Abstract
The detection of serum free light (FLC) is useful in the diagnosis of several hematological diseases. The role and biological relevance of monoclonal or polyclonal FLC elevations in predicting long-term outcome in diffuse large B-cell lymphoma (DLBCL) is unknown. We determined the relationship of the type of FLC elevations to outcome, tumor genotype, and pattern of serum cytokine elevations in 276 patients with untreated DLBCL. Elevated FLC was an adverse prognostic factor through 6 years of follow-up (monoclonal, EFS HR = 3.56, 95% CI: 1.88-6.76, p<0.0001; polyclonal, EFS HR = 2.56, 95% CI: 1.50-4.38, p=0.0006). 73% of DLBCL tumors with monoclonal FLC elevations were activated B-cell type (ABC) vs. 33% from patients with normal FLC. Only ABC-DLBCL lines secreted kappa FLC in vitro and this secretion could be inhibited by the NF-κB inhibitor bortezomib. Patients with monoclonal FLC had significantly (all p<0.001) elevated serum levels of IL-12, sIL-2Rα, IL-1R, and IP-10. Patients with polyclonal elevations of FLC had higher levels of IL-6 (p=0.033), IL-8 (p=0.025), sIL2Rα (p=0.011), and IL-1R1 (p=0.041). The combination of elevated FLC and a CXC superfamily chemokine IP-10 predicted a particularly inferior outcome characterized by late relapse. These elevated abnormal FLC and cytokines are potentially useful biomarkers for prognosis and selecting agents for untreated DLBCL.
Keywords: Free light chains, diffuse large B-cell lymphoma, cytokine, IP-10
Introduction
Normal human serum contains a small amount of free kappa and lambda immunoglobulin light chains that are not attached to the immunoglobulin heavy chain. These free light chains can be quantitated by the free light chain (FLC) assay.1,2 FLC elevations can be polyclonal (increase in one or both light chains with a normal κ:λ ratio) or monoclonal (elevation of one FLC that results in an abnormal ratio). The detection of monoclonal FLC is very useful in the diagnosis and management of patients with multiple myeloma and amyloidosis.3 The polyclonal elevation of FLC is also important and is associated with all cause mortality and impaired renal function.4,5 We have applied the serum FLC assay to hematologic lymphoid malignancies other than myeloma and found monoclonal FLC in 13% of patients with a variety of lymphoma types.6 The incidence of monoclonal FLC was highest in mantle cell (36%) and small lymphocytic (24%) lymphomas; it was found in 8% of those patients with diffuse large B-cell lymphoma (DLBCL).6 In a further study of 295 patients with new, untreated DLBCL from two independent cohorts, 32% of patients had an elevated serum FLC (polyclonal or monoclonal) and 14% were monoclonal. Either type of FLC elevation resulted in an inferior prognosis for DLBCL.7
In DLBCL, tumors can now be subtyped into activated B cell (ABC), germinal B cell (GBC) or primary mediastinal B cell type by gene expression profiling (GEP).8-10 These molecular subtypes have been shown to be prognostic in some datasets and have been very helpful in unraveling the molecular pathogenesis of DLBCL.11 DLBCL genotyping has potential treatment implications and studies are ongoing to learn if certain new treatment regimens are more effective within a specific DLBCL genotypic classification. Immunohistochemistry (IHC) algorithms have been published that demonstrated an association between IHC and gene expression classifications.11,12
In this report, we studied the relationship of the type of FLC elevation to DLBCL genotype, survival parameters, and pretreatment serum cytokine elevation. In addition, we explored the in vitro secretion of FLC and cytokines by DLBCL cells and whether pathway-specific drugs could inhibit FLC secretion. It was our hypothesis that ABC-type DLBCL tumors would be more likely to secrete monoclonal FLC because of their known increased content of cells expressing IRF-4 (MUM1) a marker of plasma cells.13
Patients and Methods
Patient
Newly diagnosed patients with DLBCL were prospectively enrolled in the University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Resource (MER)7,14 or the NCCTG clinical trial N0489.15 These studies were approved at the Institutional Review Board and all patients signed informed consent to have their samples used for research. This report contains updated FLC results from the subset of patients receiving immunochemotherapy the 295 patients from the cohort previously published.7
DLBCL Cell lines
Human DLBCL cell lines were used to study in vitro secretion of FLC by molecular subtype. The GCB lines SUDHL6 (DHL6), OCI-Ly7 (Ly7), OCI-Ly1 (Ly1), and ABC lines OCI-Ly3 (Ly3), SUDHL2 (DHL2), HBL1, and OCI-Ly10 (Ly10) were a gift from the L. Staudt lab (NCI, Bethesda) and maintained in IMDM with 20% human serum (except DHL6, which was grown in RPMI+10% FBS). CD19 cells were purified from peripheral blood mononuclear cells and used as a normal B cell control for FLC analysis. CD19 cells were further cultured in RPMI with 10% fetal bovine serum for FLC analysis.
SUDHL2 and HBL1 cell lines were treated with bortezomib (Sigma-Aldrich) or TG1013458 (Sanofi Aventis) for 24 hours and FLC analysis was performed on the supernatants.
Free light chain assay
Serum FLC was quantitated from enrollment research serum using the FREELITE assay (The Binding Site, Ltd., Birmingham, UK). The FLC assays were performed by the Mayo Clinic Clinical Immunology Lab using kits provided courtesy of The Binding Site. Abnormal κ/λ FLC ratio was a priori defined as a κ/λ FLC ratio outside of (0.26, 1.65) and elevated FLC as a κ concentration higher than 1.94 mg/dL or λ concentration higher than 2.63 mg/dL based on the published normal ranges for Mayo Medical Laboratories.16 A monoclonal elevation of FLC was defined as an elevated FLC with the corresponding FLC ratio outside the reference range (0.26-1.65). Polyclonal elevation of FLC was defined as an elevation of either or both κ or λ FLC outside the laboratory normal range, but with a normal ratio. Abnormal ratios without elevation of either FLC were considered normal based on our previous studies indicating these values were not prognostic in DLBCL.7
Immunohistochemistry (IHC)
IHC staining was performed on paraffin tissue from research tissue microarrays (TMAs). All cases were reviewed centrally by the study hematopathologists. DLBCL cases were classified into GCB or non-GCB molecular type based on the Hans, Tally, and Choi algorithms applied to paraffin-embedded tumor samples.11
30-plex ELISA from patient serum
Multiplex ELISA (30-plex) was performed as previously described on available pretreatment patient serum.17 The cytokine values have been previously published18 but the data on the relationship of cytokine elevations with monoclonal FLC secretion has not been previously reported.
Cytokine Secretion by DLBCL Cell Lines
Supernatants from various DLBCL cell line cultures were analyzed for secretion using the human sIL-2Rα, IL-12, IL-1R1 immunoassay kit (R&D Systems). The specimens were run neat and the end point read at 450 nm using a SpectraMax190 microplate reader (Molecular Devices).
Statistical analyses
Event free survival (EFS) was defined as the time from diagnosis until progression, relapse, re-treatment, or death due to any cause. Analyses of EFS were limited to patients receiving immunochemotherapy. Associations between FLC and EFS were assessed using Cox proportional hazards models and Kaplan-Meier curves. Associations between cytokines and FLC patient groups were assessed using linear models for log-2 scaled cytokine levels and adjusted for study (MER vs. ER-CHOP).
Results
Clinical outcome in monoclonal vs. polyclonal FLC elevation in DLBCL
The patient characteristics and description of FLC abnormalities for the 295 DLBCL cases have been previously reported.7 Patients with primary central nervous system lymphoma, post-transplant lymphoproliferative disorders and cutaneous DLBCL were excluded for this study, leaving 276 patients for this analysis. Ninety-four (34%) patients had an elevation of FLC - 68 (25%) polyclonal and 26 (9%) monoclonal. Updated survival curves in patients receiving immunochemotherapy (n=256) show a continued association between both monoclonal (EFS HR = 3.56, 95% CI: 1.88-6.76, p<0.0001) and polyclonal (EFS HR = 2.56, 95% CI: 1.50-4.38, p=0.0006) FLC abnormalities and inferior outcome in newly diagnosed DLBCL (Figure 1). Within the abnormal FLC category patients with monoclonal FLC elevation fared slightly worse (HR=1.38, 95% CI: 0.69-2.74) than patients with polyclonal FLC elevation (p=0.36). Of note, this extended analysis demonstrates that in patients with FLC abnormalities events continue to occur out to 6 years of follow-up whereas a plateau occurs in those DLBCL patients with normal FLC. This in part reflects the effects of co-morbidities that occurred in patients with polyclonal FLC elevations.
Figure 1.
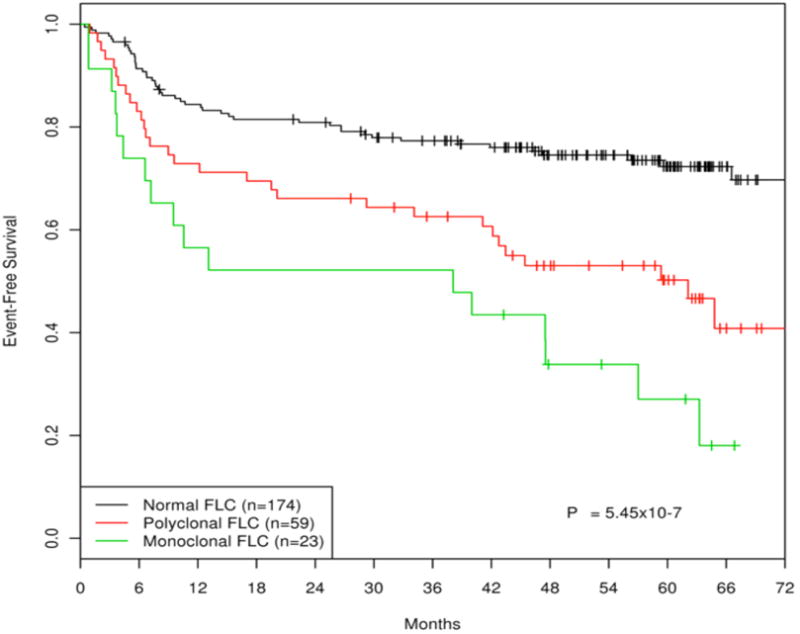
Event-free survival by normal, polyclonal and monoclonal FLC secretion of patients with untreated diffuse large B-cell lymphoma treated with chemoimmunotherapy (n=256).
Correlation of monoclonal and polyclonal FLC with DLBCL molecular subtypes
Tissue for IHC staining to determine molecular classification was available on 121 of the 276 cases (44%); 75 (63%) were GCB and 45 (38%) were non-GCB per Hans algorithm. Of these 121 cases 83 had normal FLC; 11 had monoclonal FLC; and 27 had polyclonal FLC. By the Hans, Choi, and Tally algorithms, 73% (8/11), 70% (7/10), and 90% (9/10) of these cases were ABC-type DLBCL, respectively (Table 1). The percentage of ABC patients by these same algorithms in the corresponding DLBCL patients with normal FLC secretion was 33% (p=0.0096), 38% (p=0.053), and 46% (p=0.0091), respectively. Patients with polyclonal FLC secretion had similar IHC-determined genotypes as those with normal FLC (both p >0.57). Abnormal FLC secretion was associated with inferior outcomes in both GCB and non-GCB groups.
Table 1.
Immunohistochemistry markers in 11 patients with diffuse large B-cell lymphoma and monoclonal serum immunoglobulin free light chain secretion.
| Germinal center | ABC | Either | Algorithm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Serum Kappa | Serum Lambda | Serum FLC Ratio | CD10 | GCET1 | LMO2 | MUM1 | FOXP1 | BCL6 | Hans | Choi | Tally |
| NCCTG1 | 3.38 | 2.02 | 1.67 | - | - | + | + | - | + | ABC | GCB | ABC |
| NCCTG2 | 5.57 | 0.43 | 13.1 | - | - | - | - | + | - | ABC | ABC | ABC |
| NCCTG3 | 3.68 | 1.61 | 2.29 | + | - | - | + | + | - | GCB | GCB | ABC |
| MER1 | 4.87 | 2.11 | 2.31 | - | ND | - | - | ND | - | ABC | NA | ABC |
| MER2 | 4.51 | 1.57 | 2.87 | - | - | - | - | - | - | ABC | ABC | ABC |
| MER3 | 43.6 | 1.23 | 35.4 | - | - | - | + | + | - | ABC | ABC | ABC |
| MER4 | 2.83 | 1.47 | 1.93 | - | - | - | - | + | + | GCB | ABC | ABC |
| MER5 | 5.16 | 0.36 | 14.3 | - | - | ND | - | ND | - | ABC | ABC | N/A |
| MER6 | 8.3 | 4.85 | 1.71 | - | - | - | + | - | - | ABC | ABC | ABC |
| MER7 | 1.26 | 9.67 | 0.13 | - | - | - | - | ND | - | ABC | ABC | ABC |
| MER8 | 45.7 | 5.4 | 8.46 | + | - | + | - | + | + | GCB | GCB | GCB |
Abbreviations: ABC – activated B-cell cell type; FLC, free light chain; NCCTG, North Central Cancer Treatment Group (now ALLIANCE); MER, Molecular Epidemiology Resource
Secretion of FLC by DLBCL Cell Lines
Supernatants from cell cultures of normal CD19 B cells and GCB and ABC-type DLBCL cell lines were analyzed for FLC secretion. Normal CD19 B cells did not secrete either κ or λ light chain into the culture media (Figure 2A). The GCB-type DLBCL cell lines (DHL6, Ly7, and Ly1) secreted small amounts of FLC similar to normal human serum. In contrast, the three ABC-type DLBCL cell lines tested (DHL2, HBL1, and Ly10) secreted high amounts of κ FLC with an abnormal FLC ratio (Figure 2B, 2C).
Figure 2.
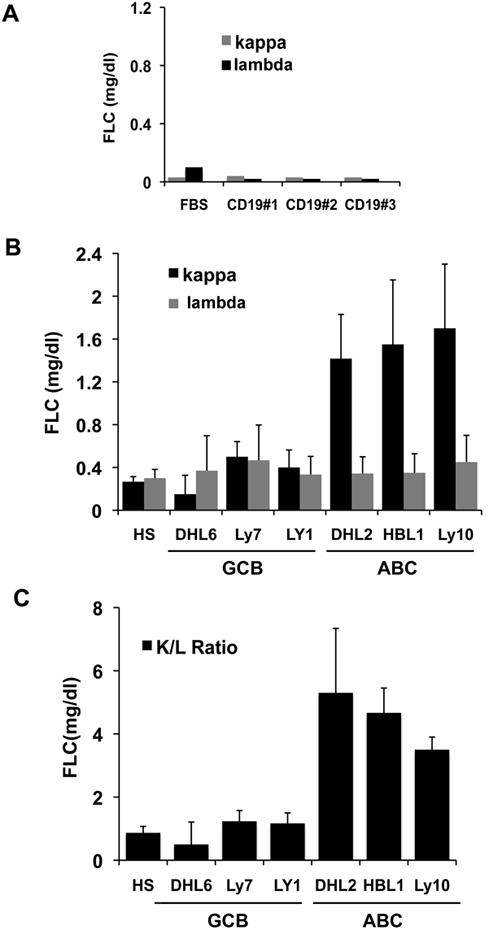
In-vitro secretion of FLC by normal B (CD19+) and DLBCL cells. (A) FLC secretion by CD19+ B-cells (n=3) from the blood of normal controls comparable to fetal bovine serum (FBS) (B) FLC (kappa and lambda) secretion by ABC and GCB DLBCL human cell lines compared to human serum control (HS). (C) FLC (kappa/lambda ratio) secretion in ABC and GCB DLBCL cell lines. Bars represent mean ± SD from three separate experiments.
Effect of NF-κB and JAK2 Pathway Inhibition on FLC secretion
The NF-κB and STAT3 pathways are activated in ABC-type DLBCL lymphoma.19-23 It has also been shown that the ABC DLBCL genotype is sensitive to NF-κB or JAK/STAT inhibitors.19,22 We investigated whether in vitro treatment of the DLBCL cell lines with bortezomib (an NF-κB inhibitor), or TG1013458 (JAK2 inhibitor) could decrease FLC secretion in ABC-type DLBCL cell lines. Treatment with sublethal doses of bortezomib, but not TG1013458, decreased κ FLC secretion in the DHL2 and HBL1 cell lines in a dose dependent manner (Figure 3A,B).
Figure 3.
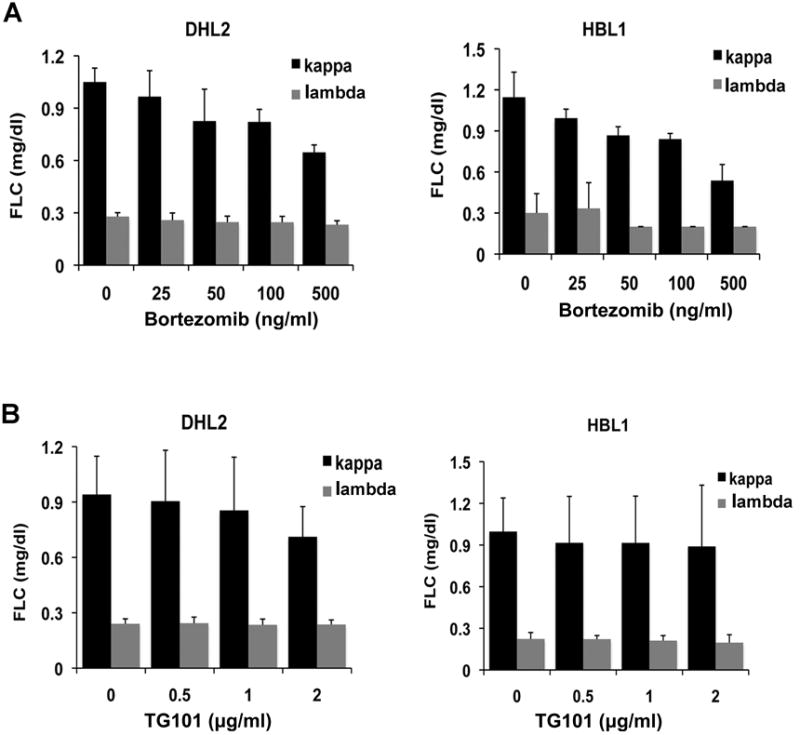
(A-B): Effect of the NF-κB inhibitor bortezomib (A) and the JAK2 inhibitor TG101 (B) on monoclonal kappa FLC secretion by the ABC DLBCL cell lines DHL2 and HBL1. Bars represent mean ± SD from three separate experiments.
Correlation of abnormal (monoclonal and polyclonal) FLC with pretreatment serum cytokine level
We have previously demonstrated that patients with DLBCL have an abnormal cytokine profile that can be prognostic for relapse and survival.17,18 Cytokine levels from pre-treatment serum were compared to the FLC data available from the same time point (Table 2). Patients with monoclonal FLC had significantly elevated concentrations of IL-12 (p=0.000087), sIL-2R (p=0.00011), IL-1R1 (p=0.00024), and IP-10 (p=0.00010). Patients with polyclonal elevations of FLC had higher levels of IL-6 (p=0.033), IL-8 (p=0.025), sIL2Rα (p=0.011), and IL-1R1 (p=0.041).
Table 2.
Cytokine values (median, min, max) are log2 transformed and batch-adjusted from pg/ml raw values. P-value is from the Wilcoxon rank-sum test comparing to normal serum immunoglobulin free light chain (FLC). Normal FLC data include 15 patients with non-elevated FLC with abnormal FLC ratio (i.e. ratio-only). Shaded cytokines are significantly correlated with monoclonal FLC.
| Normal FLC (N=157) | Polyclonal Elevated FLC (N=57) | Monoclonal Elevated FLC (N=23) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine | Median | Q1 | Q3 | Median | Q1 | Q3 | p-value | Median | Q1 | Q3 | p-value |
| EGF | 6.5 | 5.4 | 7.1 | 6.4 | 5.4 | 7.2 | 0.725 | 6.4 | 5.6 | 7.2 | 0.837 |
| Eotaxin | 6.9 | 6.4 | 7.4 | 7.0 | 6.4 | 7.5 | 0.664 | 7.0 | 6.5 | 7.4 | 0.923 |
| FGF-β | 4.5 | 4.0 | 5.2 | 4.5 | 4.0 | 5.1 | 0.812 | 4.5 | 3.8 | 5.6 | 0.762 |
| GM-CSF | 2.3 | 2.0 | 5.2 | 2.3 | 1.7 | 5.6 | 0.755 | 2.3 | 1.5 | 3.8 | 0.088 |
| G-CSF | 5.3 | 4.3 | 7.8 | 6.5 | 4.4 | 8.0 | 0.203 | 6.5 | 4.5 | 7.8 | 0.079 |
| HGF | 9.1 | 8.4 | 9.7 | 9.4 | 8.7 | 10.2 | 0.051 | 9.5 | 8.4 | 10.3 | 0.216 |
| IFN-α | 6.0 | 4.7 | 6.8 | 5.9 | 4.6 | 6.7 | 0.750 | 6.2 | 4.7 | 6.7 | 0.764 |
| IFN-γ | 2.3 | 2.0 | 3.6 | 2.7 | 2.3 | 4.4 | 0.246 | 2.3 | 2.0 | 3.8 | 0.592 |
| IL-2 | 2.7 | 2.1 | 3.8 | 2.8 | 1.6 | 3.9 | 0.520 | 3.3 | 2.0 | 4.3 | 0.602 |
| IL-4 | 4.9 | 2.3 | 6.2 | 4.9 | 2.3 | 6.4 | 0.887 | 5.3 | 2.6 | 6.4 | 0.266 |
| IL-5 | 1.6 | 1.6 | 3.4 | 2.0 | 1.6 | 3.8 | 0.316 | 1.6 | 1.6 | 3.6 | 0.847 |
| IL-6 | 2.9 | 1.6 | 4.7 | 4.4 | 1.6 | 5.9 | 0.033 | 4.6 | 1.6 | 5.5 | 0.062 |
| IL-7 | 3.3 | 3.3 | 5.4 | 3.3 | 3.3 | 4.7 | 0.201 | 3.3 | 3.3 | 6.3 | 0.997 |
| IL-8 | 5.4 | 4.5 | 6.2 | 5.7 | 5.0 | 6.7 | 0.025 | 5.8 | 5.1 | 6.6 | 0.075 |
| IL-10 | 4.6 | 3.6 | 5.6 | 4.7 | 2.9 | 5.7 | 0.734 | 5.2 | 4.1 | 5.8 | 0.051 |
| IL-13 | 4.6 | 1.7 | 5.4 | 4.8 | 1.3 | 5.4 | 0.514 | 4.6 | 1.7 | 5.3 | 0.844 |
| IL-15 | 3.3 | 3.3 | 6.3 | 3.4 | 3.3 | 6.5 | 0.686 | 3.3 | 3.3 | 7.0 | 0.367 |
| IL-17 | 4.0 | 3.0 | 4.0 | 4.0 | 3.0 | 4.0 | 0.633 | 4.0 | 4.0 | 4.0 | 0.028 |
| IL-12 | 8.0 | 7.4 | 8.6 | 8.3 | 7.7 | 8.7 | 0.240 | 8.8 | 8.3 | 9.4 | 0.000087 |
| sIL-2Rα | 9.9 | 9.0 | 11.0 | 10.6 | 9.4 | 12.0 | 0.011 | 11.5 | 10.6 | 12.9 | 0.00011 |
| IL-1R1 | 9.4 | 8.6 | 10.3 | 9.8 | 9.1 | 10.5 | 0.041 | 10.2 | 9.5 | 11.4 | 0.00024 |
| IL-1β | 3.9 | 2.9 | 4.6 | 3.9 | 2.9 | 3.9 | 0.466 | 3.9 | 3.9 | 6.2 | 0.148 |
| IP10 | 6.3 | 5.2 | 7.3 | 6.7 | 5.5 | 7.6 | 0.168 | 7.4 | 6.3 | 8.9 | 0.00010 |
| MCP-1 | 9.5 | 9.1 | 10.0 | 9.8 | 9.2 | 10.2 | 0.067 | 9.7 | 9.5 | 10.2 | 0.067 |
| MIG | 7.4 | 6.0 | 8.9 | 8.2 | 6.8 | 9.1 | 0.129 | 8.6 | 6.6 | 10.1 | 0.097 |
| MIP-1α | 5.8 | 4.7 | 6.8 | 6.0 | 5.2 | 7.0 | 0.578 | 6.3 | 5.6 | 7.0 | 0.023 |
| MIP-1β | 6.6 | 6.0 | 7.2 | 6.8 | 6.2 | 7.4 | 0.425 | 6.8 | 6.2 | 7.9 | 0.183 |
| RANTES | 12.8 | 2.9 | 13.0 | 12.7 | 2.9 | 12.9 | 0.716 | 12.8 | 12.3 | 13.0 | 0.920 |
| TNF-α | 2.3 | 1.7 | 2.4 | 2.3 | 1.3 | 2.3 | 0.996 | 2.3 | 2.0 | 2.3 | 0.723 |
| VEGF | 2.3 | 1.3 | 3.3 | 2.6 | 1.3 | 3.9 | 0.425 | 2.6 | 1.8 | 3.9 | 0.124 |
In-vitro secretion of sIL-2Rα, IL-12, IL-1R1 and IP-10 by DLBCL cell lines
Supernatants from the GCB DLBCL cell lines DHL6, Ly1 and Ly7 and the ABC cell lines DHL2, Ly3, Ly10 and HBL1 were collected and assayed for key cytokines that were significantly correlated with monoclonal FLC (Table 2); namely, IL-12, sIL-2Rα, IL-1R1 and IP-10 by individual ELISA (Figure 4). All the 3 GCB cell lines secreted detectable sIL-2Ra (range;100-445 pg/ml), whereas only one of the ABC cell lines (Ly3) secreted sIL-2Rα (284, pg/ml) as compared to Human serum alone. With respect to IL-12, only 1 GCB cell line (Ly7) and 2 ABC cell lines (DHL-2 and Ly3) secreted detectable amounts. Secretion of IL-1R1 was very low in all cell lines (<20μg/ml) with the exception in Ly3 cells (45 pg/ml). IP-10 was not secreted by none of the GCB lines (data not shown), however 2/3 ABC lines (DHL2, Ly3) secreted significant amount of IP-10 (Figure 4). In summary, sIL2Rα is secreted in-vitro primarily by GCB DLBCL cell lines; IP-10 by ABC-type, IL-12 by both types, and IL-1R1 by neither type.
Figure 4.
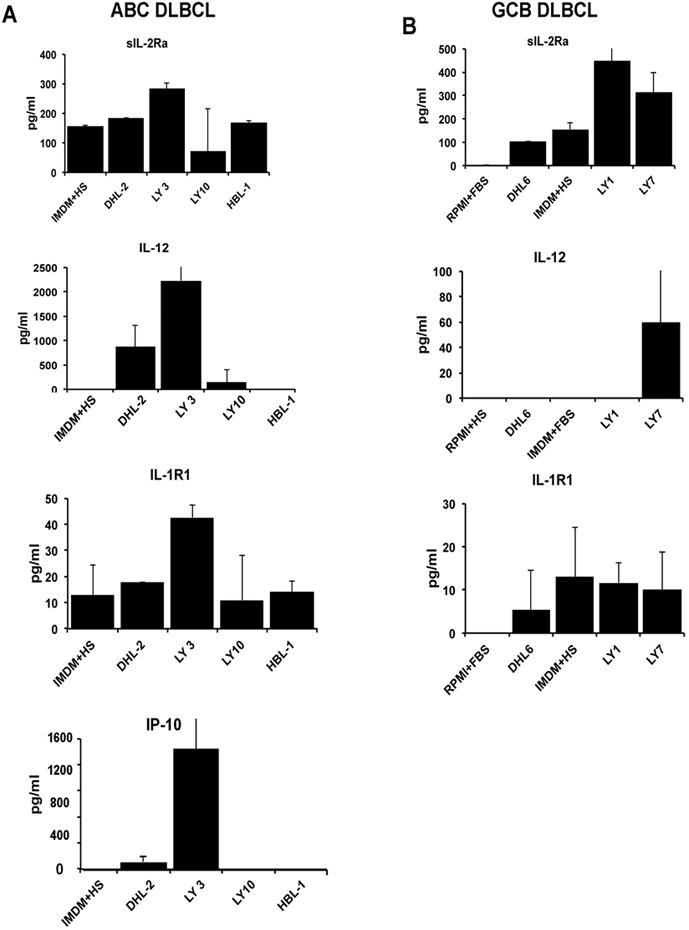
In vitro secretion of elevated cytokines (aIL-2Rα, IL-12, IL-1R1 and IP-10) correlated with monoclonal FLC in DLBCL supernatants. 3.0×106 cells from ABC (A) and GCB (B) DLBCL cell lines were cultured for 48 hours. Supernatant was then collected and ELISA was performed for sIL-2Rα, IL-12, IL-1R1 and IP-10 as described in Material and Method section. Human serum and fetal bovine serum were used as control. Bars represent mean ± SD from three separate experiments.
In-vivo correlation of IP-10 and monoclonal FLC for EFS
We have previously shown that high IP-10 is associated with inferior EFS in DLBCL and with elevated FLC in general.18 In the current study we evaluated the relationship of IP-10 with type of FLC secretion. We found that the majority (81%) of patients with monoclonal FLC also had a high IP-10 (greater than the median), compared to 51% and 45% of the patients with polyclonal or normal FLC, respectively. Patients with elevations of both IP-10 and FLC (polyclonal or monoclonal) had very poor EFS (HR=5.47, 95% CI: 3.07-9.73, p=7.36×10-9) compared to patients with both parameters low or normal (Figure 5). Patients with either high IP-10 or elevated FLC but not both had an intermediate outcome (HR=2.21, 95% CI: 1.27-3.83, p=0.0050).
Figure 5.
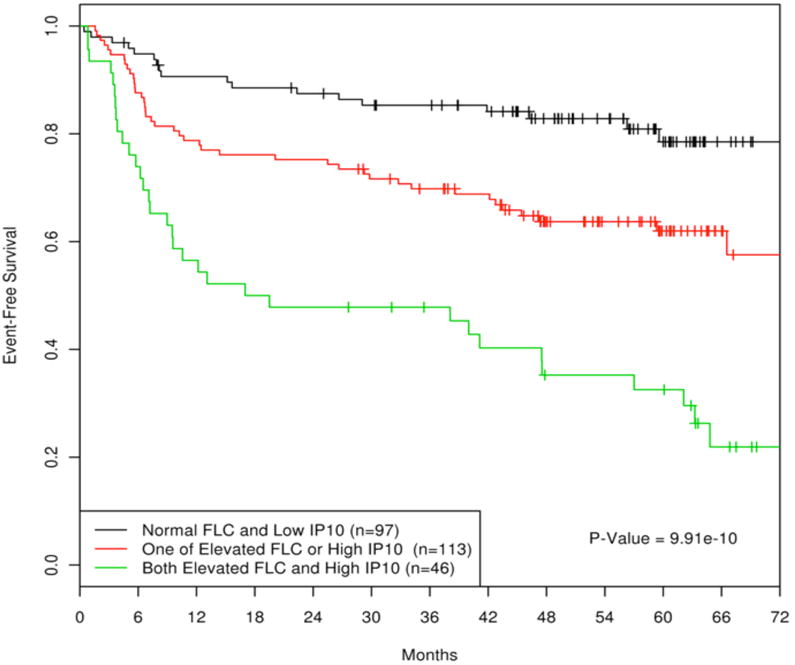
Event-free survival in DLBCL (n=256) by pre-treatment serum FLC and elevated serum IP-10.
Discussion
The era of targeted therapy has made available many new treatments for relapsed DLBCL. Agents such as epratuzumab,24 bortezomib,25 enzastaurine,26 lenalidomide,27,28 and everolimus29 have all demonstrated single-agent activity in relapsed DLBCL. These agents are now being combined with standard R-CHOP. Epratuzumab,15 bortezomib,30,31 and lenalidomide32 have all competed phase I/II trials with encouraging safety and efficacy. The current challenge is to demonstrate whether any of these regimens are superior to RCHOP alone. With the multiple agents to be tested it will be very helpful to have biomarkers that reflect tumor biology to select which patients stand to benefit from the addition of a specific new agent.
In this report we provide long-term follow-up on the prognostic relevance of elevated serum FLC and the type of elevation in untreated DLBCL. This analysis reveals that patients with elevated FLC continue to relapse even after the first two years; whereas, the relapse curve for patients with normal FLC follows the more conventional plateau after the first 2 years.33 A recent report by Jardin et al.34 provides important confirmation of the clinical utility of serum FLC abnormalities in DLBCL. They studied 407 patients with untreated DLBCL for serum FLC and immunoglobulin heavy chain/light chain pairs (HLC). Consistent with our data, patients with abnormal FLC had an inferior PFS and OS compared to those with normal ratios. The finding that abnormal IgMκ/IgMλ ratios are also prognostic is new and not previously reported for DLBCL. The production of serum FLC in patients with DLBCL could be derived from microenvironment cells based on stimuli from tumor cells or from the tumor cells themselves. In multiple myeloma the monoclonal plasma cells secrete the FLC whereas in Hodgkin lymphoma polyclonal FLC are secreted by the benign cells in the tumor microenvironment.35 Although we found no statistical difference from a prognostic standpoint between polyclonal or monoclonal FLC elevations (they both predicted inferior outcome), there was a trend for monoclonal FLC patients having the worst prognosis and there were other biological differences. The finding of a monoclonal FLC predicted for ABC-type DLBCL by IHC; normal and polyclonal FLC patterns did not inform the genotype. Only ABC-type DLBCL cell lines secreted monoclonal FLC. These results are based on a relatively small dataset and require confirmation in large randomized trials and additional DLBCL cell lines require testing. Our studies determined genotype by the typical IHC algorithms that have shown reasonable correlation with GEP.11 The tumors from patients with monoclonal FLC were for the most part classified as ABC-type by all three algorithms. Jardin et al34 also classified a subset (n=199) of their cases into GCB and non-GCB by Hans algorithm. Sixteen percent (15/94) of the patients with GCB type DLBCL had an abnormal HLC ratio compared to 23% (24/105) of non-GCB cases (p=0.2). In addition, 53 cases had GEP and 16 of these had an abnormal HLC ratio (5 were GCB and 11 were ABC, p=0.14) and 8 patients had an abnormal FLC ratio (genotype not reported). Thus, there was a trend towards abnormal ratio FLC or HLC being non-GCB type. Future prospective studies should correlate monoclonal FLC and HLC secretion with DLBCL genotype to confirm or deny this relationship. Until these studies are performed, the FLC and HLC assays will primarily be used as prognostic factors. The fact that the assay is readily available in the clinical laboratory on a daily basis makes it ideal for use as a stratification factor for randomized clinical trials. The trend of monoclonal FLC to be associated with non-GCB-type DLBCL is also potentially useful to initially stratify patients on trial until GEP classification is available.
The serum cytokine profile assayed at the same time point as the FLC also differed by the pattern of FLC abnormality. Patients with monoclonal FLC had significantly elevated concentrations of sIL-2R, IL-12, IL-1R1 and IP-10 cytokines. IL-1R1 and sIL2R elevations were found associated with both polyclonal and monoclonal FLC. Many groups have found sIL2R elevations to be associated with an adverse prognosis in DLBCL.36-41 Serum IL12 is also recognized to be elevated in DLBCL but was not a strong prognostic factor.18 The high IL-12 levels we found were more likely in patients with monoclonal FLC secretion. In B-cell lymphoma IL-12 has been demonstrated to be detrimental by causing T-cell exhaustion.42 IP-10, also known as CXCL10, is a pro-inflammatory cytokine associated with inferior DLBCL prognosis.18 In this study, IP-10 was associated with monoclonal FLC secretion and when IP-10 and abnormal FLC were combined they predicted an especially poor outcome.
In the future, treatment approaches for DLBCL are likely to use the RCHOP-21 backbone with additional agents being selected based on a specific genotype or signal pathway activation profile. Large randomized trials are ongoing that use genotype in the selection and statistical plan to test the hypothesis that new combinations are superior in the non-GCB genotype. Our cell line data demonstrating the ability of the NF-kB inhibitor bortezomib to reduce FLC secretion from ABC-type cell lines is in support of the current European trial of RCHOP + bortezomib vs. RCHOP for patients with non-GCB type DLBCL (NCT01324596). The cytokine secretion results in these cases also offer clues as to potential therapeutic interventions. The finding of elevated IL-1R1 in a subset of DLBCL patients with monoclonal FLC is potentially actionable with the IL-1R antagonist anakinra.43 sIL-2R in monoclonal FLC elevations may be amenable to the addition of basiliximab, a monoclonal antibody to the IL2R α-chain (CD25) used primarily in renal transplantation to prevent acute rejection.44,45 The addition of these inhibitors or blockers of cytokine action may potentially relieve lymphoma related symptoms (fevers, night sweats, weight loss) and fatigue in addition to improving response to therapy. However, proof of this principle will require large randomized trials with cytokine measurements pre- and post-intervention. In the meantime, abnormal FLC and cytokines/chemokines measurements are important for prognosis and stratification in clinical trials.
Acknowledgments
This work was supported in part by P50 CA97274, R01CA127433, R01CA129539 and the Predolin Foundation.
Footnotes
Authorship: Contribution: TEW interpreted the data, wrote the paper and provided clinical samples. MM performed all the statistical analysis and interpreted the data. MS performed lab based experiments. CL assisted in statistical analysis. WM performed IHC and provided clinical samples. BL provided clinical specimens. JAK performed FLC assay. MG designed and supervised the research, analyzed and interpreted data, finalized the figures and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests
References
- 1.Bradwell AR, Carr-Smith HD, Mead GP, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001;47(4):673–680. [PubMed] [Google Scholar]
- 2.Katzmann JA, Abraham RS, Dispenzieri A, Lust JA, Kyle RA. Diagnostic performance of quantitative kappa and lambda free light chain assays in clinical practice. Clin Chem. 2005;51(5):878–881. doi: 10.1373/clinchem.2004.046870. [DOI] [PubMed] [Google Scholar]
- 3.Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215–224. doi: 10.1038/leu.2008.307. Prepublished on 2008/11/21 as DOI leu2008307 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Dispenzieri A, Katzmann JA, Kyle RA, et al. Use of nonclonal serum immunoglobulin free light chains to predict overall survival in the general population. Mayo Clin Proc. 2012;87(6):517–523. doi: 10.1016/j.mayocp.2012.03.009. Prepublished on 2012/06/09 as DOI S0025-6196(12)00388-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anandram S, Assi LK, Lovatt T, et al. Elevated, combined serum free light chain levels and increased mortality: a 5-year follow-up, UK study. J Clin Pathol. 2012;65(11):1036–1042. doi: 10.1136/jclinpath-2012-200910. Prepublished on 2012/08/28 as DOI. [DOI] [PubMed] [Google Scholar]
- 6.Martin W, Abraham R, Shanafelt T, et al. Serum-free light chain-a new biomarker for patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Transl Res. 2007;149(4):231–235. doi: 10.1016/j.trsl.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Maurer MJ, Micallef IN, Cerhan JR, et al. Elevated serum free light chains are associated with event-free and overall survival in two independent cohorts of patients with diffuse large B-cell lymphoma. J Clin Oncol. 2011;29(12):1620–1626. doi: 10.1200/JCO.2010.29.4413. Prepublished on 2011/03/09 as DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. doi: 10.1038/35000501. Prepublished on 2000/02/17 as DOI. [DOI] [PubMed] [Google Scholar]
- 9.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(25):1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 10.Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198(6):851–862. doi: 10.1084/jem.20031074. Prepublished on 2003/09/17 as DOI. 10.1084/jem.20031074 jem.20031074 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer PN, Fu K, Greiner TC, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol. 2011;29(2):200–207. doi: 10.1200/JCO.2010.30.0368. Prepublished on 2010/12/08 as DOI JCO.2010.30.0368 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 13.Falini B, Fizzotti M, Pucciarini A, et al. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood. 2000;95(6):2084–2092. Prepublished on 2000/03/09 as DOI. [PubMed] [Google Scholar]
- 14.Cerhan JR, Fredericksen ZS, Wang AH, et al. Design and validity of a clinic-based case-control study on the molecular epidemiology of lymphoma. Int J Mol Epidemiol Genet. 2011;2(2):95–113. Prepublished on 2011/06/21 as DOI. [PMC free article] [PubMed] [Google Scholar]
- 15.Micallef IN, Maurer MJ, Wiseman GA, et al. Epratuzumab with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy in patients with previously untreated diffuse large B-cell lymphoma. Blood. 2011;118(15):4053–4061. doi: 10.1182/blood-2011-02-336990. Prepublished on 2011/06/16 as DOI blood-2011-02-336990 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48(9):1437–1444. [PubMed] [Google Scholar]
- 17.Gupta M, Han JJ, Stenson M, et al. Elevated serum IL-10 levels in diffuse large B-cell lymphoma: a mechanism of aberrant JAK2 activation. Blood. 2012;119(12):2844–2853. doi: 10.1182/blood-2011-10-388538. Prepublished on 2012/02/11 as DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansell SM, Maurer MJ, Ziesmer SC, et al. Elevated pretreatment serum levels of interferon-inducible protein-10 (CXCL10) predict disease relapse and prognosis in diffuse large B-cell lymphoma patients. Am J Hematol. 2012;87(9):865–869. doi: 10.1002/ajh.23259. Prepublished on 2012/06/08 as DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta M, Han JJ, Stenson M, Wellik L, Witzig TE. Regulation of STAT3 by histone deacetylase-3 in diffuse large B-cell lymphoma: implications for therapy. Leukemia. 2012;26(6):1356–1364. doi: 10.1038/leu.2011.340. Prepublished on 2011/11/26 as DOI. 10.1038/leu.2011.340 leu2011340 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta M, Maurer MJ, Wellik LE, et al. Expression of Myc, but not pSTAT3, is an adverse prognostic factor for diffuse large B-cell lymphoma treated with epratuzumab/R-CHOP. Blood. 2012;120(22):4400–4406. doi: 10.1182/blood-2012-05-428466. Prepublished on 2012/09/29 as DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding BB, Yu JJ, Yu RY, et al. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood. 2008;111(3):1515–1523. doi: 10.1182/blood-2007-04-087734. Prepublished on 2007/10/24 as DOI blood-2007-04-087734 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam LT, Wright G, Davis RE, et al. Cooperative signaling through the signal transducer and activator of transcription 3 and nuclear factor-{kappa}B pathways in subtypes of diffuse large B-cell lymphoma. Blood. 2008;111(7):3701–3713. doi: 10.1182/blood-2007-09-111948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470(7332):115–119. doi: 10.1038/nature09671. Prepublished on 2010/12/24 as DOI nature09671 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonard JP, Coleman M, Ketas JC, et al. Epratuzumab, a Humanized Anti-CD22 Antibody, in Aggressive Non-Hodgkin's Lymphoma: Phase I/II Clinical Trial Results. Clin Cancer Res. 2004;10(16):5327–5334. doi: 10.1158/1078-0432.CCR-04-0294. [DOI] [PubMed] [Google Scholar]
- 25.Dunleavy K, Pittaluga S, Czuczman MS, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009;113(24):6069–6076. doi: 10.1182/blood-2009-01-199679. Prepublished on 2009/04/22 as DOI blood-2009-01-199679 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson MJ, Kahl BS, Vose JM, et al. Phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2007;25(13):1741–1746. doi: 10.1200/JCO.2006.09.3146. Prepublished on 2007/03/29 as DOI JCO.2006.09.3146 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Wiernik PH, Lossos IS, Tuscano JM, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2008;26(30):4952–4957. doi: 10.1200/JCO.2007.15.3429. [DOI] [PubMed] [Google Scholar]
- 28.Witzig TE, Vose JM, Zinzani PL, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin's lymphoma. Ann Oncol. 2011;22(7):1622–1627. doi: 10.1093/annonc/mdq626. Prepublished on 2011/01/14 as DOI mdq626 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Witzig TE, Reeder CB, LaPlant BR, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia. 2011;25(2):341–347. doi: 10.1038/leu.2010.226. Prepublished on 2010/12/08 as DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furman RR, Martin P, Ruan J, et al. Phase 1 trial of bortezomib plus R-CHOP in previously untreated patients with aggressive non-Hodgkin lymphoma. Cancer. 2010;116(23):5432–5439. doi: 10.1002/cncr.25509. Prepublished on 2010/07/29 as DOI. [DOI] [PubMed] [Google Scholar]
- 31.Ruan J, Martin P, Furman RR, et al. Bortezomib Plus CHOP-Rituximab for Previously Untreated Diffuse Large B-Cell Lymphoma and Mantle Cell Lymphoma. J Clin Oncol. 2011;29(6):690–697. doi: 10.1200/JCO.2010.31.1142. Prepublished on 2010/12/30 as DOI JCO.2010.31.1142 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Nowakowski GS, LaPlant B, Habermann TM, et al. Lenalidomide can be safely combined with R-CHOP (R2CHOP) in the initial chemotherapy for aggressive B-cell lymphomas: phase I study. Leukemia. 2011;25(12):1877–1881. doi: 10.1038/leu.2011.165. Prepublished on 2011/07/02 as DOI. 10.1038/leu.2011.165 leu2011165 [pii] [DOI] [PubMed] [Google Scholar]
- 33.Maurer MJ, Ghesquieres H, Witzig TE, et al. Newly Diagnosed Diffuse Large B-Cell Lymphoma Patients Treated with Immunochemotherapy Who Are Alive and Progression Free 12 Months After Diagnosis Have a Subsequent Overall Survival Similar to That of the General Population. ASH Annual Meeting Abstracts. 2012;120(21):1540. [Google Scholar]
- 34.Jardin F, Delfau-Larue MH, Molina TJ, et al. Immunoglobulin heavy chain/light chain pair measurement is associated with survival in diffuse large B-cell lymphoma. Leuk Lymphoma. 2013 doi: 10.3109/10428194.2013.767456. Prepublished on 2013/01/19 as DOI. [DOI] [PubMed] [Google Scholar]
- 35.Thompson CA, Maurer MJ, Cerhan JR, et al. Elevated serum free light chains are associated with inferior event free and overall survival in Hodgkin lymphoma. Am J Hematol. 2011;86(12):998–1000. doi: 10.1002/ajh.22168. Prepublished on 2011/10/19 as DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ennishi D, Yokoyama M, Terui Y, et al. Soluble interleukin-2 receptor retains prognostic value in patients with diffuse large B-cell lymphoma receiving rituximab plus CHOP (RCHOP) therapy. Ann Oncol. 2009;20(3):526–533. doi: 10.1093/annonc/mdn677. Prepublished on 2008/12/17 as DOI. [DOI] [PubMed] [Google Scholar]
- 37.Goto N, Tsurumi H, Goto H, et al. Serum soluble interleukin-2 receptor (sIL-2R) level is associated with the outcome of patients with diffuse large B cell lymphoma treated with R-CHOP regimens. Ann Hematol. 2012;91(5):705–714. doi: 10.1007/s00277-011-1363-4. Prepublished on 2011/12/21 as DOI. [DOI] [PubMed] [Google Scholar]
- 38.Morito T, Fujihara M, Asaoku H, et al. Serum soluble interleukin-2 receptor level and immunophenotype are prognostic factors for patients with diffuse large B-cell lymphoma. Cancer Sci. 2009;100(7):1255–1260. doi: 10.1111/j.1349-7006.2009.01167.x. Prepublished on 2009/05/13 as DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oki Y, Kato H, Matsuo K, et al. Prognostic value of serum soluble interleukin-2 receptor level in patients with diffuse large B cell lymphoma, treated with CHOP- or RCHOP-based therapy. Leuk Lymphoma. 2008;49(7):1345–1351. doi: 10.1080/10428190802108888. Prepublished on 2008/05/03 as DOI. [DOI] [PubMed] [Google Scholar]
- 40.Tomita N, Sakai R, Fujisawa S, et al. SIL index, comprising stage, soluble interleukin-2 receptor, and lactate dehydrogenase, is a useful prognostic predictor in diffuse large B-cell lymphoma. Cancer Sci. 2012;103(8):1518–1523. doi: 10.1111/j.1349-7006.2012.02331.x. Prepublished on 2012/05/17 as DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamauchi T, Matsuda Y, Takai M, et al. Early relapse is associated with high serum soluble interleukin-2 receptor after the sixth cycle of R-CHOP chemotherapy in patients with advanced diffuse large B-cell lymphoma. Anticancer Res. 2012;32(11):5051–5057. Prepublished on 2012/11/17 as DOI. [PubMed] [Google Scholar]
- 42.Yang ZZ, Grote DM, Ziesmer SC, et al. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J Clin Invest. 2012;122(4):1271–1282. doi: 10.1172/JCI59806. Prepublished on 2012/03/20 as DOI. 10.1172/JCI59806 59806 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lust JA, Lacy MQ, Zeldenrust SR, et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1{beta}-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84(2):114–122. doi: 10.4065/84.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teixeira C, El Bouazzaoui Z, Guerra J, et al. Are there real advantages of induction therapy with basiliximab in renal transplantation? Transplant Proc. 2013;45(3):1073–1075. doi: 10.1016/j.transproceed.2013.02.088. Prepublished on 2013/04/30 as DOI. [DOI] [PubMed] [Google Scholar]
- 45.Chapman TM, Keating GM. Basiliximab: a review of its use as induction therapy in renal transplantation. Drugs. 2003;63(24):2803–2835. doi: 10.2165/00003495-200363240-00009. Prepublished on 2003/12/11 as DOI. [DOI] [PubMed] [Google Scholar]


