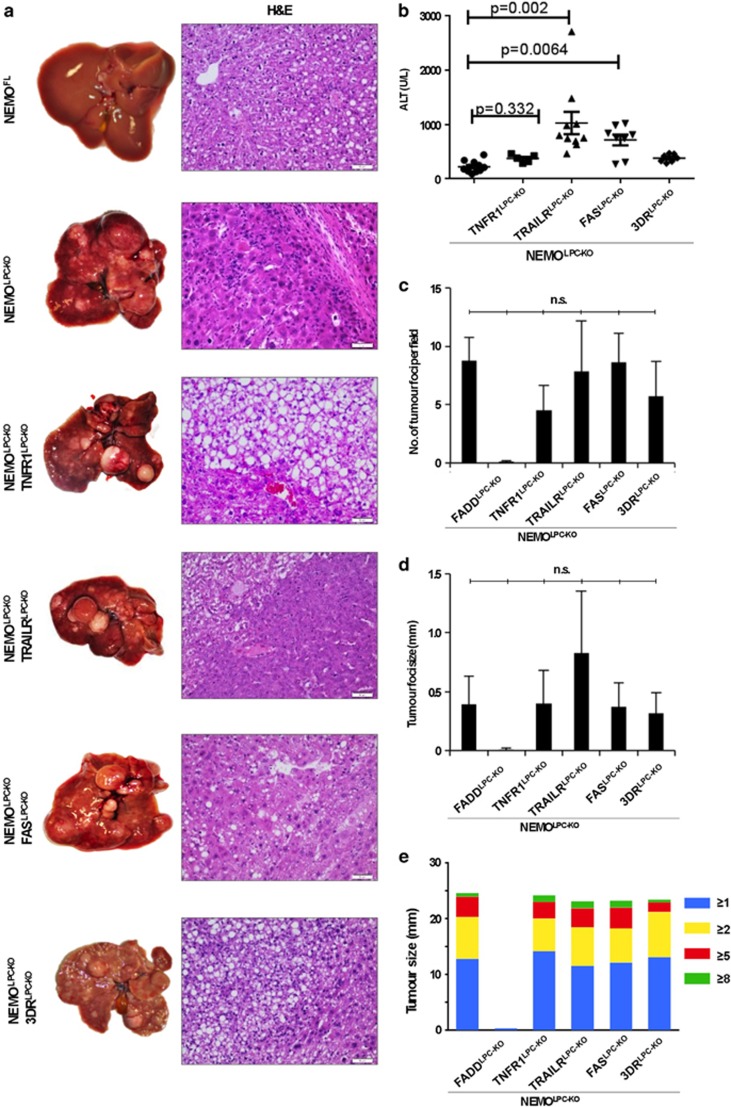
The results from the analysis of 8-week-old mice showed that TNFR1, TRAIL-R and Fas expression in LPCs is not required for the spontaneous death of hepatocytes and the development of hepatitis in NEMOLPC-KO mice. To assess whether death receptors are implicated in the development of HCC in this model, we examined the livers of NEMOLPC-KO/TNFR1LPC-KO, NEMOLPC-KO/TRAILRLPC-KO, NEMOLPC-KO/FASLPC-KO and NEMOLPC-KO/3DRLPC-KO mice at the age of 1 year. All mouse lines exhibited elevated serum ALT levels at the age of 1 year, with NEMOLPC-KO/TRAILRLPC-KO and NEMOLPC-KO/FASLPC-KO showing higher ALT levels compared with the NEMOLPC-KO mice (Figure 6b). Macroscopic and microscopic assessments of livers from mice at the age of 1 year revealed that NEMOLPC-KO/TNFR1LPC-KO, NEMOLPC-KO/TRAILRLPC-KO, NEMOLPC-KO/FASLPC-KO and NEMOLPC-KO/3DRLPC-KO mice developed HCC (Figure 6a). Macroscopic and microscopic quantifications did not reveal significant differences in the number or size of tumours found in the livers of NEMOLPC-KO/TNFR1LPC-KO, NEMOLPC-KO/TRAILRLPC-KO, NEMOLPC-KO/FASLPC-KO and NEMOLPC-KO/3DRLPC-KO mice compared with NEMOLPC-KO mice (Figures 6c–e and Supplementary Figure S1). Taken together, these results demonstrate that signalling by the three main death receptors, namely TNFR1, Fas and TRAIL-R, in LPCs is not required for spontaneous hepatocyte apoptosis, chronic hepatitis and HCC development in NEMOLPC-KO mice.
Figure 6.
Individual or combined LPC-specific ablation of TNFR1, FAS and TRAIL-R does not prevent HCC development in NEMOLPC-KO mice. (a) Macroscopic images of livers from 1-year-old mice with the indicated genotypes and corresponding H&E-stained images of liver sections. (b) Serum ALT values of 1-year-old mice. (c) Graph showing number of dysplastic/tumour foci per liver section in 1-year-old mice. (d) Graph showing size of tumour foci determined microscopically in liver sections. (e) Graph showing size of tumours in 1-year-old mice counted and classified into groups based on the analysis of macroscopic liver images
NK cells and T and B lymphocytes are not required for the spontaneous development of hepatitis in NEMOLPC-KO mice
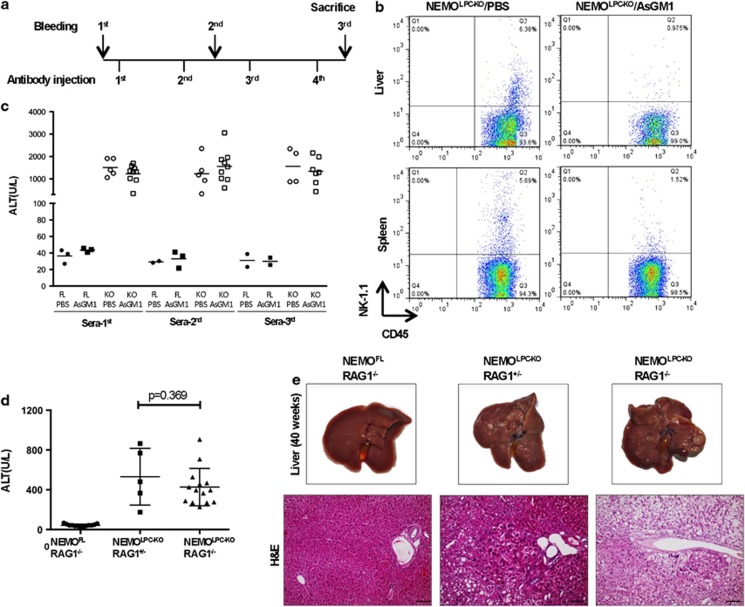
NK cells have been implicated in inducing liver damage in NEMOLPC-KO mice.24 To address the role of NK cells in the development of hepatitis in NEMOLPC-KO mice, we performed immunodepletion with anti-Asialo GM1 (α-ASGM1) antibodies in vivo (Figure 7a). As shown in Figure 7b, after four consecutive α-ASGM1 antibody injections NK cells were efficiently depleted from the spleen and liver. However, analysis of serum ALT levels and also immunohistological analysis of liver sections obtained from the mice at the end of the experiment revealed that NK cell depletion did not prevent apoptosis and compensatory proliferation of hepatocytes, oval cell activation and inflammation (Figure 7c and Supplementary Figure S3). Thus, NK cells are not required for spontaneous hepatitis development in NEMOLPC-KO mice.
Figure 7.
T, B and NK cells are dispensable for the induction of liver pathology in NEMOLPC-KO mice. (a) Experimental scheme for depleting NK cells in NEMOLPC-KO mice. (b) FACS plots of immune cells isolated from the liver and spleen of mice injected with PBS or anti-AsialoGM1 antibody. (c) Serum ALT levels measured at three different time points; before depletion, intermittently after two rounds of AsialoGM1 antibody administrations, and finally after killing. (d) Serum ALT levels measured in 8-week-old NEMOLPC-KO/RAG1−/− mice. (e) Representative macroscopic images and H&E-stained liver sections of 42-week-old mice with the indicated genotypes
Cells of the adaptive immune system and in particular T cells are implicated in inducing liver damage in different models of hepatitis but also in human patients. We therefore addressed the role of B and T lymphocytes in NEMOLPC-KO mice by crossing them to Rag1−/− mice. Analysis of serum ALT levels and immunohistological examination of livers from 8-week-old NEMOLPC-KO/Rag1−/−mice revealed that the absence of B and T cells did not protect NEMOLPC-KO mice from hepatocyte apoptosis and liver inflammation (Figure 7d). Moreover, macroscopic and histological examination of livers from 42-week-old animals revealed that NEMOLPC-KO/Rag1−/− mice developed liver tumours similarly to their NEMOLPC-KO/Rag1+/−littermates (Figure 7e). Therefore, B and T lymphocytes are dispensable for the development of hepatitis and liver tumours in NEMOLPC-KO mice.
Discussion
Signalling pathways activated downstream of death receptors, such as TNFR1, regulate cell death and inflammation and have been implicated in the pathogenesis of acute and chronic liver diseases and in liver carcinogenesis.25, 26 Studies in knockout mice lacking NF-κB signalling components revealed an essential role of NF-κB in protecting the fetal liver from TNF-induced cell death.21, 22 We therefore hypothesized that the spontaneous apoptosis of NEMO-deficient hepatocytes that results in chronic hepatitis and HCC development in NEMOLPC-KO mice is driven by death receptor signalling.27
Consistent with our earlier results showing that FADD deficiency could prevent spontaneous and LPS/TNF-induced apoptosis of NEMO-deficient hepatocytes,15 we show here that LPC-specific ablation of FADD also prevents HCC development in NEMOLPC-KO mice demonstrating that FADD-dependent hepatocyte apoptosis is essential for hepatocarcinogenesis in this model. In addition, LPC-specific knockout of caspase-8 could prevent hepatocyte apoptosis and HCC development in NEMOLPC-KO mice.28 However, whereas caspase-8 deficiency caused massive necrotic hepatocyte death in NEMOLPC-KO mice resulting in severe cholestatic liver disease,28 we found that FADD deficiency caused only very mild focal hepatocyte necrosis without any signs of biliary damage and cholestasis. This difference could reflect different functions of FADD and caspase-8 in regulating necrotic hepatocyte death, or alternatively a different health status of the mice considering that commensal or opportunistic microorganisms could be involved in triggering necrosis of hepatocytes.
On the basis of the well-established role of FADD and caspase-8 as mediators of death receptor-induced apoptosis, we were surprised that our extensive genetic studies failed to confirm an important role of death receptor signalling in triggering hepatocyte apoptosis, chronic hepatitis and HCC in NEMOLPC-KO mice. First, we found that conventional knockout of TNF or conditional LPC-specific individual ablation of TNFR1, Fas or TRAIL-R did not prevent liver damage and HCC in NEMOLPC-KO mice. Then, to address potential functional redundancies between the three death receptors we generated NEMOLPC-KO mice with combined LPC-specific deficiency of the three death receptors and found that even their simultaneous absence could not prevent spontaneous hepatocyte apoptosis, chronic hepatitis and HCC in NEMOLPC-KO mice. In contrast, triple death receptor deficiency could protect NEMOLPC-KO mice from LPS-induced liver injury, showing that different mechanisms trigger LPS-induced and spontaneous hepatocyte apoptosis in NEMOLPC-KO mice.
An earlier study suggested that NK cells acting via TRAIL-R mediate hepatocyte apoptosis in NEMOLPC-KO mice.24 Our results do not support a role for NK cells or TRAIL-R in mediating spontaneous hepatocyte apoptosis, hepatitis and HCC in NEMOLPC-KO mice. Consistent with our results using LPC-specific TRAIL-R knockout, a recent study showed that systemic TRAIL-R deficiency could not prevent hepatocyte apoptosis, hepatitis and HCC in NEMOLPC-KO mice.29 In the same study, systemic TNFR1 deficiency could significantly protect NEMOLPC-KO mice from hepatocyte apoptosis and compensatory proliferation, liver inflammation and fibrosis, and also inhibited liver tumour progression. It is therefore surprising that LPC-specific knockout of TNFR1, and even in combination with Fas and TRAIL-R, failed to protect NEMOLPC-KO mice from hepatocyte death, hepatitis and HCC development. Considering that haematopoietic chimera experiments suggested that TNFR1 deficiency in radioresistant cells prevents hepatitis and HCC in NEMOLPC-KO mice,29 one possibility is that TNFR1 acts in liver stromal cells that are not targeted by the Alfp-Cre transgene, such as stellate cells or endothelial cells to mediate hepatocyte apoptosis and liver tumour progression. However, in that case TNFR1 signalling must be driven by a ligand other than TNF as TNF deficiency failed to prevent or ameliorate liver damage and HCC development in NEMOLPC-KO mice.
In conclusion, our experiments demonstrated that TNFR1, Fas and TRAIL-R signalling in LPCs is dispensable for spontaneous hepatocyte apoptosis, chronic hepatitis and HCC development in NEMOLPC-KO mice. These findings are surprising and raise questions as to which upstream pathway(s) trigger(s) FADD/caspase-8-dependent apoptosis of NEMO-deficient hepatocytes. In addition to TNFR1, Fas and TRAIL-R, DR3 is another death receptor capable of inducing NF-κB activation and FADD/caspase-8-dependent apoptosis upon stimulation with its ligand TL1A.16, 30 However, DR3 is mainly expressed in T cells and has not been reported to function in hepatocytes, and we did not detect upregulation of its expression in the NEMO-deficient liver suggesting that DR3 is not involved in driving hepatocyte apoptosis in NEMOLPC-KO mice. Together, our results suggest that as yet unidentified death receptor, NK cell and B- and T-cell-independent mechanisms trigger the spontaneous FADD/caspase-8-mediated apoptosis of NEMO-deficient hepatocytes. Further studies addressing how NEMO controls the activation of FADD/caspase-8-mediated apoptosis will be required to shed light on the mechanisms triggering spontaneous hepatitis and cancer in NEMOLPC-KO mice and may be relevant for the pathogenesis of human chronic liver diseases and the development of HCC.
Materials and methods
Generation and handling of mice
The following mouse lines were used in this study: AlfpCre,31 NEMO floxed,10 FADD floxed,32 Tnf−/−,23 TNFR1 floxed,33 TRAIL-R floxed,34 Fas floxed35 and Rag1−/−.36 All mice were maintained in C57BL/6 background. Animals were bred at the animal facility of the Institute for Genetics, University of Cologne, Germany. All animal procedures were conducted in accordance with European, national and institutional guidelines and protocols and were approved by local government authorities (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, Germany).
Quantitative real-time polymerase chain reaction (q-RT PCR)
Isolation of total RNA and cDNA synthesis were performed as previously described.9 The gene-specific Taq Man assays were performed. mRNA expression was normalized to the expression of the housekeeping gene TBP. For the determination of deletion efficiency, genomic DNA both from isolated hepatocytes or whole-liver tissue samples was used to perform a SYBR Green q-RT PCR. Deletion efficiency was calculated as the ratio of copies of the deleted product to that of the control PCR product.
Serum levels of alanine amino transferase
About 100 μl of blood was collected either from the submandibular vein or from the caval vein during the final killing. ALT was measured using standard assays in a Roche-Cobas C111 biochemical analyser.
Hepatocyte and immune cell isolation from liver and spleen and FACS analysis
Primary hepatocytes were isolated by perfusing the liver via the caval vein with EBSS+100 mM EGTA followed by collagenase solution (EBSS+15 mg Collagenase D+2 mg Trypsin inhibitor) at 37 degrees. The liver was gently scraped in DMEM+1% FCS, filtered through 70-μm nylon filter, mixed with equal volume of 80% Percoll/HBSS solution and spun at 200 × g for 7 min. Hepatocytes settled at the bottom were washed with PBS.
To determine the NK cell depletion efficiency using the anti-AsialoGM1 antibody, the liver and spleen immune cell populations were isolated and analysed using FACS. To isolate the immune cells from the spleen and liver, the tissues were mechanically dissociated in D-PBS using a syringe plunger, passed through a 70-μm cell strainer, and centrifuged at 1500 r.p.m. for 5 min. Liver immune cells were separated from hepatocytes by Percoll gradient centrifugation at 800 × g for 20 min. Erythrocytes were lysed in 0.15 M NH4Cl, washed three times in D-PBS and diluted in Stain Buffer (FBS; BD, San Diego, CA, USA; 554656). The cells were pre-incubated with rat anti-mouse CD16/CD32 (Mouse Fc Block; BD, 553141) antibody for 10 min, and then incubated with CD45 (BD, 553081) and NK1.1 (BD, 550627) antibodies for 30 min in the dark. After two washes, the cells were resuspended in PBS and analysed using a BD FACScalibur (BD Biosciences, San Jose, CA, USA), whereas the data were analysed using Windows Multiple Document Interface (WinMDI) 2.9 for Flow Cytometry. Surface expression of Fas in hepatocytes was analysed by FACS using PE-labelled hamster anti-mouse Fas antibody (BD Pharmingen, San Diego, CA, USA; cat. no. 554258).
LPS and TNF responses
Age- and sex-matched animals between 12- and 15-week old were injected intraperitoneally with 2.5 μg of LPS (From Escherichia coli 0111:B4, Sigma-Aldrich, Munich, Germany; L2630) or 10 ng recombinant murine TNF per gram of body weight. Animals were killed 10 h after LPS and 5 h after TNF administration, and blood and liver were collected for analysis.
Analysis of livers and immunohistochemistry
Livers were assessed macroscopically and photographed. In addition, tumour size (diameter), architecture and histology were determined using 5-μm-thick sections of formalin-fixed (O/N), paraffin-embedded liver tissues stained with haematoxylin and eosin (H&E). Fibrosis was determined with Masson's Trichrome staining. Immunohistochemical staining of sections was performed after antigen retrieval in Na-Citrate buffer with 0.005% tween and boiling in a pressure cooker for 20 min. Antibodies used were active caspase-3 (R&D Systems, Minneapolis, MN, USA; clone AF835), Ki-67 (Dako Cytomation, Glostrup, Denmark; M724901), F4/80 (home-made), Ly6G/Gr1 (BD, 551459) and CK19 (Developmental Studies Hybridoma Bank, Iowa City, IA, USA; TROMA-III). Biotinylated secondary antibodies, Avidin/Biotin blocking kit (Vector Labs, Burlingame, CA, USA; SP-2001), HRP-conjugated biotin (ABC Elite Kit, Vector Labs, PK6100) and DAB substrate (Dako Cytomation; K3466) were used in all stainings. Immunostainings for cleaved Caspase-3 in livers from TNF-injected mice were performed on cryosections (Tissue-Tek, Sakura Finetek, Torrance, CA, USA; cat. no. 4583).
Immunoblotting
Tissue lysates were prepared by homogenizing liver tissue in buffer (150 mM NaCl, 1% NP-40, 0.1% SDS in a 50 mM Tris buffer at pH 7.5 including the Protease inhibitor tablets (complete, Roche, Mannheim, Germany, 05892970001) and phosphatase inhibitors (PhosSTOP Roche, 04906837001). Immunodetection was performed using ECL reagent from GE Healthcare, Buckinghamshire, UK; RPN 2106). Antibodies used were as follows: anti-NEMO (homemade),15 anti-cleaved caspase-3 (Cell Signalling, Danvers, MA, USA – 9661) and Actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA; sc-1616).
Acknowledgments
MP acknowledges funding from the ERC (2012-ADG_20120314), the DFG (SFB670, SFB829, SPP1656), the European Commission (FP7 grants 223404 (Masterswitch) and 223151 (InflaCare)), the Deutsche Krebshilfe (Grant 110302), the Else Kröner-Fresenius-Stiftung and the Helmholtz Alliance Preclinical Comprehensive Cancer Center. VK was supported by a Marie Curie Career Development Fellowship (FP7-PEOPLE-2010-IEF; Proposal No.: 275767).
Glossary
- NEMO
NF-kappa B essential modulator
- TNF
tumour necrosis factor
- FADD
Fas-associated with death domain
- Trail
TNF-related apoptosis-inducing ligand
- LPS
Lipopolysaccharide
- LPC
liver parenchymal cells
- DR5
death receptor 5
- ALT
alanine aminotransferase
- RIP
receptor interacting protein
- Ifng
interferon gamma
- Ccnd1
cyclin D1
- CK-19
cytokeratin 19
- TBP
TATA-binding protein
- EBSS
Eagle's balanced salt solution
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by V Dixit
Supplementary Material
References
- Shariff MI, Cox IJ, Gomaa AI, Khan SA, Gedroyc W, Taylor-Robinson SD. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Exp Rev Gastroenterol Hepatol. 2009;3:353–367. doi: 10.1586/egh.09.35. [DOI] [PubMed] [Google Scholar]
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56:704–713. doi: 10.1016/j.jhep.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedde T, Schwabe RF. NF-kappaB in the liver—linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Karin M. NF-kappaB signaling, liver disease and hepatoprotective agents. Oncogene. 2008;27:6228–6244. doi: 10.1038/onc.2008.300. [DOI] [PubMed] [Google Scholar]
- Luedde T, Heinrichsdorff J, de Lorenzi R, De Vos R, Roskams T, Pasparakis M. IKK1 and IKK2 cooperate to maintain bile duct integrity in the liver. Proc Natl Acad Sci USA. 2008;105:9733–9738. doi: 10.1073/pnas.0800198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris C, Godfrey VL, Krahn-Senftleben G, Takahashi T, Roberts JL, Schwarz T, et al. Female mice heterozygous for IKK gamma/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol Cell. 2000;5:969–979. doi: 10.1016/s1097-2765(00)80262-2. [DOI] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Bloch W, Courtois G, Addicks K, Israel A, Rajewsky K, et al. NEMO/IKK gamma-deficient mice model incontinentia pigmenti. Mol Cell. 2000;5:981–992. doi: 10.1016/s1097-2765(00)80263-4. [DOI] [PubMed] [Google Scholar]
- Rudolph D, Yeh W, Wakeham A, Rudolph B, Nallainathan D, Potter J, et al. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev. 2000;14:854–862. [PMC free article] [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16:295–308. doi: 10.1016/j.ccr.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Liedtke C, Bangen JM, Freimuth J, Beraza N, Lambertz D, Cubero FJ, et al. Loss of caspase-8 protects mice against inflammation-related hepatocarcinogenesis but induces non-apoptotic liver injury. Gastroenterology. 2011;141:2176–2187. doi: 10.1053/j.gastro.2011.08.037. [DOI] [PubMed] [Google Scholar]
- Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, Bloch W, et al. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity. 2011;35:572–582. doi: 10.1016/j.immuni.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Marino M, Takahashi T, Yoshida T, Sakakura T, Old L, et al. Absence of tumor necrosis factor rescues RelA-deficient mice from embryonic lethality. Proc Natl Acad Sci USA. 1999;96:2994–2999. doi: 10.1073/pnas.96.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraza N, Malato Y, Sander L, Al-Masaoudi M, Freimuth J, Riethmacher D, et al. Hepatocyte-specific NEMO deletion promotes NK/NKT cell- and TRAIL-dependent liver damage. J Exp Med. 2009;206:1727–1737. doi: 10.1084/jem.20082152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 2014;5:e996. doi: 10.1038/cddis.2013.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattenberg JM, Schuchmann M, Galle PR. Cell death and hepatocarcinogenesis: Dysregulation of apoptosis signaling pathways. J Gastroenterol Hepatol. 2011;26 (Suppl 1:213–219. doi: 10.1111/j.1440-1746.2010.06582.x. [DOI] [PubMed] [Google Scholar]
- Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- Liedtke C, Bangen J-M, Freimuth J, Beraza N, Lambertz D, Cubero F, et al. Loss of caspase-8 protects mice against inflammation-related hepatocarcinogenesis but induces non-apoptotic liver injury. Gastroenterology. 2011;141:2176–2187. doi: 10.1053/j.gastro.2011.08.037. [DOI] [PubMed] [Google Scholar]
- Cubero F, Singh A, Borkham-Kamphorst E, Nevzorova Y, Al Masaoudi M, Haas U, et al. TNFR1 determines progression of chronic liver injury in the IKKγ/Nemo genetic model. Cell Death Differ. 2013;20:1580–1592. doi: 10.1038/cdd.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan F, Richard AC, RM. Siegel. TL1A and DR3, a TNF family ligand-receptor pair that promotes lymphocyte costimulation, mucosal hyperplasia, and autoimmune inflammation. Immunol Rev. 2011;244:188–196. doi: 10.1111/j.1600-065X.2011.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C, Opherk C, Anlag K, Schütz G, Tronche F. Hepatocyte-specific expression of Cre recombinase. Genesis. 2000;26:151–153. doi: 10.1002/(sici)1526-968x(200002)26:2<151::aid-gene17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Mc Guire C, Volckaert T, Wolke U, Sze M, de Rycke R, Waisman A, et al. Oligodendrocyte-specific FADD deletion protects mice from autoimmune-mediated demyelination. J Immunol. 2010;185:7646–7653. doi: 10.4049/jimmunol.1000930. [DOI] [PubMed] [Google Scholar]
- Van Hauwermeiren F, Armaka M, Karagianni N, Kranidioti K, Vandenbroucke R, Loges S, et al. Safe TNF-based antitumor therapy following p55TNFR reduction in intestinal epithelium. J Clin Invest. 2013;123:2590–2603. doi: 10.1172/JCI65624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse-Wilde A, Voloshanenko O, Bailey SL, Longton GM, Schaefer U, Csernok AI, et al. TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development. J Clin Invest. 2008;118:100–110. doi: 10.1172/JCI33061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Hampel B, Yagita H, Rajewsky K. T cell-specific ablation of Fas leads to Fas ligand-mediated lymphocyte depletion and inflammatory pulmonary fibrosis. J Exp Med. 2004;199:1355–1365. doi: 10.1084/jem.20032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.