Abstract
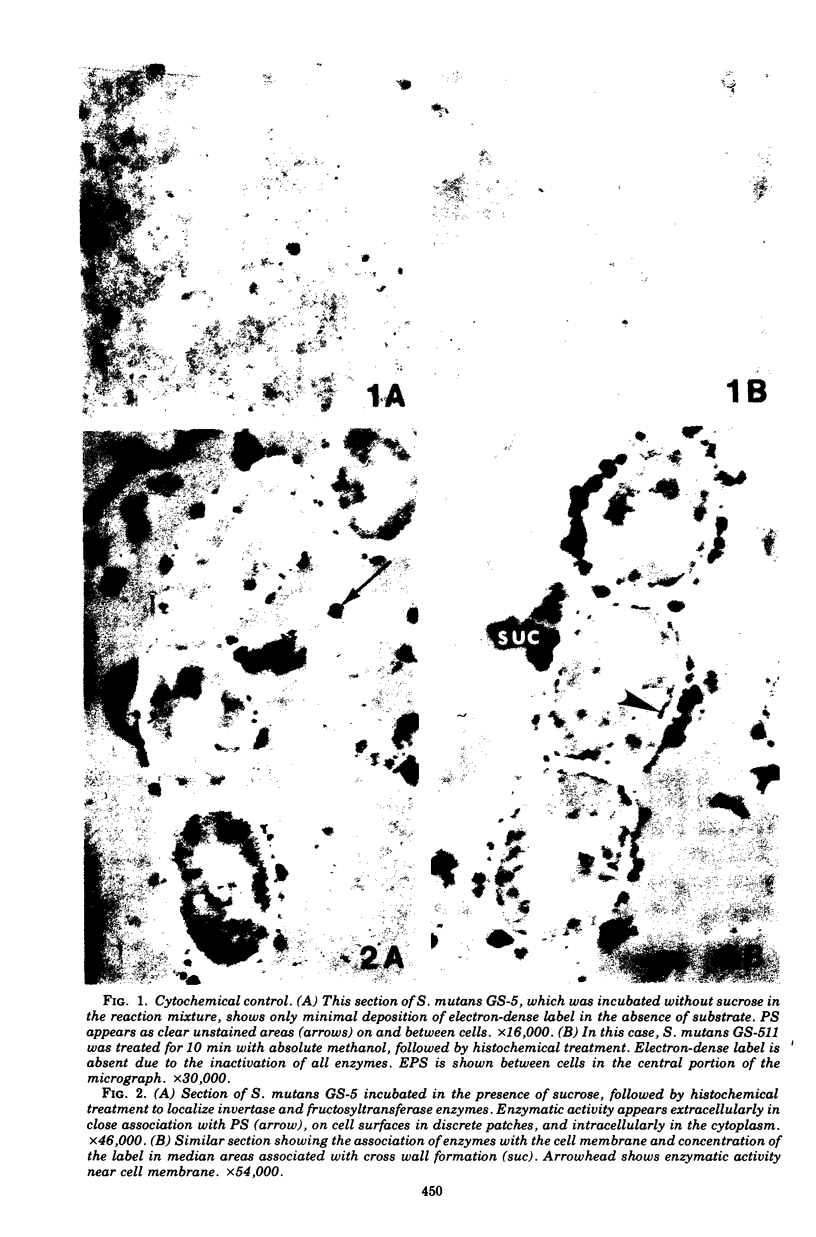
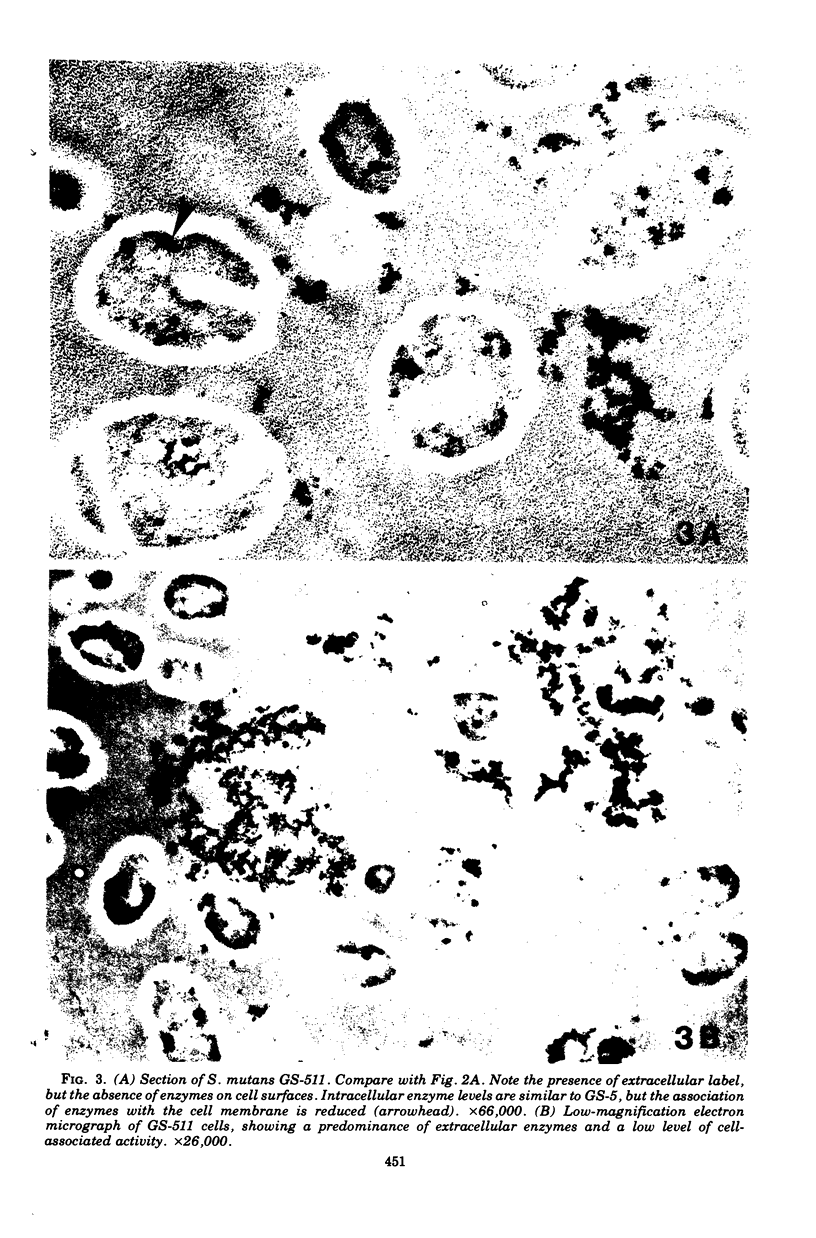
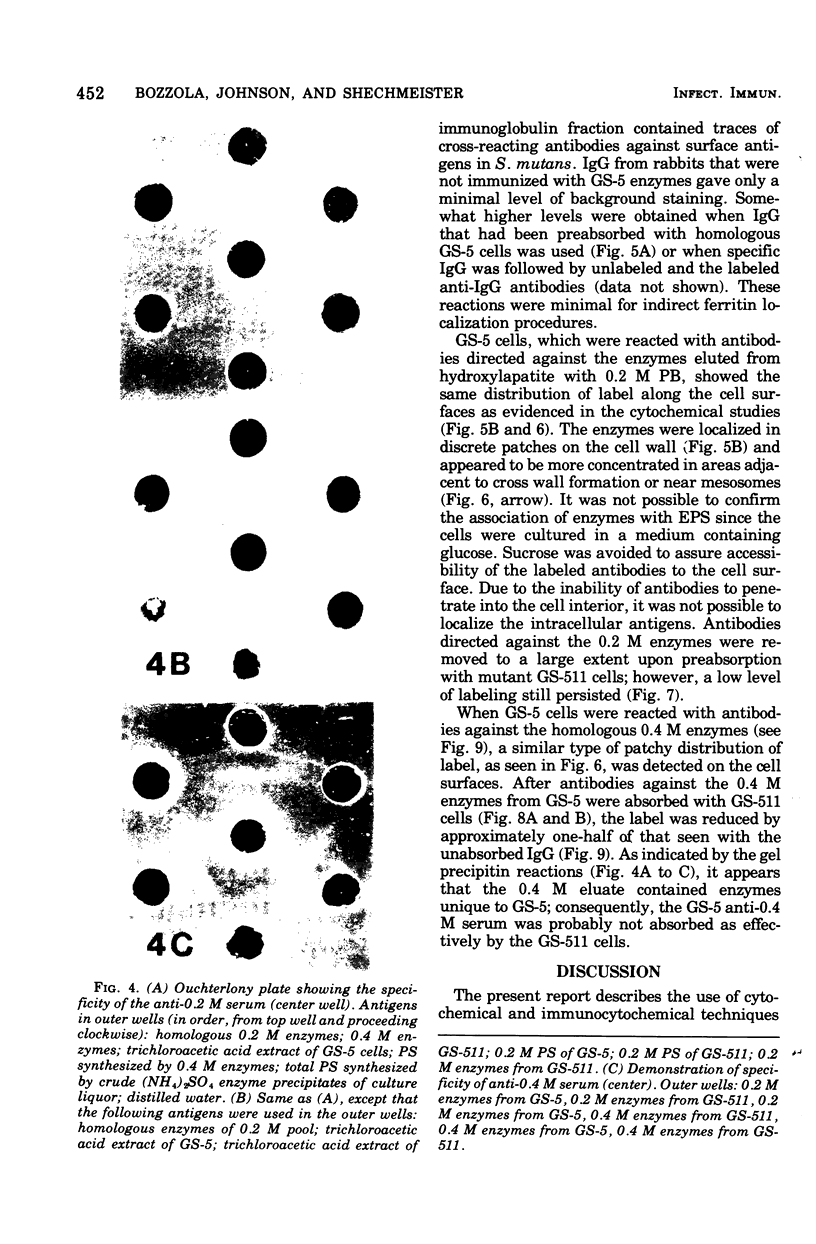
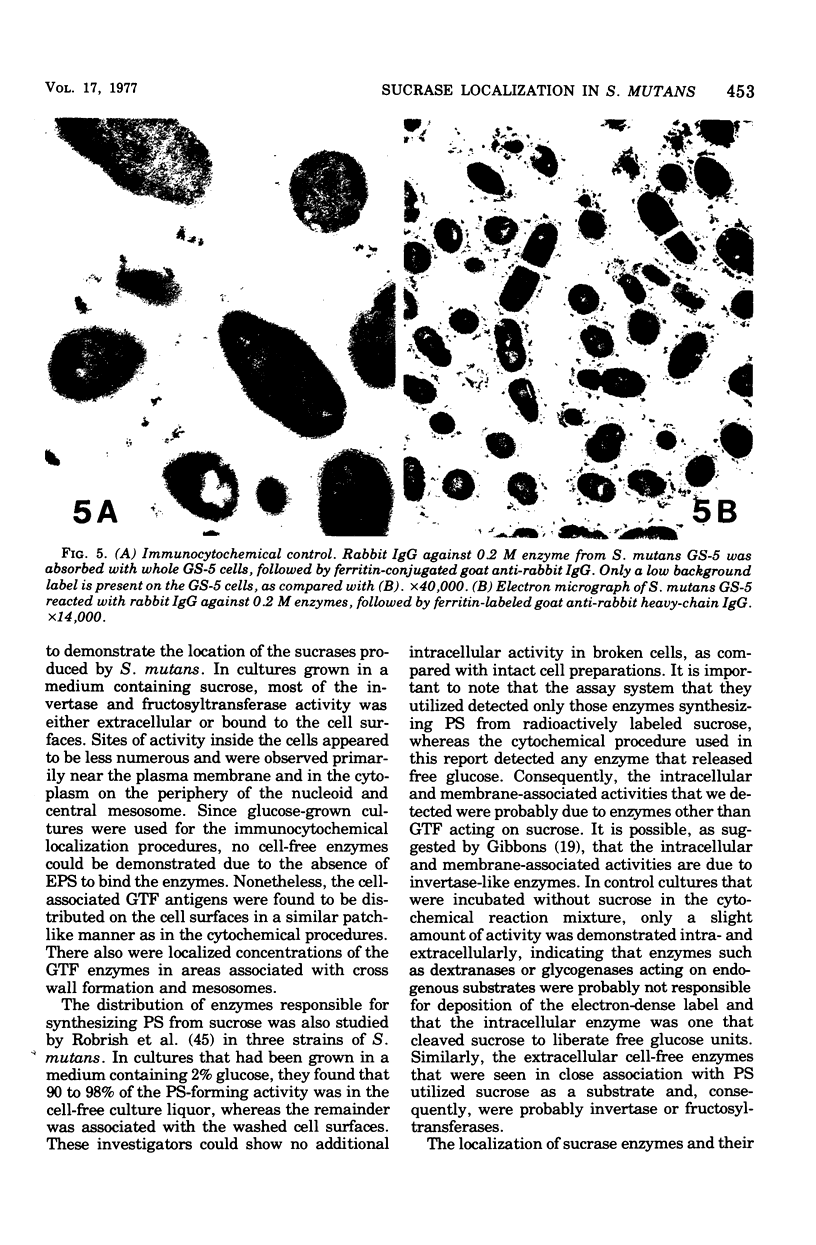
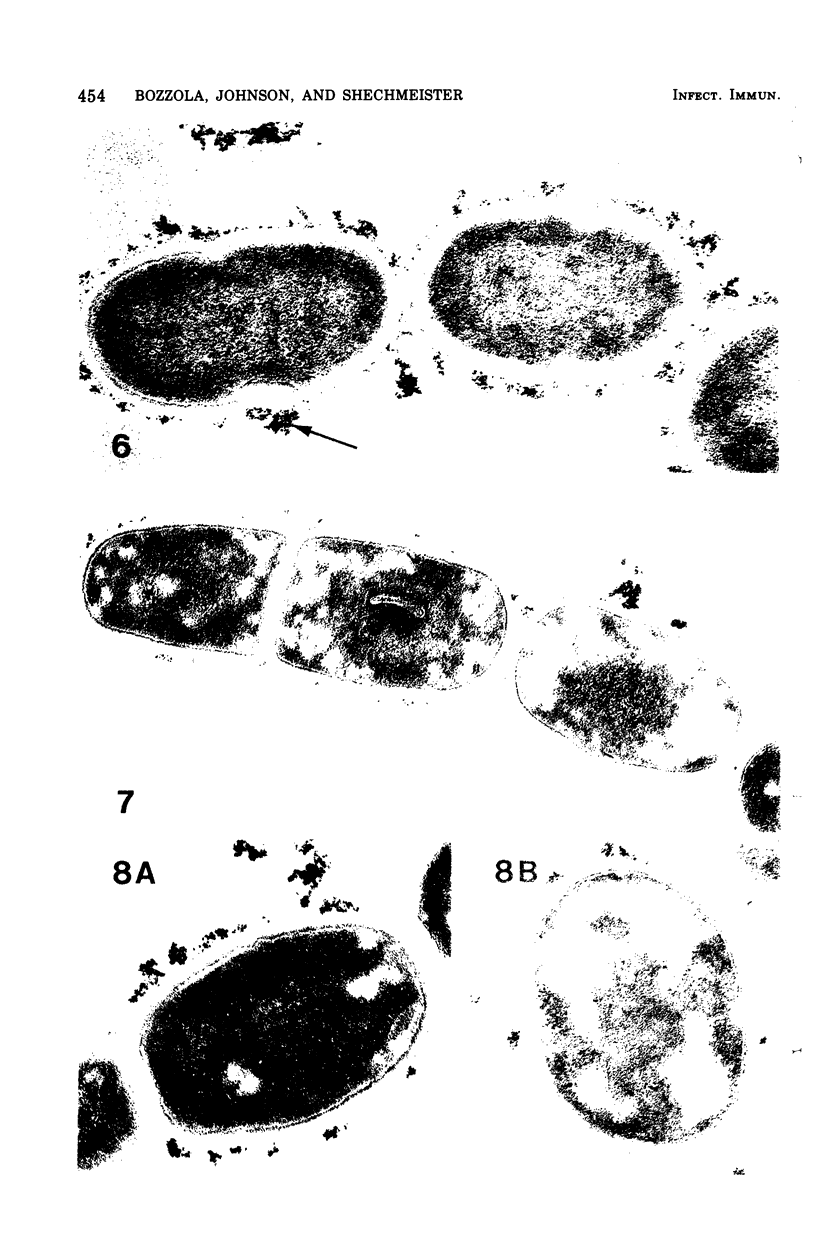
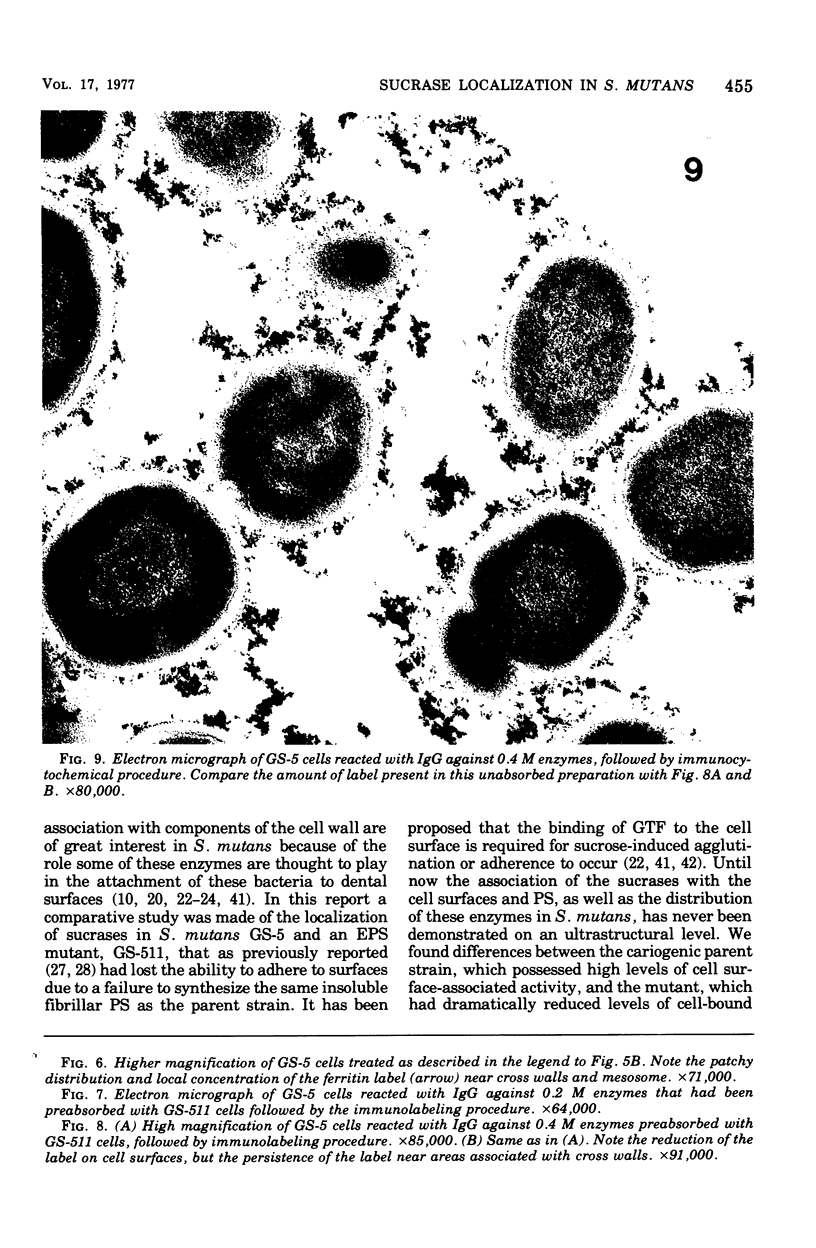
Electron microscopy and cytochemical and immunocytochemical procedures were used to study the ultrastructural distribution of sucrase enzymes in two strains of Streptococcus mutans. In a strongly adherent and virulent parent strain, GS-5, most of the invertase and fructosyltransferase activities were demonstrated extracellularly or bound to the cell surfaces. Intracellularly, enzymatic sites were detected near the plasma membrane on the periphery of the nucleoid and central mesosome. In GS-511, a mutant of diminished virulence and adherence, most of the enzymatic activity was not located on the cell surfaces, but was found away from the cell walls and associated with extracellular polysaccharides. Intracellularly, GS-511 manifested the same distribution of invertase and fructosyltransferase as did GS-5; however, the close association of these enzymes with the plasma membrane was not shown in GS-511. In both strains, extracellular areas near regions associated with cross wall formation appeared to show localized concentrations of these sucrases. Antibodies against partially purified glucosyltransferase (GTF) enzymes from GS-5 were used to localize GTF by immunocytochemical techniques. Indirect ferritin localization procedures showed that the extracellular and cell-bound GTF enzymes were distributed in similar locations as the fructosyltransferase and invertase enzymes. By absorption of the antiserum with whole GS-511 cells, the location of extracellular GTF and surface antigens unique to GS-5 was demonstrated. The dramatically reduced levels of cell-bound sucrase activity in GS-511 indicates the significant role of these enzymes in adherence and cariogenicity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowen W. H., Cohen B., Cole M. F., Colman G. Immunization against dental caries. Br Dent J. 1975 Jul 15;139(2):45–58. [PubMed] [Google Scholar]
- Bowen W. H. The induction of rampant dental caries in monkeys (Macaca irus). Caries Res. 1969;3(3):227–237. doi: 10.1159/000259597. [DOI] [PubMed] [Google Scholar]
- Bozzola J. J., Johnson M. C., Shechmeister I. L. In situ multiple sampling of attached bacteria for scanning and transmission electron microscopy. Stain Technol. 1973 Nov;48(6):317–325. doi: 10.3109/10520297309116648. [DOI] [PubMed] [Google Scholar]
- Burckhardt J. J., Guggenheim B. Interactions of antisera, sera, and oral fluid with glucosyltransferases. Infect Immun. 1976 Apr;13(4):1009–1022. doi: 10.1128/iai.13.4.1009-1022.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Newbrun E., Krasse B. Purification and properties of dextransucrase from Streptococcus sanguis. Arch Oral Biol. 1969 May;14(5):469–478. doi: 10.1016/0003-9969(69)90140-x. [DOI] [PubMed] [Google Scholar]
- Charlton G., Fitzgerald D. B., Keyes P. H. Hydrogen ion activity in dental plaques of hamsters during metabolism of sucrose, glucose and fructose. Arch Oral Biol. 1971 Jun;16(6):655–661. doi: 10.1016/0003-9969(71)90069-0. [DOI] [PubMed] [Google Scholar]
- Charlton G., Fitzgerald R. J., Keyes P. H. Determination of saliva and dental plaque pH in hamsters with glass micro-electrodes. Arch Oral Biol. 1971 Jun;16(6):649–654. doi: 10.1016/0003-9969(71)90068-9. [DOI] [PubMed] [Google Scholar]
- Chludzinski A. M., Germaine G. R., Schachtele C. F. Purification and properties of dextransucrase from Streptococcus mutans. J Bacteriol. 1974 Apr;118(1):1–7. doi: 10.1128/jb.118.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley P., Wood J. M., Saxton C. A., Leach S. A. The polymerisation of dietary sugars by dental plaque. Caries Res. 1967;1(2):112–129. doi: 10.1159/000259506. [DOI] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer Dirks O. The relationship between extracellular polysaccharide-producing streptococci and smooth surface caries in 13-year-old children. Caries Res. 1969;3(2):190–199. doi: 10.1159/000259582. [DOI] [PubMed] [Google Scholar]
- Duany L. F., Zinner D. D., Landy J. J. Bone loss and caries in rats infected with human streptococci. J Dent Res. 1971 Mar-Apr;50(2):460–465. doi: 10.1177/00220345710500025801. [DOI] [PubMed] [Google Scholar]
- Edwardsson S. Characteristics of caries-inducing human streptococci resembling Streptococcus mutans. Arch Oral Biol. 1968 Jun;13(6):637–646. doi: 10.1016/0003-9969(68)90142-8. [DOI] [PubMed] [Google Scholar]
- Evans R. T., Genco R. J. Inhibition of glucosyltransferase activity by antisera to known serotypes of Streptococcus mutans. Infect Immun. 1973 Feb;7(2):237–241. doi: 10.1128/iai.7.2.237-241.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerlad R. J. Dental caries research in gnotobiotic animals. Caries Res. 1968;2(2):139–146. doi: 10.1159/000259552. [DOI] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Acceleration of dextransucrase activity of Streptococcus mutans by secretory immunoglobulin A. J Bacteriol. 1974 Jun;118(3):805–809. doi: 10.1128/jb.118.3.805-809.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Purification and properties of dextransucrase and invertase from Streptococcus mutans. J Bacteriol. 1974 Jun;118(3):796–804. doi: 10.1128/jb.118.3.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. B. Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol. 1967 Jan;12(1):11–23. doi: 10.1016/0003-9969(67)90137-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Berman K. S., Knoettner P., Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966 Jun;11(6):549–560. doi: 10.1016/0003-9969(66)90220-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Presence of an invertase-like enzyme and a sucrose permeation system in strains of Streptococcus mutans. Caries Res. 1972;6(2):122–131. doi: 10.1159/000259784. [DOI] [PubMed] [Google Scholar]
- Guggenheim B. Extracellular polysaccharides and microbial plaque. Int Dent J. 1970 Dec;20(4):657–678. [PubMed] [Google Scholar]
- Guggenheim B., Newbrun E. Extracellular glucosyltransferase activity of an HS strain of Streptococcus mutans. Helv Odontol Acta. 1969 Oct;13(2):84–97. [PubMed] [Google Scholar]
- Johnson M. C., Bozzola J. J., Shechmeister I. L. Morphological study of Streptococcus mutans and two extracellular polysaccharide mutants. J Bacteriol. 1974 Apr;118(1):304–311. doi: 10.1128/jb.118.1.304-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. C., Bozzola J. J., Shechmeister I. L., Shklair I. L. Biochemical study of the relationship of extracellular glucan to adherence and cariogenicity in Streptococcus mutans and an extracellular polysaccharide mutant. J Bacteriol. 1977 Jan;129(1):351–357. doi: 10.1128/jb.129.1.351-357.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes P. H. Research in dental caries. J Am Dent Assoc. 1968 Jun;76(6):1357–1373. doi: 10.14219/jada.archive.1968.0186. [DOI] [PubMed] [Google Scholar]
- Kraehenbuhl J. P., Jamieson J. D. Solid-phase conjugation of ferritin to Fab-fragments of immunoglobulin G for use in antigen localization on thin sections. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1771–1775. doi: 10.1073/pnas.69.7.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasse B. Human streptococci and experimental caries in hamsters. Arch Oral Biol. 1966 Apr;11(4):429–436. doi: 10.1016/0003-9969(66)90107-5. [DOI] [PubMed] [Google Scholar]
- Krasse B., Jordan H. V., Edwardsson S., Svensson I., Trell L. The occurrence of certain "caries-inducing" streptococci in human dental plaque material with special reference to frequency and activity of caries. Arch Oral Biol. 1968 Aug;13(8):911–918. doi: 10.1016/0003-9969(68)90006-x. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K., Ingersoll L. Differential inhibition of Streptococcus mutans in vitro adherence by anti-glucosyltransferase antibodies. Infect Immun. 1976 Jun;13(6):1775–1777. doi: 10.1128/iai.13.6.1775-1777.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Properties of a mutant of Streptococcus mutans altered in glucosyltransferase activity. Infect Immun. 1976 Feb;13(2):345–353. doi: 10.1128/iai.13.2.345-353.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. II. Nature of the binding site and the adsorption of dextran-levan synthetase enzymes on the cell-wall surface of the streptococcus. Infect Immun. 1974 Feb;9(2):419–429. doi: 10.1128/iai.9.2.419-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of the Adherence of Streptococcus mutans to Smooth Surfaces III. Purification and Properties of the Enzyme Complex Responsible for Adherence. Infect Immun. 1974 Nov;10(5):1135–1145. doi: 10.1128/iai.10.5.1135-1145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- Robrish S. A., Reid W., Krichevsky M. I. Distribution of enzymes forming polysaccharide from sucrose and the composition of extracellular polysaccharide synthesized by Streptococcus mutans. Appl Microbiol. 1972 Aug;24(2):184–190. doi: 10.1128/am.24.2.184-190.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherp H. W. Dental caries: prospects for prevention. Science. 1971 Sep 24;173(4003):1199–1205. doi: 10.1126/science.173.4003.1199. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINNER D. D., JABLON J. M., ARAN A. P., SASLAW M. S. EXPERIMENTAL CARIES INDUCED IN ANIMALS BY STREPTOCOCCI OF HUMAN ORIGIN. Proc Soc Exp Biol Med. 1965 Mar;118:766–770. doi: 10.3181/00379727-118-29964. [DOI] [PubMed] [Google Scholar]