Abstract
It has long been known that individuals will engage in voluntary inhalation of volatile solvents for their rewarding effects. However, research into the neurobiology of these agents has lagged behind that of more commonly used drugs of abuse such as psychostimulants, alcohol and nicotine. This imbalance has begun to shift in recent years as the serious effects of abused inhalants, especially among children and adolescents, on brain function and behavior have become appreciated and scientifically documented. In this review, we discuss the physicochemical and pharmacological properties of toluene, a representative member of a large class of organic solvents commonly used as inhalants. This is followed by a brief summary of the clinical and pre-clinical evidence showing that toluene and related solvents produce significant effects on brain structures and processes involved in the rewarding aspects of drugs. This is highlighted by tables highlighting toluene’s effect on behaviors (reward, motor effects, learning, etc.) and cellular proteins (e.g. voltage and ligand-gated ion channels) closely associated the actions of abused substances. These sections demonstrate not only the significant progress that has been made in understanding the neurobiological basis for solvent abuse but also reveal the challenges that remain in developing a coherent understanding of this often overlooked class of drugs of abuse.
General
Toluene (toluol, methylbenzene, phenylmethane) is an organic solvent widely used in many industrial processes including plastic production, chemical synthesis and gasoline manufacturing. A volatile liquid (i.e., it becomes vapor at room temperature), toluene produces psychoactive effects when intentionally inhaled in pure form or from numerous commercial products, such as solvents, gasoline, paints, varnishes, paint thinner, adhesives and inks, among other products [1].
Physicochemical properties
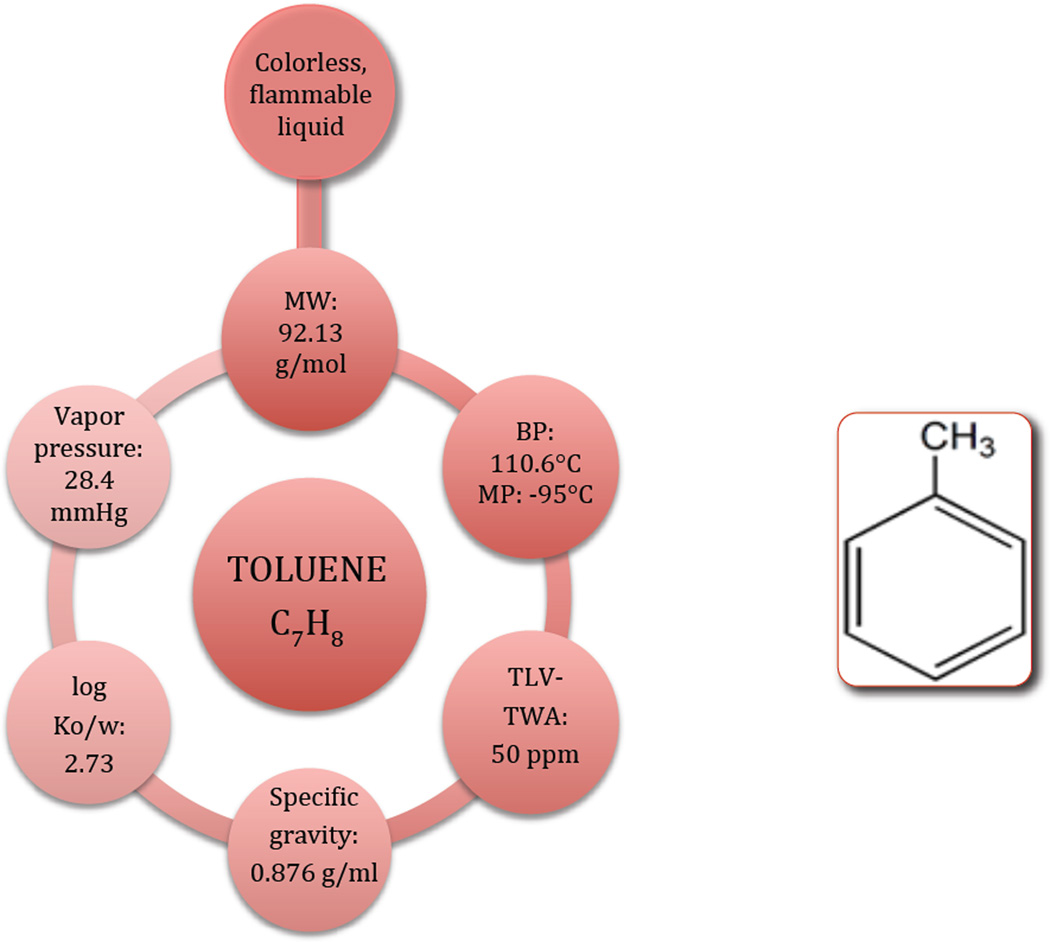
Toluene’s structure and physicochemical properties are shown in Fig. 1. An aromatic hydrocarbon, toluene is lighter than water in its liquid form, but three times heavier than air as a vapor, has high affinity for lipids (log octanol/water partition coefficient = 2.73) and is flammable with a low flash point (the lowest temperature at which it can vaporize to form an ignitable mixture in air) of 4.4 °C [2].
Figure 1.
Chemical and physical properties of toluene. BP: boiling point; MP: melting point; TLV-TWA: Threshold limit value–Time-weighted average: an allowable exposure concentration averaged over a normal 8-hour workday or 40-hour workweek; log Ko/w: octanol/water partition coefficient.
Exposure
Individuals can be exposed to low toluene concentrations when they use household/school products or fill the car with gasoline, but these activities generally do not pose significant health risks when performed in well-ventilated areas. Occupational exposure in workplaces such as factories, workshops or refineries usually occurs several hours a day, five days a week. Regulations exist to prevent physiological and behavioral adverse consequences and although they vary among countries, safe exposure limits are usually in the range of 10 to 100 ppm. The immediately dangerous to life and health limit (IDLH) has been estimated at 500 ppm [3]. In spite of this, people who misuse toluene-based products are exposed to concentrations of several thousands ppm following an intermittent pattern of inhalation [4, 5].
Several methods are used for voluntarily inhaling toluene-based products. “Huffing” refers to breathing fumes from a solvent-soaked rag or tissue paper that is held in a hand and placed near the nose and mouth; “sniffing” is the direct nasal inhalation from containers; “bagging” refers to breathing fumes from substances placed in a bag; and “cuffing” means inhaling vapors from cuffs or sleeves soaked with solvents and raised to the mouse and nose [6]. In Australia, “chroming” is used as synonymous with inhaling paint sprays which contain toluene and propellant gases [7]. Using any of these methods, inhalant effects appear very quickly, usually within seconds, and they last from 15 to 60 min. In order to increase the duration of effects, users repeat the exposure to maintain the desired level of intoxication.
Metabolism
Toluene is rapidly absorbed through the lungs. Gastrointestinal and dermal absorption also occurs. Once absorbed, it is distributed to highly perfused lipid rich organs. Because of its high affinity for lipids, toluene can readily cross the brain blood barrier and the placenta. As perfusion in the brain is very high, brain’s toluene concentration is also high in this region. Most inhaled toluene (95%) is metabolized in the liver first to benzyl alcohol and benzoic acid, which is then conjugated with glycine to form hippuric acid. Conversion to cresol is a minor pathway [8]. Hippuric acid is dissociated in hippurate anions and protons. Protons are titrated by bicarbonate and some of the anions are excreted in the urine with ammonium. After binge toluene exposure, there is an excess of hippuric acid, which can produce not only the excretion of ammonium, but also of sodium and potassium combined with hippurate anions, resulting is metabolic acidosis and hypokalemia. Low levels of potassium are associated with weakness, muscle spasticity, cardiac arrhythmias and other serious complications. The rate-limiting step in toluene metabolism is conversion to benzyl alcohol through cytochrome P-450 in the liver. Several P450 isoenzymes are involved in toluene metabolism, among which CYP2E1 has been described as the most active in forming benzyl alcohol [9] and one which can be induced by repeated toluene exposure [10].
Effects: the clinical evidence
Toluene is the most commonly misused solvent and also the best studied, both in terms of behavioral effects and action mechanisms. Acute and chronic toluene’s effects are summarized in Table 1. Briefly, toluene intoxication resembles ethanol intoxication in some aspects because toluene produces an initial euphoria and excitation, followed by a more prolonged inhibition. Motor incoordination, dizziness, relaxation and lightheadedness are also characteristic of toluene intoxication. Unlike other central nervous system (CNS) depressant drugs, toluene produces illusions and hallucinations [11, 12]. Of particular concern is that even acute inhalation can lead to life threatening conditions due to poor oxygenation, cardiac arrhythmias and other complications associated with hypokalemia. “Sudden sniffing death” has been documented since the 1970s [13, 14] and can be caused by cardiac arrhythmias, hypothermia, hypoxia or a combination of these factors [15].
Table 1.
Effects of toluene exposure
| Acute effects | Chronic effects |
|---|---|
|
|
Long-term effects of toluene inhalation vary depending on age, patterns of use (duration and frequency), misused products and concomitant exposure to other drugs. Chronic irritation of eyes and respiratory airways is common. Heavy long-term toluene abuse has been associated with general cognitive impairment (memory deficits, difficulty to concentrate, etc.), decreased IQ, increased impulsivity and impaired judgment [16, 17]. Imaging studies have shown that toluene chronic exposure can lead to neurobiological abnormalities, which have been related to white matter damage (leukoencephalopathy). Interestingly, in a study using proton magnetic resonance spectroscopy, axonal damage, rather than demyelination was found [18].
A well-described complication of toluene exposure is renal tubular acidosis. Although it can happen after an acute binge episode of intentional toluene inhalation, it is more frequent in chronic users [10]. Renal failure can also occur, and it is attributed to acute tubular necrosis caused by hypotension or possibly rhabdomyolysis. Liver toxicity may also be a consequence of toluene exposure [19].
Toluene produces tinnitus and can cause hearing loss after chronic exposure. Hearing frequencies affected by toluene are different from those affected by noise but both factors can act synergistically to diminish hearing [20].
Due to its lipophilic nature, toluene crosses biological membranes easily including the placental barrier. If inhalation occurs during pregnancy, the fetus can be affected with developmental disorders, physical malformations or even death. A fetal solvent syndrome (FSS), analogous to the fetal alcohol spectrum disorder (FASD) has been described. Thus, infants born from mothers who misused toluene-based products can have smaller heads, a thin upper lip, lower set ears and other signs similar to what has been described for FASD. Follow-up studies of children exposed during gestation to solvents show growth retardation, language impairment and cerebellar dysfunction (reviewed in [15, 21]).
Although the clinical effects of toluene are relatively well known, many studies have analyzed the damages caused by inhalation of toluene-based products rather than toluene itself. Human studies are limited by ethical concerns and the occurrence of confounding variables such as malnourishment and concomitant use of other drugs. Because of this, animal studies have been very valuable to determine cause-effects relationships between toluene exposure, behavior and sites of action. Also, under controlled experimental conditions it has been possible to establish concentration- dependent effects.
Preclinical evidence
-Neurobehavioral studies
Most behavioral studies have been done in rodents. Of special interest for this review are those that used binge patterns of toluene exposure either acutely or chronically, but many of these effects have also been described for conditions of prolonged exposures to low toluene concentrations. Some of the most representative studies are summarized in Table 2.
Table 2.
Preclinical studies on toluene’s effects
| Acute Effects | Preparation | Species | Refs. |
|---|---|---|---|
| Reinforcing properties | Intravenous self-administration | Mice | [25] |
| Conditioned place preference | Rats | [23] | |
| Mice | [22] | ||
| Discriminative stimulus effects | Toluene acts as a discriminative stimulus | Mice | [27, 28] |
| Amphetamine-like effects | Mice | [32] | |
| CNS depressant-like effects | Mice | [29, 30] | |
| Anxiolytic-like | Burying behavior test (decreased cumulative time burying the prod) | Mice | [35, 36] |
| Elevated plus maze (increased number of entries and time spent in open arms) | Mice | [34] | |
| Geller-Seifter conflict test (active response reinstatement after punishment) | Rats | [31, 37] | |
| Motor Incoordination | Rota rod test | Rats | [50] |
| Anticonvulsant | PTZ-induced seizures (decreased percentage of convulsing animals) | Rats | [37] |
| NMDA-induced seizures (decreased percentage of convulsing animals; protection against death) | Mice | [38, 41] | |
| Nicotine-, picrotoxin- and bicucullin-induced seizures (increased seizure threshold) | Mice | [41] | |
| Antidepressant-like | Forced swimming test and tail suspension test (decreased immobility). | Mice | [44] |
| Altered locomotion | Open field test Low concentrations: increased locomotion. High concentrations: decreased locomotion. |
Rats | [45, 47, 48, 52, 58] |
| Impaired Learning and Memory | Passive avoidance test (long-term memory) | Rats | [52] |
| Novel object recognition test (reduced novel object exploration) | Rats | [52] | |
| Mice | [49, 53] | ||
| Pronociception | Hot plate test and tail flick tests (increased latency to response) | Mice | [36, 54] |
| Antinociception | Foot-shock test (increased threshold to elicit a response. | Rats | [52] |
| Impulsivity-like | Waiting for reward task | Mice | [65] |
| Social interaction | Social interaction test (reduced contact with a partner) | Mice | [53] |
| Chronic exposure | |||
| Impaired learning and memory | Morris water maze; object recognition, passive avoidance test | Rat | [52, 66] |
| Sensitization to hyperlocomotion | Open field test | Mice | [67] |
| Prenatal exposure | |||
| Increased locomotor activity | Open field test | Rat | [62] |
| Delayed reflexes | Postnatal test battery (surface righting, air righting, auditory startle) | Rat | [62] |
| Impulsivity-like | Waiting for reward task | Rat | [65] |
| Sensitization to hyperlocomotion | Open field test (amphetamine induced locomotion) | Rat | [68] |
Being a misused drug, toluene has reinforcing effects. This has been shown using the conditioned preference place procedure [22–24], i.v. self-administration [25] and intracranial self-stimulation [26]. Inhaled toluene acts as a robust discriminative stimulus [27, 28] and also produces CNS depressant-like [29–31] amphetamine-like [32] and PCP-like discriminative effects [33]. Similar to other CNS depressant drugs, toluene has anxiolytic-like properties [34–36], anticonvulsant effects [37–41] and impairs locomotor coordination [42, 43]. It also exerts antidepressant-like actions [44] and a biphasic locomotor response; i.e., increased and decreased activity at low and high concentrations, respectively [45–48]. The detrimental effects on learning, short-term and long-term memory produced by toluene are also well documented [49–53]. A species-specific effect is observed regarding nociception because toluene increases the response to a noxious stimulus in mice [54] but has antinociceptive effects in rats [52]. Hypothermia [55–57] and tachycardia have also been described after toluene exposure [58].
There are relatively few studies concerning tolerance development and sensitization after chronic toluene exposure. The most consistent finding is sensitization to hyperlocomotion effects [48, 50, 59]. As in humans, prenatal exposure to toluene has been associated with malformations [60], growth retardation [61], delayed reflexes [62] and attention deficit in pups (López-Rubalcava et al., this issue). Some of these deleterious effects of prenatal toluene exposure can be enhanced by stress [63, 64]. Exposure to toluene during gestation also results in deficient body weight gain and poor lactation in dams [64].
-Sites of action for toluene
The molecular and cellular targets for abused inhalants including toluene have been investigated using a variety of in vitro and in vivo preparations. Not surprisingly, many of these studies have focused on defining the effects of toluene on ion channels that are critically involved in regulating neuronal excitability. As summarized below in Table 3, results from these studies indicate that both voltage-gated and ligand-gated ion channels are affected by concentrations of toluene associated with voluntary inhalation of these substances. In addition, these studies suggest that toluene and other related solvents possess a surprising degree of selectivity given their rather simple chemical structure. For example, toluene was shown to significantly inhibit the NMDA subtype of glutamate-activated ion channels while having little effect on the closely related AMPA subtype of ionotropic glutamate receptor [69, 70]. Moreover, within the NMDA family, receptors composed of the GluN2B subunit were considerably more sensitive to toluene inhibition than other NMDA subtypes. A similar effect was observed for nicotinic acetylcholine receptors (nAchRs) where α4β2 receptors were much more sensitive to toluene inhibition than α7 nAchRs [71]. Amongst the P2X family of ATP-gated channels, toluene inhibits the function of some subtypes (P2X2, P2X4) but enhances currents through P2X3 containing receptors [72]. These findings suggest that there are distinct sites of action for toluene on individual channel subunits and suggest that regional and anatomical differences in subunit expression are important determinants of solvent action.
Table 3.
Summary of the effects of toluene on recombinant and native ion channels.
| Receptor Name | Subunit Composition | Effect | Ref. |
|---|---|---|---|
| AMPA | GluA1; GluA1/2 | None | [69] |
| GluA6 | Increase | [69] | |
| Native neuron | None/Decreasea | [70, 73] | |
| NMDA | GluN1/N2A | Decrease | [69] |
| GluN1/N2B | Decrease | [69] | |
| GluN1/N2C | Decrease | [69] | |
| Native neuron | Decrease | [74] | |
| GABA | α1/β1 | Increase | [75] |
| Native neuron | Increaseb | [73, 76, 77] | |
| Glycine | α1 | Increase | [75] |
| 5HT3 | 5HT3 | Increase | [77] |
| nAchR | α4/β2 | Decrease | [71, 74] |
| α4/β4 | Decrease | [71] | |
| α3/β2 | Decrease | [71, 74] | |
| α3/β4 | Decrease | [71] | |
| α7 | Decrease | [71, 74] | |
| Native neuron | Decrease | [71] | |
| ATP | P2X2 | Increase | [72] |
| P2X2/3 | Increase | [72] | |
| P2X3 | Decreased | [72] | |
| P2X4 | Increase | [72] | |
| P2X4/6 | Increase | [72] | |
| Sodium Channels | Nav1.5 (cardiac) | Decrease | [78] |
| Native cardiac | Decrease | [78] | |
| Nav1.4 (skeletal) | Decrease | [79] | |
| Native neuron | None | [73] | |
| Ca++ Channels | Cav1/Cav2 | Decrease | [80, 81] |
| Native neuron | Decrease | [81] | |
| K+ Channels | mSlo | Decrease | [82] |
| Girk2 | Decrease | [82] | |
| Girk1/2; Girk1/4 | None | [82] | |
| Gap Junction | Native (HEK cell) | Decrease | [83] |
After the identification of some of toluene’s molecular actions, several research groups studied metabolic and neurochemical changes associated with toluene exposure. For example, using microPET, [84] it has been shown that acute and repeated toluene exposure markedly reduces metabolic function in rat brain. This effect was regionally specific, with the hippocampus, pons and thalamus as the more affected areas. Other researchers have found that toluene produces increases in dopamine release and dopaminergic neurons’ activity ([85–88], reviewed in Woodward and Beckley., this issue), regional brain changes in glutamate, glutamine and monoamine levels [89–91], as well as changes in NMDA and GABAA receptor densities or subunit composition [92–94]. Toluene’s apoptotic effects have also been described [95] and, interestingly, these actions can be lessened by placing animals in enriched environments [96]. Recent studies show that repeated toluene also results in epigenetic changes that might have a long-term impact in gene expression and behavior [52, 97]. Other effects of toluene such as increased oxidative stress seem to contribute to the detrimetal effects of prolonged toluene exposure [98].
In conclusion, the last 15 years have provided extensive evidence of the molecular, cellular and systemic actions of toluene and have firmly established solvents as important drugs of abuse. Despite these advances, it remains an interesting challenge to identify the most relevant molecular mechanisms that underlie specific effects of toluene and related solvents. There is recent evidence indicating that different neurotransmitter systems are activated at different doses/concentrations of toluene [55] and it is likely that similar differences exist regarding acute versus chronic exposures to these solvents. Of particular interest in understanding the long-term effects of inhalant use is how exposure to these agents during adolescence impacts normal brain development and cognition and behavior in the adult. This is particularly relevant for frontal cortical areas that undergo significant maturation during the time that many solvent users are experimenting with these agents. In addition, there is clear evidence that stress affects these same frontal areas suggesting that the deleterious effects of inhalants may be exacerbated by environmental and psychosocial factors (homelessness, poor family structure, etc.) often associated with use of abused inhalants. From an experimental standpoint, there is also a need to conduct studies utilizing relevant mixtures of solvents. To date, most animal based reports have used single compounds with toluene being considered the representative volatile solvent. But it is clear that many individuals who misuse inhalants are exposed to complex mixtures of solvents that may produce effects that are different from those observed with toluene alone. While this presents a more challenging experimental design, it likely to be more informative and may identify novel sites or mechanisms of action that would not be revealed with studies of single solvents. Finally, efforts are needed to better understand how other commonly used drugs of abuse may affect the actions of abused inhalants. With the recent discovery of the neural targets of toluene and related solvents, it is clear that there is substantial overlap in the cellular and molecular actions of these agents with other drugs such as alcohol, nicotine, and marijuana. These finding suggest that the effects of abused inhalants on brain circuits that underlie reward, cognition and behavioral control may be amplified or altered by chronic use of these other commonly abused substances.
Acknowledgments
This work was partially supported to grant R01 DA013951 (JJW) and _____ scholarship from Conacyt, Mexico (MTR-G). The authors thank Monserrat Armenta-Reséndiz for preparation of Fig. 1.
References
- 1.Balster RL, Cruz SL, Howard MO, Dell CA, Cottler LB. Classification of abused inhalants. Addiction. 2009;104(6):878–882. doi: 10.1111/j.1360-0443.2008.02494.x. [DOI] [PubMed] [Google Scholar]
- 2.ASTDR. Toluene Chemical and Physical Properties [Google Scholar]
- 3.Administration, O.S.a.H. Toluene. [11/14/2013]; Available from: https://www.osha.gov/SLTC/toluene/exposure_limits.html.
- 4.Marjot R, McLeod AA. Chronic non-neurological toxicity from volatile substance abuse. Hum Toxicol. 1989;8(4):301–306. doi: 10.1177/096032718900800408. [DOI] [PubMed] [Google Scholar]
- 5.Sharp CW, Rosenber N, Beauvais F. Inhalant- Related Disorders. In: Tasman A, editor. Psychiatry. John Wiley & Sons; 2008. pp. 1127–1148. [Google Scholar]
- 6.Cruz SL, Bowen SE. Inhalant abuse. In: Ubach MM, Mondragon-Ceballos R, editors. Neural Mecahnisms of Action of Drugs of Abuse and Natural Reinforcers. Kerala, India: Research Signpost; 2008. pp. 61–87. [Google Scholar]
- 7.Takagi MJ, Yücel M, Lubman DI. The dark side of sniffing: paint colour affects intoxication experiences among adolescent inhalant users. Drug Alcohol Review. 2010;29(4):452–455. doi: 10.1111/j.1465-3362.2009.00162.x. [DOI] [PubMed] [Google Scholar]
- 8.Arlien-Soborg P. Solvent neurotoxicity. Boca Raton, Florida: CRC Press; 1992. p. 382. [Google Scholar]
- 9.Nakajima T, Wang RS, Elovaara E, Gonzalez FJ, Gelboin HV, Raunio H, Pelkonen O, Vainio H, Aoyama T. Toluene metabolism by cDNA-expressed human hepatic cytochrome P450. Biochem Pharmacol. 1997;53(3):271–277. doi: 10.1016/s0006-2952(96)00652-1. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima T, Wang RS. Induction of cytochrome P450 by toluene. Int J Biochem. 1994;26(12):1333–1340. doi: 10.1016/0020-711x(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 11.MacLean S. Global selves: marginalised young people and aesthetic reflexivity in inhalant drug use. Journal of Youth Studies. 2007;10(4):399–418. [Google Scholar]
- 12.Cruz SL, Dominguez M. Misusing volatile substances for their hallucinatory effects: a qualitative pilot study with Mexican teenagers and a pharmacological discussion of their hallucinations. Subst Use Misuse. 2011;46(Suppl 1):84–94. doi: 10.3109/10826084.2011.580222. [DOI] [PubMed] [Google Scholar]
- 13.Bass M. Sudden sniffing death. JAMA. 1970;212(12):2075–2079. [PubMed] [Google Scholar]
- 14.Bowen SE, Daniel J, Balster RL. Deaths associated with inhalant abuse in Virginia from 1987 to 1996. Drug Alcohol Depend. 1999;53(3):239–245. doi: 10.1016/s0376-8716(98)00139-2. [DOI] [PubMed] [Google Scholar]
- 15.Bowen SE. Two serious and challenging medical complications associated with volatile substance misuse: sudden sniffing death and fetal solvent syndrome. Subst Use Misuse. 2011;46(Suppl 1):68–72. doi: 10.3109/10826084.2011.580220. [DOI] [PubMed] [Google Scholar]
- 16.Yucel M, Takagi M, Walterfang M, Lubman DI. Toluene misuse and long-term harms: a systematic review of the neuropsychological and neuroimaging literature. Neurosci Biobehav Rev. 2008;32(5):910–926. doi: 10.1016/j.neubiorev.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Howard MO, Bowen SE, Garland EL, Perron BE, Vaughn MG. Inhalant use and inhalant use disorders in the United States. Addict Sci Clin Pract. 2011;6(1):18–31. [PMC free article] [PubMed] [Google Scholar]
- 18.Aydin K, Sencer S, Demir T, Ogel K, Tunaci A, Minareci O. Cranial MR findings in chronic toluene abuse by inhalation. AJNR Am J Neuroradiol. 2002;23(7):1173–1179. [PMC free article] [PubMed] [Google Scholar]
- 19.Malaguarnera G, Cataudella E, Giordano M, Nunnari G, Chisari G, Malaguarnera M. Toxic hepatitis in occupational exposure to solvents. World J Gastroenterol. 2012;18(22):2756–2766. doi: 10.3748/wjg.v18.i22.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuente A, McPherson B. Organic solvents and hearing loss: The challenge for audiology. Int J Audiol. 2006;45(7):367–381. doi: 10.1080/14992020600753205. [DOI] [PubMed] [Google Scholar]
- 21.Hannigan JH, Bowen SE. Reproductive toxicology and teratology of abused toluene. Syst Biol Reprod Med. 2010;56(2):184–200. doi: 10.3109/19396360903377195. [DOI] [PubMed] [Google Scholar]
- 22.Funada M, Sato M, Makino Y, Wada K. Evaluation of rewarding effect of toluene by the conditioned place preference procedure in mice. Brain Res Brain Res Protoc. 2002;10(1):47–54. doi: 10.1016/s1385-299x(02)00182-4. [DOI] [PubMed] [Google Scholar]
- 23.Gerasimov MR, Collier L, Ferrieri A, Alexoff D, Lee D, Gifford AN, Balster RL. Toluene inhalation produces a conditioned place preference in rats. Eur J Pharmacol. 2003;477(1):45–52. doi: 10.1016/j.ejphar.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 24.Lee DE, Gerasimov MR, Schiffer WK, Gifford AN. Concentration-dependent conditioned place preference to inhaled toluene vapors in rats. Drug Alcohol Depend. 2006;85(1):87–90. doi: 10.1016/j.drugalcdep.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Blokhina EA, Dravolina OA, Bespalov AY, Balster RL, Zvartau EE. Intravenous self-administration of abused solvents and anesthetics in mice. Eur J Pharmacol. 2004;485(1–3):211–218. doi: 10.1016/j.ejphar.2003.11.068. [DOI] [PubMed] [Google Scholar]
- 26.Tracy ME, Slavova-Hernandez GG, Shelton KL. Assessment of reinforcement enhancing effects of toluene vapor and nitrous oxide in intracranial self-stimulation. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shelton KL. Inhaled toluene vapor as a discriminative stimulus. Behav Pharmacol. 2007;18(3):219–229. doi: 10.1097/FBP.0b013e328157f460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shelton KL, Slavova-Hernandez G. Characterization of an inhaled toluene drug discrimination in mice: effect of exposure conditions and route of administration. Pharmacol Biochem Behav. 2009;92(4):614–620. doi: 10.1016/j.pbb.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rees DC, Coggeshall E, Balster RL. Inhaled toluene produces pentobarbital-like discriminative stimulus effects in mice. Life Sci. 1985;37(14):1319–1325. doi: 10.1016/0024-3205(85)90247-4. [DOI] [PubMed] [Google Scholar]
- 30.Bowen SE. Time course of the ethanol-like discriminative stimulus effects of abused inhalants in mice. Pharmacol Biochem Behav. 2009;91(3):345–350. doi: 10.1016/j.pbb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geller I, Hartmann RJ, Mendez V, Gause EM. Toluene inhalation and anxiolytic activity: possible synergism with diazepam. Pharmacol Biochem Behav. 1983;19(5):899–903. doi: 10.1016/0091-3057(83)90102-8. [DOI] [PubMed] [Google Scholar]
- 32.Bowen SE. Increases in amphetamine-like discriminative stimulus effects of the abused inhalant toluene in mice. Psychopharmacology (Berl) 2006;186(4):517–524. doi: 10.1007/s00213-006-0381-8. [DOI] [PubMed] [Google Scholar]
- 33.Bowen SE, Wiley JL, Jones HE, Balster RL. Phencyclidine- and diazepam- like discriminative stimulus effects of inhalants in mice. Exp Clin Psychopharmacol. 1999;7(1):28–37. doi: 10.1037//1064-1297.7.1.28. [DOI] [PubMed] [Google Scholar]
- 34.Bowen SE, Wiley JL, Balster RL. The effects of abused inhalants on mouse behavior in an elevated plus-maze. Eur J Pharmacol. 1996;312(2):131–136. doi: 10.1016/0014-2999(96)00459-1. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Rubalcava C, Hen R, Cruz SL. Anxiolytic-like actions of toluene in the burying behavior and plus-maze tests: differences in sensitivity between 5-HT(1B) knockout and wild-type mice. Behav Brain Res. 2000;115(1):85–94. doi: 10.1016/s0166-4328(00)00241-2. [DOI] [PubMed] [Google Scholar]
- 36.Paez-Martinez N, Cruz SL, Lopez-Rubalcava C. Comparative study of the effects of toluene, benzene, 1,1,1-trichloroethane, diethyl ether, and flurothyl on anxiety and nociception in mice. Toxicol Appl Pharmacol. 2003;193(1):9–16. doi: 10.1016/s0041-008x(03)00335-1. [DOI] [PubMed] [Google Scholar]
- 37.Wood RW, Coleman JB, Schuler R, Cox C. Anticonvulsant and antipunishment effects of toluene. J Pharmacol Exp Ther. 1984;230(2):407–412. [PubMed] [Google Scholar]
- 38.Cruz SL, Gauthereau MY, Camacho-Munoz C, Lopez-Rubalcava C, Balster RL. Effects of inhaled toluene and 1,1,1-trichloroethane on seizures and death produced by N-methyl-D-aspartic acid in mice. Behav Brain Res. 2003;140(1–2):195–202. doi: 10.1016/s0166-4328(02)00323-6. [DOI] [PubMed] [Google Scholar]
- 39.Chan MH, Chen HH. Toluene exposure increases aminophylline-induced seizure susceptibility in mice. Toxicol Appl Pharmacol. 2003;193(2):303–308. doi: 10.1016/j.taap.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Chen HH, Lee YF. Neonatal toluene exposure selectively alters sensitivity to different chemoconvulsant drugs in juvenile rats. Pharmacol Biochem Behav. 2002;73(4):921–927. doi: 10.1016/s0091-3057(02)00943-7. [DOI] [PubMed] [Google Scholar]
- 41.Chan MH, Lee CC, Chen HH. Effects of toluene on seizures induced by convulsants acting at distinct ligand-gated ion channels. Toxicol Lett. 2006;160(3):179–184. doi: 10.1016/j.toxlet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Tegeris JS, Balster RL. A comparison of the acute behavioral effects of alkylbenzenes using a functional observational battery in mice. Fundam Appl Toxicol. 1994;22(2):240–250. doi: 10.1006/faat.1994.1028. [DOI] [PubMed] [Google Scholar]
- 43.Chan MH, Chung SS, Stoker AK, Markou A, Chen HH. Sarcosine attenuates toluene-induced motor incoordination, memory impairment, and hypothermia but not brain stimulation reward enhancement in mice. Toxicol Appl Pharmacol. 2012;265(2):158–165. doi: 10.1016/j.taap.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cruz SL, Soberanes-Chavez P, Paez-Martinez N, Lopez-Rubalcava C. Toluene has antidepressant-like actions in two animal models used for the screening of antidepressant drugs. Psychopharmacology (Berl) 2009;204(2):279–286. doi: 10.1007/s00213-009-1462-2. [DOI] [PubMed] [Google Scholar]
- 45.Bowen SE, Balster RL. A direct comparison of inhalant effects on locomotor activity and schedule-controlled behavior in mice. Exp Clin Psychopharmacol. 1998;6(3):235–247. doi: 10.1037//1064-1297.6.3.235. [DOI] [PubMed] [Google Scholar]
- 46.Chan MH, Chien TH, Lee PY, Chen HH. Involvement of NO/cGMP pathway in toluene-induced locomotor hyperactivity in female rats. Psychopharmacology (Berl) 2004;176(3–4):435–439. doi: 10.1007/s00213-004-1900-0. [DOI] [PubMed] [Google Scholar]
- 47.Riegel AC, French ED. Acute toluene induces biphasic changes in rat spontaneous locomotor activity which are blocked by remoxipride. Pharmacol Biochem Behav. 1999;62(3):399–402. doi: 10.1016/s0091-3057(98)00062-8. [DOI] [PubMed] [Google Scholar]
- 48.Batis JC, Hannigan JH, Bowen SE. Differential effects of inhaled toluene on locomotor activity in adolescent and adult rats. Pharmacol Biochem Behav. 2010;96(4):438–448. doi: 10.1016/j.pbb.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Win-Shwe TT, Fujimaki H. Acute administration of toluene affects memory retention in novel object recognition test and memory function-related gene expression in mice. J Appl Toxicol. 2012;32(4):300–304. doi: 10.1002/jat.1693. [DOI] [PubMed] [Google Scholar]
- 50.Lo PS, Wu CY, Sue HZ, Chen HH. Acute neurobehavioral effects of toluene: involvement of dopamine and NMDA receptors. Toxicology. 2009;265(1–2):34–40. doi: 10.1016/j.tox.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Museridze DP, Tsaishvili TS, Svanidze IK, Gedevanishvli NS, Didimova EV, Gvinadze NN, Gegenava LG. Effect of Toluene Intoxication on Spatial Behavior and Learning of Rats within Early Stages of Postnatal Development. Neurophysiology. 2010;42(2):118–123. [Google Scholar]
- 52.Huerta-Rivas A, Lopez-Rubalcava C, Sanchez-Serrano SL, Valdez-Tapia M, Lamas M, Cruz SL. Toluene impairs learning and memory, has antinociceptive effects, and modifies histone acetylation in the dentate gyrus of adolescent and adult rats. Pharmacol Biochem Behav. 2012;102(1):48–57. doi: 10.1016/j.pbb.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 53.Lin BF, Ou MC, Chung SS, Pang CY, Chen HH. Adolescent toluene exposure produces enduring social and cognitive deficits in mice: an animal model of solvent- induced psychosis. World J Biol Psychiatry. 2010;11(6):792–802. doi: 10.3109/15622970903406234. [DOI] [PubMed] [Google Scholar]
- 54.Cruz SL, Paez-Martinez N, Pellicer F, Salazar LA, Lopez-Rubalcava C. Toluene increases acute thermonociception in mice. Behav Brain Res. 2001;120(2):213–220. doi: 10.1016/s0166-4328(00)00375-2. [DOI] [PubMed] [Google Scholar]
- 55.Paez-Martinez N, Aldrete-Audiffred J, Gallardo-Tenorio A, Castro-Garcia M, Estrada-Camarena E, Lopez-Rubalcava C. Participation of GABAA, GABA(B) receptors and neurosteroids in toluene-induced hypothermia: evidence of concentration-dependent differences in the mechanism of action. Eur J Pharmacol. 2013;698(1–3):178–185. doi: 10.1016/j.ejphar.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Chan MH, Lee CC, Lin BF, Wu CY, Chen HH. Metabotropic glutamate receptor 5 modulates behavioral and hypothermic responses to toluene in rats. Pharmacol Biochem Behav. 2012;103(2):418–424. doi: 10.1016/j.pbb.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 57.Gordon CJ, Gottipolu RR, Kenyon EM, Thomas R, Schladweiler MC, Mack CM, Shannahan JH, Wallenborn JG, Nyska A, MacPhail RC, Richards JE, Devito M, Kodavanti UP. Aging and susceptibility to toluene in rats: a pharmacokinetic, biomarker, and physiological approach. J Toxicol Environ Health A. 2010;73(4):301–318. doi: 10.1080/15287390903421144. [DOI] [PubMed] [Google Scholar]
- 58.Gordon CJ, Samsam TE, Oshiro WM, Bushnell PJ. Cardiovascular effects of oral toluene exposure in the rat monitored by radiotelemetry. Neurotoxicol Teratol. 2007;29(2):228–235. doi: 10.1016/j.ntt.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 59.Bowen SE, Mohammadi MH, Batis JC, Hannigan JH. Gestational toluene exposure effects on spontaneous and amphetamine-induced locomotor behavior in rats. Neurotoxicol Teratol. 2007;29(2):236–246. doi: 10.1016/j.ntt.2006.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bowen SE, Batis JC, Mohammadi MH, Hannigan JH. Abuse pattern of gestational toluene exposure and early postnatal development in rats. Neurotoxicol Teratol. 2005;27(1):105–116. doi: 10.1016/j.ntt.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 61.Jarosz PA, Fata E, Bowen SE, Jen KL, Coscina DV. Effects of abuse pattern of gestational toluene exposure on metabolism, feeding and body composition. Physiol Behav. 2008;93(4–5):984–993. doi: 10.1016/j.physbeh.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 62.Hass U, Lund SP, Hougaard KS, Simonsen L. Developmental neurotoxicity after toluene inhalation exposure in rats. Neurotoxicol Teratol. 1999;21(4):349–357. doi: 10.1016/s0892-0362(99)00013-6. [DOI] [PubMed] [Google Scholar]
- 63.Hougaard KS, Andersen MB, Hansen AM, Hass U, Werge T, Lund SP. Effects of prenatal exposure to chronic mild stress and toluene in rats. Neurotoxicol Teratol. 2005;27(1):153–167. doi: 10.1016/j.ntt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 64.Soberanes-Chavez P, Lopez-Rubalcava C, de Gortari P, Cruz SL. Exposure to toluene and stress during pregnancy impairs pups' growth and dams' lactation. Neurotoxicol Teratol. 2013;40C:9–16. doi: 10.1016/j.ntt.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 65.Bowen SE, Hannigan JH, Cooper PB. Abuse pattern of gestational toluene exposure alters behavior in rats in a "waiting-for-reward" task. Neurotoxicol Teratol. 2009;31(2):89–97. doi: 10.1016/j.ntt.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 66.von Euler G, Ogren SO, Li XM, Fuxe K, Gustafsson JA. Persistent effects of subchronic toluene exposure on spatial learning and memory, dopamine-mediated locomotor activity and dopamine D2 agonist binding in the rat. Toxicology. 1993;77(3):223–232. doi: 10.1016/0300-483x(93)90162-l. [DOI] [PubMed] [Google Scholar]
- 67.Bowen SE, Kimar S, Irtenkauf S. Comparison of toluene-induced locomotor activity in four mouse strains. Pharmacol Biochem Behav. 2010;95(2):249–257. doi: 10.1016/j.pbb.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bowen SE, Charlesworth JD, Tokarz ME, Wright MJ, Jr, Wiley JL. Decreased sensitivity in adolescent vs. adult rats to the locomotor activating effects of toluene. Neurotoxicol Teratol. 2007;29(6):599–606. doi: 10.1016/j.ntt.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cruz SL, Mirshahi T, Thomas B, Balster RL, Woodward JJ. Effects of the abused solvent toluene on recombinant N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1998;286(1):334–340. [PubMed] [Google Scholar]
- 70.Bale AS, Tu Y, Carpenter-Hyland EP, Chandler LJ, Woodward JJ. Alterations in glutamatergic and gabaergic ion channel activity in hippocampal neurons following exposure to the abused inhalant toluene. Neuroscience. 2005;130(1):197–206. doi: 10.1016/j.neuroscience.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 71.Bale AS, Smothers CT, Woodward JJ. Inhibition of neuronal nicotinic acetylcholine receptors by the abused solvent, toluene. Br J Pharmacol. 2002;137(3):375–383. doi: 10.1038/sj.bjp.0704874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Woodward JJ, Nowak M, Davies DL. Effects of the abused solvent toluene on recombinant P2X receptors expressed in HEK293 cells. Brain Res Mol Brain Res. 2004;125(1–2):86–95. doi: 10.1016/j.molbrainres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 73.Beckley JT, Woodward JJ. The abused inhalant toluene differentially modulates excitatory and inhibitory synaptic transmission in deep-layer neurons of the medial prefrontal cortex. Neuropsychopharmacology. 2011;36(7):1531–1542. doi: 10.1038/npp.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bale AS, Meacham CA, Benignus VA, Bushnell PJ, Shafer TJ. Volatile organic compounds inhibit human and rat neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Toxicol Appl Pharmacol. 2005;205(1):77–88. doi: 10.1016/j.taap.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 75.Beckstead MJ, Weiner JL, Eger EI, 2nd, Gong DH, Mihic SJ. Glycine and gamma-aminobutyric acid(A) receptor function is enhanced by inhaled drugs of abuse. Mol Pharmacol. 2000;57(6):1199–1205. [PubMed] [Google Scholar]
- 76.MacIver MB. Abused inhalants enhance GABA-mediated synaptic inhibition. Neuropsychopharmacology. 2009;34(10):2296–2304. doi: 10.1038/npp.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lopreato GF, Phelan R, Borghese CM, Beckstead MJ, Mihic SJ. Inhaled drugs of abuse enhance serotonin-3 receptor function. Drug Alcohol Depend. 2003;70(1):11–15. doi: 10.1016/s0376-8716(02)00330-7. [DOI] [PubMed] [Google Scholar]
- 78.Cruz SL, Orta-Salazar G, Gauthereau MY, Millan-Perez Pena L, Salinas-Stefanon EM. Inhibition of cardiac sodium currents by toluene exposure. Br J Pharmacol. 2003;140(4):653–660. doi: 10.1038/sj.bjp.0705481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gauthereau MY, Salinas-Stefanon EM, Cruz SL. A mutation in the local anaesthetic binding site abolishes toluene effects in sodium channels. Eur J Pharmacol. 2005;528(1–3):17–26. doi: 10.1016/j.ejphar.2005.10.069. [DOI] [PubMed] [Google Scholar]
- 80.Tillar R, Shafer TJ, Woodward JJ. Toluene inhibits voltage-sensitive calcium channels expressed in pheochromocytoma cells. Neurochem Int. 2002;41(6):391–397. doi: 10.1016/s0197-0186(02)00048-7. [DOI] [PubMed] [Google Scholar]
- 81.Shafer TJ, Bushnell PJ, Benignus VA, Woodward JJ. Perturbation of voltage-sensitive Ca2+ channel function by volatile organic solvents. J Pharmacol Exp Ther. 2005;315(3):1109–1118. doi: 10.1124/jpet.105.090027. [DOI] [PubMed] [Google Scholar]
- 82.Del Re AM, Dopico AM, Woodward JJ. Effects of the abused inhalant toluene on ethanol-sensitive potassium channels expressed in oocytes. Brain Res. 2006;1087(1):75–82. doi: 10.1016/j.brainres.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 83.Del Re AM, Woodward JJ. Inhibition of gap junction currents by the abused solvent toluene. Drug Alcohol Depend. 2005;78(2):221–224. doi: 10.1016/j.drugalcdep.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 84.Schiffer WK, Lee DE, Alexoff DL, Ferrieri R, Brodie JD, Dewey SL. Metabolic correlates of toluene abuse: decline and recovery of function in adolescent animals. Psychopharmacology (Berl) 2006;186(2):159–167. doi: 10.1007/s00213-006-0359-6. [DOI] [PubMed] [Google Scholar]
- 85.Riegel AC, French ED. An electrophysiological analysis of rat ventral tegmental dopamine neuronal activity during acute toluene exposure. Pharmacol Toxicol. 1999;85(1):37–43. doi: 10.1111/j.1600-0773.1999.tb01061.x. [DOI] [PubMed] [Google Scholar]
- 86.Riegel AC, Zapata A, Shippenberg TS, French ED. The abused inhalant toluene increases dopamine release in the nucleus accumbens by directly stimulating ventral tegmental area neurons. Neuropsychopharmacology. 2007;32(7):1558–1569. doi: 10.1038/sj.npp.1301273. [DOI] [PubMed] [Google Scholar]
- 87.Gerasimov MR, Schiffer WK, Marstellar D, Ferrieri R, Alexoff D, Dewey SL. Toluene inhalation produces regionally specific changes in extracellular dopamine. Drug Alcohol Depend. 2002;65(3):243–251. doi: 10.1016/s0376-8716(01)00166-1. [DOI] [PubMed] [Google Scholar]
- 88.Beckley JT, Evins CE, Fedarovich H, Gilstrap MJ, Woodward JJ. Medial prefrontal cortex inversely regulates toluene-induced changes in markers of synaptic plasticity of mesolimbic dopamine neurons. J Neurosci. 2013;33(2):804–813. doi: 10.1523/JNEUROSCI.3729-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Win-Shwe TT, Mitsushima D, Nakajima D, Ahmed S, Yamamoto S, Tsukahara S, Kakeyama M, Goto S, Fujimaki H. Toluene induces rapid and reversible rise of hippocampal glutamate and taurine neurotransmitter levels in mice. Toxicol Lett. 2007;168(1):75–82. doi: 10.1016/j.toxlet.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 90.Perrine SA, O'Leary-Moore SK, Galloway MP, Hannigan JH, Bowen SE. Binge toluene exposure alters glutamate, glutamine and GABA in the adolescent rat brain as measured by proton magnetic resonance spectroscopy. Drug Alcohol Depend. 2011;115(1–2):101–106. doi: 10.1016/j.drugalcdep.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ladefoged O, Strange P, Moller A, Lam HR, Ostergaard G, Larsen JJ, Arlien-Soborg P. Irreversible effects in rats of toluene (inhalation) exposure for six months. Pharmacol Toxicol. 1991;68(5):384–390. doi: 10.1111/j.1600-0773.1991.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 92.Williams JM, Stafford D, Steketee JD. Effects of repeated inhalation of toluene on ionotropic GABA A and glutamate receptor subunit levels in rat brain. Neurochem Int. 2005;46(1):1–10. doi: 10.1016/j.neuint.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 93.Lee YF, Lo PS, Wang YJ, Hu A, Chen HH. Neonatal toluene exposure alters N-methyl-D-aspartate receptor subunit expression in the hippocampus and cerebellum in juvenile rats. Neuropharmacology. 2005;48(2):195–203. doi: 10.1016/j.neuropharm.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 94.Chen HH, Wei CT, Lin YR, Chien TH, Chan MH. Neonatal toluene exposure alters agonist and antagonist sensitivity and NR2B subunit expression of NMDA receptors in cultured cerebellar granule neurons. Toxicol Sci. 2005;85(1):666–674. doi: 10.1093/toxsci/kfi100. [DOI] [PubMed] [Google Scholar]
- 95.Zhvania MG, Chilachava LR, Japaridze NJ, Gelazonia LK, Lordkipanidze TG. Immediate and persisting effect of toluene chronic exposure on hippocampal cell loss in adolescent and adult rats. Brain Res Bull. 2012;87(2–3):187–192. doi: 10.1016/j.brainresbull.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 96.Paez-Martinez N, Flores-Serrano Z, Ortiz-Lopez L, Ramirez-Rodriguez G. Environmental enrichment increases doublecortin-associated new neurons and decreases neuronal death without modifying anxiety-like behavior in mice chronically exposed to toluene. Behav Brain Res. 2013;256:432–440. doi: 10.1016/j.bbr.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 97.Sanchez-Serrano SL, Cruz SL, Lamas M. Repeated toluene exposure modifies the acetylation pattern of histones H3 and H4 in the rat brain. Neurosci Lett. 2011;489(3):142–147. doi: 10.1016/j.neulet.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 98.Kodavanti PR, Royland JE, Richards JE, Besas J, Macphail RC. Toluene effects on oxidative stress in brain regions of young-adult, middle-age, and senescent Brown Norway rats. Toxicol Appl Pharmacol. 2011;256(3):386–398. doi: 10.1016/j.taap.2011.04.012. [DOI] [PubMed] [Google Scholar]