Abstract
OBJECTIVE
To determine whether specific macrophage immune functions of the newly born are insensitive to the actions of therapeutic levels of dexamethasone (DEX), previously measured in infants with bronchopulmonary dysplasia (BPD), compared to betamethasone (BETA) and exogenous or endogenous interleukin-10 (IL-10).
STUDY DESIGN
Macrophages were differentiated from cord blood monocytes (N=18). A serial dose response (around 10−8M), in vitro study was used to examine the effect of DEX, BETA and IL-10, on pro-inflammatory (PI) cytokine release, phagocytosis and respiratory burst.
RESULTS
Exogenous IL-10 (10−8M) significantly (p<0.05) inhibited the endotoxin-stimulated release of IL-6, IL-8 and tumor necrosis factor by 63% to 82% with no significant effect by DEX and BETA. There was no inhibition by these 3 agents at 10−8M on phagocytosis and respiratory burst. Inhibition of endogenous IL-10 with a monoclonal antibody significantly raised endotoxin-stimulated cytokine release by at least 4 fold.
CONCLUSION
Macrophages were relatively insensitive to therapeutic levels of DEX and BETA with regard to PI cytokine release. This study provides rationale for translational, preclinical research using airway instillation of IL-10 for the treatment of BPD.
Keywords: dexamethasone, betamethasone, bronchopulmonary dysplasia, inflammation, cytokines, phagocytosis, respiratory burst
INTRODUCTION
Bronchopulmonary dysplasia (BPD) is one of the most important causes of morbidity and mortality from neonatal intensive care units 1. Persistent lung inflammation, with an imbalance of pro-inflammatory versus anti-inflammatory mediators, plays a central role in the pathogenesis of BPD along with an arrest of lung growth 2, 3, 4, 5. Late anti-inflammatory therapy with dexamethasone (DEX) has been the mainstay of therapy for neonates that are at high risk for severe BPD and/or death 6. The optimal dose of postnatal dexamethasone that reduces the incidence of BPD without increasing neurodevelopmental injury is not known but appears to occur when higher cumulative doses of dexamethasone are administered 7. DEX treatment for BPD is only partially effective and may be associated with potential serious short and long term side effects, therefore alternative therapies are being actively investigated 8, 9.
During the early evolution of BPD, there are abnormally high levels of polymorphonuclear leukocytes followed by monocytes recruited into the lung 10, 11, 12, 13. Circulating monocytes are the principal precursor of alveolar macrophages 14. By one to two weeks after birth, the principal inflammatory cells in the airway fluid of babies with evolving BPD are macrophages associated high levels of pro-inflammatory cytokines such as interleukin-6 (IL-6), interleukin-8 (IL-8) and tumor necrosis factor (TNF) 15. In contrast, the potent anti-inflammatory cytokine (IL-10) is either absent or detected at very low levels from airway fluid of babies developing BPD 16, 17, 18. Interestingly, previous work has shown that the endotoxin-stimulated monocyte of the newborn, the cell precursor of the alveolar macrophage is insensitive to glucocorticoids compared to exogenous interleukin-10 (IL-10) on an equimolar basis, in the therapeutic dose range of dexamethasone for BPD 19, 20.
Accordingly, we hypothesized that for macrophages of the newly born: 1) endogenous and exogenous IL-10 produces a greater inhibition of pro-inflammatory cytokine release compared to equimolar levels of dexamethasone and betamethasone; but 2) these three anti-inflammatory agents, at therapeutic levels of DEX for BPD, may have an unwanted inhibitory effect on two major macrophage innate immune functions, phagocytosis and respiratory burst.
MATERIALS AND METHODS
Subjects
Cord blood (approximately 60 ml) was obtained from placentas after elective, term cesarean section deliveries, without medical complications. Blood was collected in heparinized preservative –free tubes for transport to the laboratory, followed by immediate cell isolation. The study was approved by the Internal Review Board of the North Shore-Long Island Jewish Health System. Consent was not required.
Cell Isolation and Culture
Monocytes were isolated from cord blood as previously described 19, 20 with >95% viability by trypan blue inclusion and >90% (CD14+) purity by flow cytometry. Briefly, peripheral blood mononuclear leukocytes (monocytes and lymphocytes) were isolated from cord blood using Ficoll-Paque PLUS (Amersham-GE Healthcare, Piscataway, NJ) density centrifugation. Monocytes were separated from lymphocytes using Percoll (Amersham-GE Healthcare) and the MACS monocyte isolation kit II supplemented with CD15 microbeads (Miltenyi Biotec, Auburn, CA). Monocytes were resuspended in RPMI at 1×106 cells per well. Monocytes were allowed to adhere for one hour at 37°C.
Macrophage Differentiation
RPMI media was removed and adherent monocytes were re-incubated at 37°C + 5% CO2 with RPMI 1640 supplemented with penicillin-streptomycin, 10% fetal calf serum, glutamine, and granulocyte macrophage colony-stimulating factor (GM-CSF, 10ng/ml) for 7 days 21. Media was changed on Day 4. Cells were examined histologically for differentiation using a Zeiss Apotome inverted microscope at 20x resolution. At the time of each experiment, cell viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-diphenyl tetrazolium bromide (MTT) assay.
Cytokine Release and Inhibition
Macrophages (N=6 cord blood samples) were pre-incubated for 1 h with PBS vehicle or serial concentrations of recombinant IL-10 (R&D Systems, Minneapolis, MN), dexamethasone (DEX), or betamethasone (BETA) (American Regent Shirley, NY). For the dose response experiments, equimolar, serial doses of 10−10M to 10−6M of these 3 agents were used, based on work demonstrating that the plasma concentration range of dexamethasone in neonates being treated for BPD was 10−8 to 10−7 M. 22, 23 Macrophages were then stimulated with LPS (1ng/ml in PBS) (Sigma-Aldrich, St. Louis, MO) for 4 and18 h. PBS was used as a negative control. Separately, macrophages were incubated with anti-IL-10 antibody or IgG as a control (R&D Systems, Minneapolis, MN) prior to LPS stimulation to determine the effect of endogenous IL-10 on PI cytokine release. For the time course experiments (N=7), macrophages were pre-incubated with equimolar concentrations (10−8M) of IL-10, DEX or BETA for 1 h, then stimulated with LPS (1ng/ml) for 4 and 18 h. Cell culture supernatant was collected and analyzed for cytokine release of IL-6, IL-8 and TNF alpha using ELISA kits (R&D Systems, Minneapolis, MN).
Phagocytosis
Macrophage phagocytosis was measured by the CytoSelect™ 96-Well Phagocytosis Zymosan Colorimetric Assay (Cell Biolabs Inc, San Diego, CA), according to the manufacturer’s protocol. Briefly, monocytes (N=7 cord blood samples) were incubated in 96 well plates (5×105 cells/well) and differentiated into macrophages as described above. At 7 days, differentiated macrophages were pre-incubated for 1h at 37°C with 10−8M IL-10, DEX or BETA, with PBS as a control. Cells were then stimulated with prelabeled zymosan (5×106 particles per well) for 2 h. Engulfed zymosan was detected at an absorbance of 405 nanometers.
Respiratory Burst
Monocytes (N=5 cord blood samples) (105 cells/well) were incubated in black, clear bottom 96 well plates and differentiated into macrophages as described above. Macrophage respiratory burst was measured by using the OxiSelect™ Intracellular ROS Assay Kit (Cell Biolabs Inc., San Diego, CA). Differentiated macrophages were washed with PBS and pre-incubated for 1 h at 37°C with 10−8M IL-10, DEX or BETA, with PBS as a control. At 30 min, cell permeable fluorogenic probe 2′,7″-dichlorohydrofluoroescin diacetate (DCFH-DA) was added to the cell media. After 1 h cells were washed and stimulated with Zymosan A from Saccharomyces cerevisiae (0.5 mg/ml)(Sigma-Aldrich, St. Louis, MO). Cells incubated with hydrogen peroxide served as a positive control during the assay. The DCFH oxidized to highly fluorescent 2′7′-Dichlorohydrofluoroscein (DCF) by ROS generated during the incubation with zymosan, was read at 0 h (baseline) and 1 h, using a fluorescent plate reader with 480nm excitation and 530nm emission.
Statistical Analyses
Data was analyzed by one way ANOVA with a Bonferroni correction for multiple comparisons over time, between concentrations of inhibitors and for the effects of IL-10 and glucocorticoids on phagocytosis and respiratory burst. An overall p value of 0.05 was considered statistically significant.
RESULTS
Figure 1 demonstrates the effect of serial increases DEX on release of three pro-inflammatory cytokines over 18 h from endotoxin-stimulated macrophages of the newborn. The only significant inhibition was observed for IL-8 release when macrophages were pretreated with DEX at 10−6M. Figure 2 demonstrates the results of the same dose-response design for BETA. However a significant inhibition of IL-8 and TNFα, was observed at 10−7M.
Figure 1. Dose-response of dexamethasone on pro-inflammatory cytokine release in endotoxin-stimulated macrophages of the newly born.
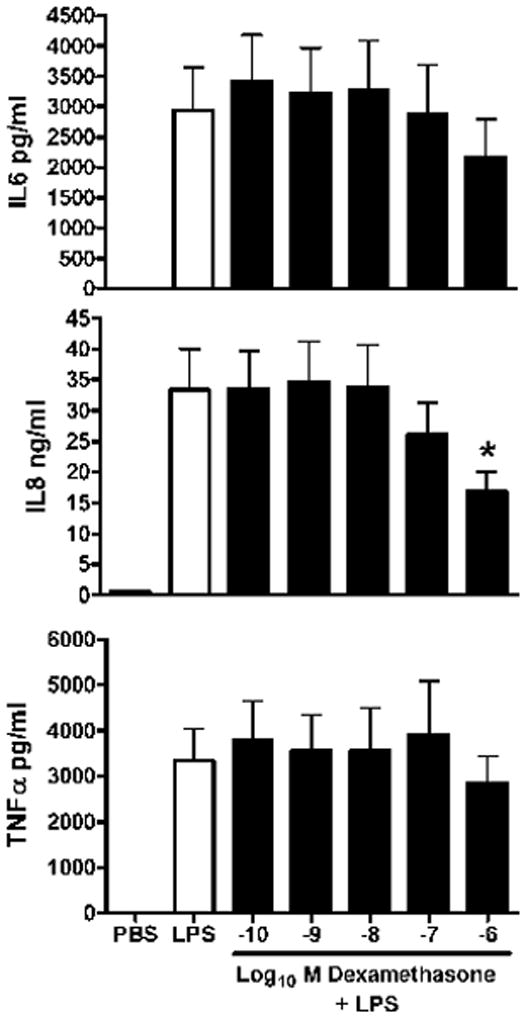
Cytokines were measured from cell culture media after pre-incubation with serial levels of dexamethasone for 1 h followed by endotoxin stimulation for 18 h. Values are mean+/−SE, N= 7, * = different from LPS alone, p< 0.01.
Figure 2. Dose-response of betamethasone on pro-inflammatory cytokine release in endotoxin-stimulated macrophages of the newly born.
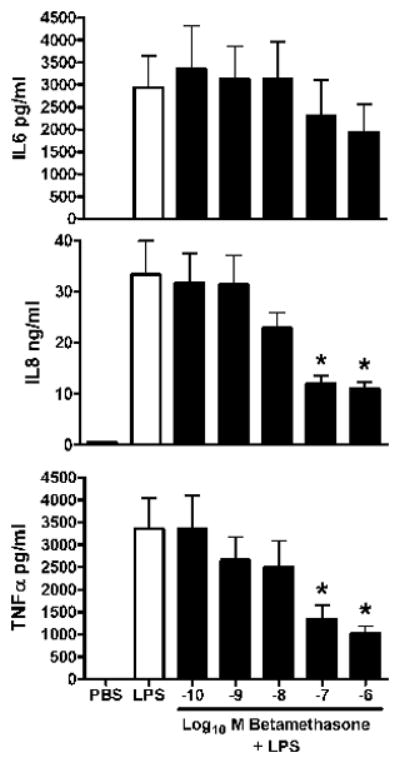
Cytokines were measured from cell culture media after pre-incubation with serial doses of betamethasone for 1 h followed by endotoxin stimulation for 18 h. Values are mean+/−SE, N=7, *= different from LPS alone, p< 0.01.
Figure 3 demonstrates the effect of serial increases of IL-10 on the same 3 cytokines released from endotoxin-stimulated macrophages of the newborn over 18 h. Release of all 3 cytokines were inhibited by over 50% with IL-10 at 10−10M and with further inhibition occurred to over 80% with 10−8M. In addition, this figure demonstrates the effect of pretreatment of endotoxin-stimulated macrophages with a monoclonal antibody to IL-10 (IL-10 mAb) and IgG as a control. Over a 5 fold increase in cytokine release was observed over 18 h for each cytokine with pre-incubation of macrophages with IL-10 mAb before LPS stimulation. There was no effect of pre-incubation of IgG (as a control for IL-10 mAb) on the release of these cytokines compared to endotoxin alone.
Figure 3. Dose-response of interleukin-10 (IL-10) on pro-inflammatory cytokine release in endotoxin-stimulated macrophages of the newly born.
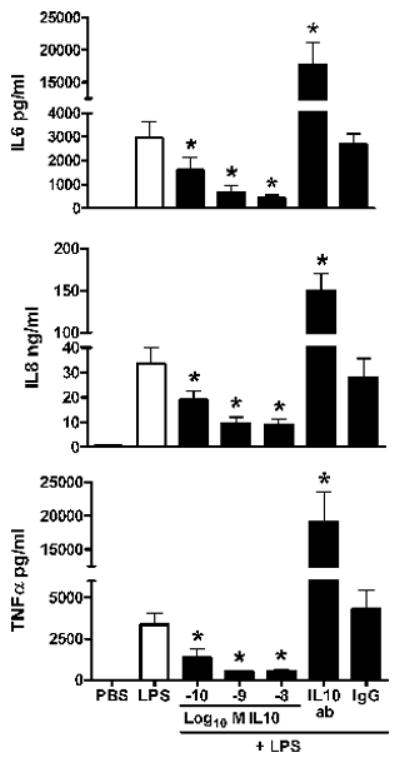
Cytokines were measured from cell culture media after pre-incubation with serial doses of IL-10 for 1 h followed by endotoxin stimulation for 18 h. Values are mean+/−SE, N=7, * = different from LPS alone, p< 0.01.
The time-related effects of equimolar levels (10−8M) of DEX, BETA, and exogenous IL-10 on LPS-stimulated macrophages (106 cells) release for 3 pro-inflammatory cytokines are shown in Figure 4. Cytokine levels at 4 and 18 h of LPS stimulation with an anti-inflammatory agent were compared to cells exposed to LPS alone. DEX had no effect on any cytokine release. Betamethasone inhibited IL-8 release by 25% at 18 h. Exogenous IL-10 inhibited the release of all three cytokines at 4 h (IL-6 by 84%, IL-8 by 74%, and TNF by 64%). At 18 h, exogenous IL-10 inhibited the release of the 3 pro-inflammatory cytokines (IL-6 by 82%, IL-8 by 72%, and TNF by 83%).
Figure 4. Time course of pro-inflammatory cytokine release from endotoxin-stimulated macrophages of the newly born: effect of anti-inflammatory agents and endogenous IL-10.
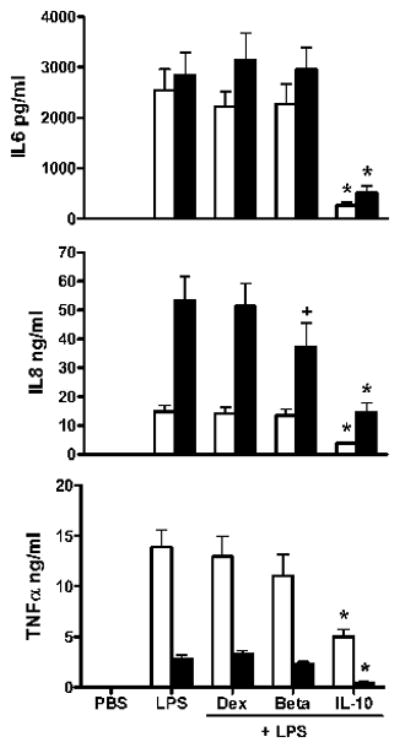
Cytokines were measured in cell culture media after pre-incubation with 10−8 M levels of interleukin-10 (IL-10), dexamethasone (DEX), betamethasone (BETA), IL-10 monoclonal antibody (IL-10 ab) or IgG control antibody followed by 4 h (open bars) or 18 h (black bars) of endotoxin stimulation. Values are mean+/−SE, N =7, *= different from LPS alone p<0.01, or += p<0.05.
There was no effect of 10−8M DEX, BETA or IL-10 on phagocytosis as shown in Figure 5. A marked effect was observed with cytochalasin D. There was also no effect of 10−8 M DEX, BETA or IL-10 on respiratory burst as shown in Figure 6. Zymosan alone produced a significant increase in superoxide release as measured by DCF.
Figure 5. Effect of interleukin-10 (IL-10), dexamethasone (Dex) or betamethasone (Beta) on phagocytosis by macrophages of the newly born.
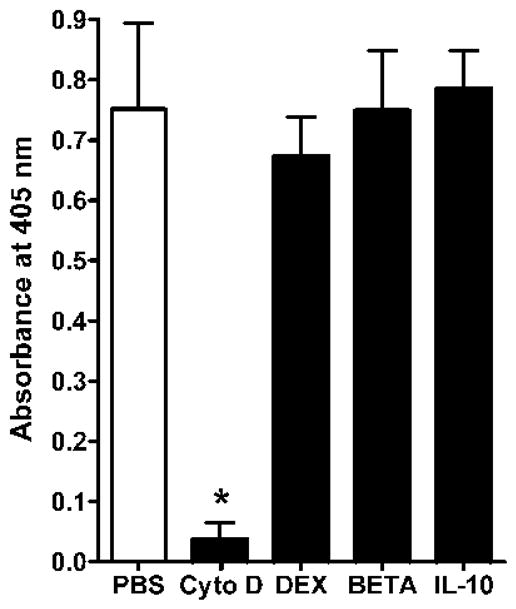
Cytochalsin D was used as a positive control stimulus. Cells were pre-incubated for 1 h with each anti-inflammatory agent at 10−8 M. Values are mean+/−SE, N=7 *= different from PBS, p<0.01.
Figure 6. Effect of interleukin-10 (IL-10), dexamethasone (Dex) or betamethasone (Beta) on respiratory burst by macrophages of the newly born.
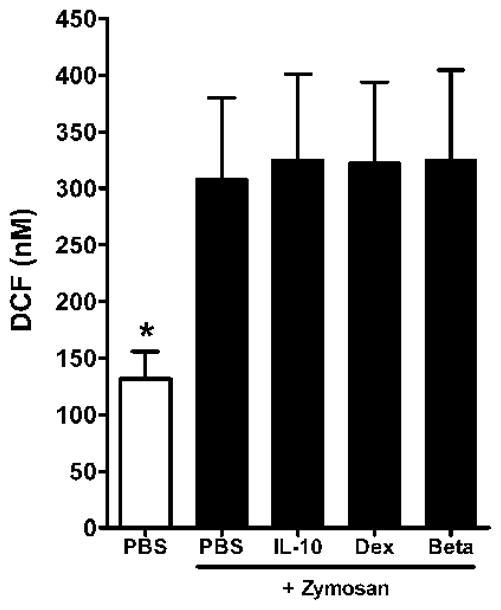
Cells were pre-incubated for 1 h with each anti-inflammatory agent at 10−8 M. Values are mean+/−SE, N=5, *= different from all other conditions with zymosan, p<0.01.
DISCUSSION
The present study used macrophages derived from cord blood of the newly born, to compare the effect of equimolar levels of exogenous IL-10 versus dexamethasone and betamethasone on pro-inflammatory cytokine release, phagocytosis and respiratory burst. At plasma levels of dexamethasone detected in babies being treated for BPD (approximately 10−8 M), only IL-10 produced a significant reduction in pro-inflammatory cytokine release and there was no effect of these three agents on phagocytosis and respiratory burst.
By 10 days after birth in the evolution of BPD, macrophages are the principal cells found within the airway fluid and the major source of pro-inflammatory (PI) cytokines 15. IL-10, the potent anti-inflammatory (AI) cytokine can be produced by MONOs and macrophages but not by PMNs of the newborn 24. However, IL-10 is absent or found at very low levels in the airway fluid of babies with evolving BPD suggesting an imbalance in PI and AI cytokines as one underlying mechanism of this disorder 16, 17, 18
MONOs, the principal precursors of alveolar macrophages of the newborn demonstrate a striking insensitivity to DEX compared to IL-10 at therapeutic levels of DEX 20. In the present study, under similar experimental conditions to previous monocyte studies, we found a similar glucocorticoid insensitivity observed for macrophages of the newborn. This insensitivity may explain, in part, why the beneficial anti-inflammatory effect of exogenous DEX in present day treatment of BPD is only partly effective6. Corticosteroid insensitivity has been reported in several common and serious, adult pulmonary disorders, including chronic obstructive pulmonary disease and severe asthma 25. Patients with asthma, who also smoke, are insensitive to corticosteroids 25. Research directed to mononuclear cells and macrophages have started to uncover mechanisms associated with corticosteroid insensitivity 25, 26, 27, 28.
Due to the harmful side effects and limited efficacy of corticosteroids, alternative therapy for BPD is needed. Novel anti-inflammatory therapy for the prevention or treatment of BPD, should be selective in its effect on immune functions of the newborn, so as to minimize the risk of the newborn to infection. We found that IL-10 at 10−8 M resulted in marked inhibition of PI cytokine release in macrophages unlike the corticosteroids. Neither IL-10, DEX nor BETA had. significant effects on the important macrophage functions of phagocytosis and respiratory burst. In previous work IL-10 was found to be a deactivator or activator of phagocytosis depending on the neutrophil or monocyte cell type from adults and concentration of IL-10 29. In mouse peritoneal macrophages, glucocorticoids enhanced the respiratory burst via a nongenomic mechanism30.
The present in vitro studies used clinical benchmarks for LPS stimulation and the testing of equimolar levels of the three anti-inflammatory agents on macrophage functions. LPS was used at a concentration of 1ng/ml to benchmark against levels found in amniotic fluid during the fetal inflammatory response syndrome (0.6 to 48ng/ml)31. Measurements of plasma DEX levels during the treatment BPD were in the 10−7 to 10−8 M range in an era when relatively higher doses of DEX were used to treat BPD on a long tapering dose regimen 22, 23. All dose comparisons in the present study for IL-10, DEX and BETA were made on an equimolar basis. Among the many pro-inflammatory cytokines found in airway fluid of babies with evolving BPD, IL-6 and IL-8 are found at the highest levels by 10 days 15, 16. No significant inhibition of these PI cytokines was observed at 10−8 M by DEX and BETA. IL-1β has also been also found in the airway of neonates developing BPD 15, 16, however interestingly, we could not detect IL-1β under our experimental conditions (data not shown). Other investigators have found variable production of IL-1β by macrophages 32.
A limitation of this study may be the use of cord blood from healthy term infants after elective cesarean section since BPD occurs more frequently in preterm infants after respiratory distress syndrome than term infants with hypoxic respiratory failure 1, 33. The design for the present study required the collection of enough volume of blood for monocyte isolation. The design also avoided the potential confounding effects of antenatal steroids, maternal medications and maternal disorders associated with prematurity that are not present when corticosteroids are used for BPD, usually beyond several weeks after birth34. This study does not address whether there are developmental differences in macrophage insensitivity to glucocorticoids or Il-10. Additionally, we chose to use in vitro differentiation of monocytes to study macrophages because alveolar macrophages of the premature infants could not provide enough cells to perform the present studies. However, cell surface antigens and functions of GM-CSF-induced, monocyte-derived macrophages from adults have almost identical characteristics as alveolar macrophages from adults 21.
In conclusion, the present in vitro study demonstrates a relative insensitivity of DEX, and to a lesser degree BETA, with regard to pro-inflammatory cytokine release from monocyte-derived macrophages of the newly born, compared to equimolar levels of IL-10. These findings may help explain why DEX, when used at a low dose and short course, for selected patients with advanced BPD, has limited efficacy34, 35. None of these three agents, had an inhibitory effect on macrophage respiratory burst or phagocytosis, which may play a role in the pathogenesis of BPD, at equimolar therapeutic levels of DEX. The relatively selective effect of these three agents on pro-inflammatory cytokine release from macrophages of the newborn, could tip the balance of inflammation towards recovery without jeopardizing the newborn to infection. IL-10 has been used to treat adults with chronic inflammatory disorders36. Therefore, the present study provides rationale for translational research using airway instillation of IL-10 as potential therapy for BPD, in a manner similar to preclinical and clinical work for surfactant laced with budesonide37.
Acknowledgments
Funded by: R03-HD048508 (NICHD) and Ikaria (Grant Program for Fellows)
Footnotes
Conflict of Interest: The authors do not have any competing financial interests in relation to the work described.
References
- 1.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23(2):167–172. doi: 10.1097/MOP.0b013e3283423e6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speer CP. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med. 2006;11(5):354–362. doi: 10.1016/j.siny.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Mokres LM, Parai K, Hilgendorff A, Ertsey R, Alvira CM, Rabinovitch M, et al. Prolonged mechanical ventilation with air induces apoptosis and causes failure of alveolar septation and angiogenesis in lungs of newborn mice. Am J Physiol Lung Cell Mol Physiol. 2010;298(1):L23–35. doi: 10.1152/ajplung.00251.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mourani PM, Abman SH. Pulmonary vascular disease in bronchopulmonary dysplasia: pulmonary hypertension and beyond. Curr Opin Pediatr. 2013;25(3):329–337. doi: 10.1097/MOP.0b013e328360a3f6. [DOI] [PubMed] [Google Scholar]
- 6.Halliday HL, Ehrenkranz RA, Doyle LW. Late (>7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2009;(1):Cd001145. doi: 10.1002/14651858.CD001145.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Onland W, Offringa M, De Jaegere AP, van Kaam AH. Finding the optimal postnatal dexamethasone regimen for preterm infants at risk of bronchopulmonary dysplasia: a systematic review of placebo-controlled trials. Pediatrics. 2009;123(1):367–377. doi: 10.1542/peds.2008-0016. [DOI] [PubMed] [Google Scholar]
- 8.O’Reilly M, Thebaud B. The promise of stem cells in bronchopulmonary dysplasia. Semin Perinatol. 2013;37(2):79–84. doi: 10.1053/j.semperi.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Ghanta S, Leeman KT, Christou H. An update on pharmacologic approaches to bronchopulmonary dysplasia. Semin Perinatol. 2013;37(2):115–123. doi: 10.1053/j.semperi.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson JC, Chi EY, Wilson CB, Truog WE, Teh EC, Hodson WA. Sequence of inflammatory cell migration into lung during recovery from hyaline membrane disease in premature newborn monkeys. Am Rev Respir Dis. 1987;135(4):937–940. doi: 10.1164/arrd.1987.135.4.937. [DOI] [PubMed] [Google Scholar]
- 11.Kwong KY, Jones CA, Cayabyab R, Lecart C, Khuu N, Rhandhawa I, et al. The effects of IL-10 on proinflammatory cytokine expression (IL-1beta and IL-8) in hyaline membrane disease (HMD) Clin Immunol Immunopathol. 1998;88(1):105–113. doi: 10.1006/clin.1997.4510. [DOI] [PubMed] [Google Scholar]
- 12.Merritt TA, Cochrane CG, Holcomb K, Bohl B, Hallman M, Strayer D, et al. Elastase and alpha 1-proteinase inhibitor activity in tracheal aspirates during respiratory distress syndrome. Role of inflammation in the pathogenesis of bronchopulmonary dysplasia. J Clin Invest. 1983;72(2):656–666. doi: 10.1172/JCI111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munshi UK, Niu JO, Siddiq MM, Parton LA. Elevation of interleukin-8 and interleukin-6 precedes the influx of neutrophils in tracheal aspirates from preterm infants who develop bronchopulmonary dysplasia. Pediatr Pulmonol. 1997;24(5):331–336. doi: 10.1002/(sici)1099-0496(199711)24:5<331::aid-ppul5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Schibler KR. The Mononuclear Phagocyte System. In: Polin RAFW, Abman SH, editors. Fetal and Neonatal Physiology. Vol. 2. Saunders; Philadelphia: 2011. p. 153. [Google Scholar]
- 15.Kotecha S, Wilson L, Wangoo A, Silverman M, Shaw RJ. Increase in interleukin (IL)-1 beta and IL-6 in bronchoalveolar lavage fluid obtained from infants with chronic lung disease of prematurity. Pediatr Res. 1996;40(2):250–256. doi: 10.1203/00006450-199608000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Ambalavanan N, Carlo WA, D’Angio CT, McDonald SA, Das A, Schendel D, et al. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123(4):1132–1141. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones CA, Cayabyab RG, Kwong KY, Stotts C, Wong B, Hamdan H, et al. Undetectable interleukin (IL)-10 and persistent IL-8 expression early in hyaline membrane disease: a possible developmental basis for the predisposition to chronic lung inflammation in preterm newborns. Pediatr Res. 1996;39(6):966–975. doi: 10.1203/00006450-199606000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oei J, Lui K, Wang H, Henry R. Decreased interleukin-10 in tracheal aspirates from preterm infants developing chronic lung disease. Acta Paediatr. 2002;91(11):1194–1199. doi: 10.1111/j.1651-2227.2002.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 19.Chusid LA, Pereira-Argenziano L, Miskolci V, Vancurova I, Davidson D. Transcriptional control of cytokine release from monocytes of the newborn: effects of endogenous and exogenous interleukin-10 versus dexamethasone. Neonatology. 2010;97(2):108–116. doi: 10.1159/000235807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson D, Patel H, Degoy A, Gershkovich I, Vancurova I, Miskolci V. Differential effect of exogenous interleukin-10 versus glucocorticoids on gene expression and pro-inflammatory cytokine release by polymorphonuclear leukocytes and monocytes of the newly born. American Journal of Translational Research. 2013;5(1):103–115. [PMC free article] [PubMed] [Google Scholar]
- 21.Akagawa KS. Functional heterogeneity of colony-stimulating factor-induced human monocyte-derived macrophages. Int J Hematol. 2002;76(1):27–34. doi: 10.1007/BF02982715. [DOI] [PubMed] [Google Scholar]
- 22.Lugo RA, Nahata MC, Menke JA, McClead RE., Jr Pharmacokinetics of dexamethasone in premature neonates. Eur J Clin Pharmacol. 1996;49(6):477–483. doi: 10.1007/BF00195934. [DOI] [PubMed] [Google Scholar]
- 23.Schild PN, Charles BG. Determination of dexamethasone in plasma of premature neonates using high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1994;658(1):189–192. doi: 10.1016/0378-4347(94)00192-8. [DOI] [PubMed] [Google Scholar]
- 24.Davidson D, Zaytseva A, Miskolci V, Castro-Alcaraz S, Vancurova I, Patel H. Gene Expression Profile of Endotoxin-stimulated Leukocytes of the Term New Born: Control of Cytokine Gene Expression by Interleukin-10. PLoS One. 2013;8(1):e53641. doi: 10.1371/journal.pone.0053641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373(9678):1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- 26.Bhavsar P, Hew M, Khorasani N, Torrego A, Barnes PJ, Adcock I, et al. Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared with non-severe asthma. Thorax. 2008;63(9):784–790. doi: 10.1136/thx.2007.090027. [DOI] [PubMed] [Google Scholar]
- 27.Mercado N, To Y, Ito K, Barnes PJ. Nortriptyline reverses corticosteroid insensitivity by inhibition of. J Pharmacol Exp Ther. 2011;337(2):465–470. doi: 10.1124/jpet.110.175950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Culpitt SV, Rogers DF, Shah P, De Matos C, Russell RE, Donnelly LE, et al. Impaired inhibition by dexamethasone of cytokine release by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167(1):24–31. doi: 10.1164/rccm.200204-298OC. [DOI] [PubMed] [Google Scholar]
- 29.Buchwald UK, Geerdes-Fenge HF, Vockler J, Ziege S, Lode H. Interleukin-10: effects on phagocytosis and adhesion molecule expression of granulocytes and monocytes in a comparison with prednisolone. Eur J Med Res. 1999;4(3):85–94. [PubMed] [Google Scholar]
- 30.Shi WL, Ma Q, Zhang LD, Huang JL, Zhou J, Liu L, et al. Corticosterone rapidly promotes respiratory burst of mouse peritoneal macrophages. Chin Med J (Engl) 2011;124(19):3127–3132. [PubMed] [Google Scholar]
- 31.Romero R, Roslansky P, Oyarzun E, Wan M, Emamian M, Novitsky TJ, et al. Labor and infection. II. Bacterial endotoxin in amniotic fluid and its relationship to the onset of preterm labor. Am J Obstet Gynecol. 1988;158(5):1044–1049. doi: 10.1016/0002-9378(88)90216-5. [DOI] [PubMed] [Google Scholar]
- 32.Herzyk DJ, Allen JN, Marsh CB, Wewers MD. Macrophage and monocyte IL-1 beta regulation differs at multiple sites. Messenger RNA expression, translation, and post-translational processing. J Immunol. 1992;149(9):3052–3058. [PubMed] [Google Scholar]
- 33.Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000;342(7):469–474. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- 34.Halliday HL, Ehrenkranz RA, Doyle LW. Delayed (>3 weeks) postnatal corticosteroids for chronic lung disease in preterm. Cochrane Database Syst Rev. 2003;(1):Cd001145. doi: 10.1002/14651858.CD001145. [DOI] [PubMed] [Google Scholar]
- 35.Watterberg K. Evidence-based neonatal pharmacotherapy: postnatal corticosteroids. Clin Perinatol. 2012;39(1):47–59. doi: 10.1016/j.clp.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeh TF, Lin HC, Chang CH, Wu TS, Su BH, Li TC, et al. Early intratracheal instillation of budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants: a pilot study. Pediatrics. 2008;121(5):e1310–1318. doi: 10.1542/peds.2007-1973. [DOI] [PubMed] [Google Scholar]


